Published online Oct 27, 2024. doi: 10.4254/wjh.v16.i10.1199
Revised: August 13, 2024
Accepted: September 14, 2024
Published online: October 27, 2024
Processing time: 225 Days and 11.1 Hours
Occult hepatitis B infection (OBI) is characterized by the detection of hepatitis B virus (HBV) DNA in serum (usually HBV DNA < 200 IU/mL) or the liver but negativity for hepatitis B surface antigen (HBsAg). The diagnosis of OBI relies on the sensitivity of assays used in the detection of HBV DNA and HBsAg. HBsAg assays with inadequate sensitivity or inability to detect HBV S variants may lead to misdiagnosis of OBI in people with overt HBV infection.
We report a HBsAg-negative but hepatitis B envelope antigen-positive patient who had a significant HBV DNA level. The patient was initially diagnosed as having OBI. However, sequence analysis revealed a unique insertion of amino acid residues at positions 120-124 in the S protein, which affects the formation of a disulfide bond that is associated with the formation of a loop. It is well known that there is an overlap between the S protein and Pol protein. We found that this new insertion site occurred in polymerase/reverse transcriptase domain, indi
An insertion of amino acid residues at positions 120-124 of the S protein affects the formation of immunodominant epitopes and results in negative HBsAg levels.
Core Tip: We report a hepatitis B surface antigen-negative but hepatitis B envelope antigen-positive patient who had a significant hepatitis B virus (HBV) DNA level. The patient was initially diagnosed as having OBI. However, sequence analysis revealed a unique insertion of amino acid residues at positions 120-124 in the S protein, which affects the formation of a disulfide bond that is associated with the formation of a loop. It is well known that there is an overlap between the S protein and Pol protein. We found that this new insertion site occurred in polymerase/reverse transcriptase domain, indicating that this insertion might be involved in HBV pathogenicity. The patient was finally diagnosed with a false OBI.
- Citation: Yang SS, Fu F, Xuan QK, Zhang ZX, Li ZJ, Li GB, Yu XY. Hepatitis B surface antigen-negative but hepatitis B envelope antigen-positive false occult hepatitis B virus infection: A case report. World J Hepatol 2024; 16(10): 1199-1207
- URL: https://www.wjgnet.com/1948-5182/full/v16/i10/1199.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i10.1199
In order to investigate the biological and clinical implications of occult hepatitis B virus infection (OBI), an international workshop on OBI, supported by the European association for the study of the liver (EASL), was convened in Taormina, Italy in 2008[1]. An article titled “Update of the statements on biology and clinical impact of OBI” was published in the J Hepatol in 2019[2]. Because of the low prevalence of OBI and the gradual updates to the Taormina statement on OBI, clinicians are often unfamiliar with the diagnosis and disease characteristics of OBI.
Even if patients are negative for hepatitis B surface antigen (HBsAg), there may exist hepatitis B virus (HBV) DNA in the liver and/or blood, which is a typical feature of OBI[2]. According to a recent meta-analysis, OBI has a prevalence of only 0.82% [95% confidence interval (CI): 0.69-0.96] in the general population worldwide. A similar prevalence as low as 0.88% has also been reported in the general population in China[3]. As reported in the 2019 Taormina statement on OBI, the prevalence rates of OBI vary greatly, from as low as less than 1% to as high as 87%. However, the prevalence of OBI can be affected by various factors, such as the specific risk group studied, the methodologies used for sampling, the sensitivity of the assays employed, and the prevalence of HBsAg in the geographical district where the study was carried out. Patients who are coinfected with hepatitis C virus (15%-33%), who undergo dialysis (27%), or with hepatocellular carcinoma (62%), or with cryptogenic cirrhosis (32%), or recipients of liver transplantation (64%), have been found to have higher rates of HBV infection.
With respect to the diagnostic criteria for OBI, both the 2008 and 2019 Taormina statements on OBI reported that HBV DNA could be detected in blood or the liver of HBsAg-negative individuals, in spite of requirement of a very low HBV DNA level (< 200 IU/mL, about 1000 copies/mL). Cases with serum HBV DNA levels similar to those that are often discovered in different stages of serologically evident (overt) HBV infection are thought as having false OBI. For these cases, it is necessary to identify the S-gene variants in order to make an accurate diagnosis and to determine their potential clinical implications[1]. Several HBV variants with replacements, deletions, or insertions in the α determinant have been reported worldwide. Immune prophylaxis failure and false-negative HBsAg results can be caused by these variants. Multivalent anti-HBs antibodies in HBsAg assays should be used to detect these variants successfully.
In this report, we describe a patient with OBI who tested negative for HBsAg but positive for hepatitis B envelope antigen (HBeAg) and exhibited an HBV DNA level suggesting overt infection for 4 years. The identification of S antigen variation proved that the patient was indeed a false OBI case.
A 70-year-old woman was hospitalized for cerebral ischemia caused by hypertension in December 2017. Upon routine examination, abnormal hepatitis B marker patterns were detected. HBV serological markers were detected using commercially available qualitative enzyme immunoassay kits (Elecsys HBsAg II quant II, Cobas). The patient was found to be HBsAg-negative and anti-HBc/anti-HBe-negative, but he was positive for anti-HBs (> 1000 mIU/mL) and HBeAg (60.64 S/Co). Serum HBV DNA level was detected using a real-time polymerase chain reaction (PCR) kit (S3013E HBV fast-HBV DNA quantitative fluorescence diagnostic kit, Sansure Biotech Inc.) on a Roche Z480 real-time PCR system, and the result was 3.82 × 104 IU/mL.
The patient had hypertension and OBI.
The patient had a past history of hypertension. She had no history of blood transfusions, jaundice, or liver disease. In addition, she had never received any treatment with interferons or anti-HBV nucleoside/nucleotide analogs. Besides, she had not any risk factors for HBV infection, including hepatitis C virus or human immunodeficiency virus infections, or intravenous drug use.
The patient denied any personal and family history of HBV infection.
All liver function tests including alanine transaminase (ALT) (13 U/L), aspartate aminotransferase (AST) (26 U/L), total bilirubin (9.7 μmol/L), prothrombin time (12.2 s), international normalized ratio (1.01), fibrinogen (3.4 g/L), activated partial thromboplastin time (24.6 s), thrombin time (18.5 seconds), and alpha-fetoprotein (2.16 ng/mL) were generally normal. All autoimmunity indices did not exceed normal limits. When she stayed in the cardiovascular department, because no HBsAg was detected and ALT and AST levels were normal, she was classified as having OBI and did not receive special treatment. The patient was hospitalized every year between 2017-2022 due to coronary atherosclerotic heart disease. Due to her history of OBI, her HBV markers were re-examined each time when she was hospitalized. HBV markers, liver function, and liver B-ultrasound examinations were recorded during these 4 years (Table 1). The patient’s HBV DNA level measured last time in July 2021 was 2.72 × 104 IU/mL, which was similar to the level found originally in 2017.
| Index | Normal range | December 2017 | March 2018 | April 2019 | June 2019 | August 2019 | October 2019 | June 2020 | July 2021 |
| ALT (U/L) | < 40 | 13 | 36 | 11 | 14 | 31 | 24 | 17 | 13 |
| AST (U/L) | < 35 | 26 | 23 | 17 | 18 | 25 | 18 | 14 | 14 |
| TBIL (μmol/L) | < 15 | 9.7 | 6.2 | 6.2 | 9.7 | 9.8 | 8.6 | 9 | 8.2 |
| PT (s) | 11-13 | 12.2 | 17.9 | 12.5 | |||||
| INR | 0.8-1.5 | 1.01 | 1.55 | 0.97 | 1.09 | 0.96 | 0.93 | 0.93 | 1.06 |
| FIB (g/L) | 1.8-3.5 | 3.4 | 4.37 | 2.72 | |||||
| APTT (s) | 25-31.3 | 24.6 | 30.8 | 22.9 | |||||
| TT (s) | 14-21 | 18.5 | 16.3 | 17.8 | |||||
| AFP (ng/mL) | ≤ 7 | 2.16 | 1.77 | 1.67 | 1.75 | 1.86 | 1.79 | 2 | 1.7 |
| HBsAg (IU/mL) | < 1 | 0.650 | 0.377 | 0.287 | 0.330 | 0.371 | 0.288 | 0.394 | 0.397 |
| Anti-HBs (mIU/mL) | < 10 | > 1000 | > 1000 | > 1000 | > 1000 | > 1000 | > 1000 | > 1000 | > 1000 |
| HBeAg (S/Co) | < 1.0 | 60.64 | 134.4 | 62.69 | 27.9 | 17.72 | 12.79 | 10.4 | 11.82 |
| Anti-HBe (S/Co) | > 1.0 | 1.3 | 1.39 | 1.1 | 0.994 | 1.01 | 1.06 | 1.09 | 1.03 |
| Anti-HBc (S/Co) | > 1.0 | 0.009 | 0.007 | 0.007 | 0.008 | 0.008 | 0.007 | 0.007 | 0.006 |
| HBV DNA (IU/mL) | 3.82 × 104 | 6.82 × 105 | 2.72 × 104 | ||||||
| HCV DNA (IU/mL) | < 50 | < 50 | < 50 | < 50 | < 50 | < 50 | < 50 | < 50 | < 50 |
| HIV antibody | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| HLA | HLA-A2 | HLA-A2 | HLA-A2 | ||||||
| Hepatic steatosis on B-ultrasound | - | Yes | - | Yes | Yes | - | - | Yes |
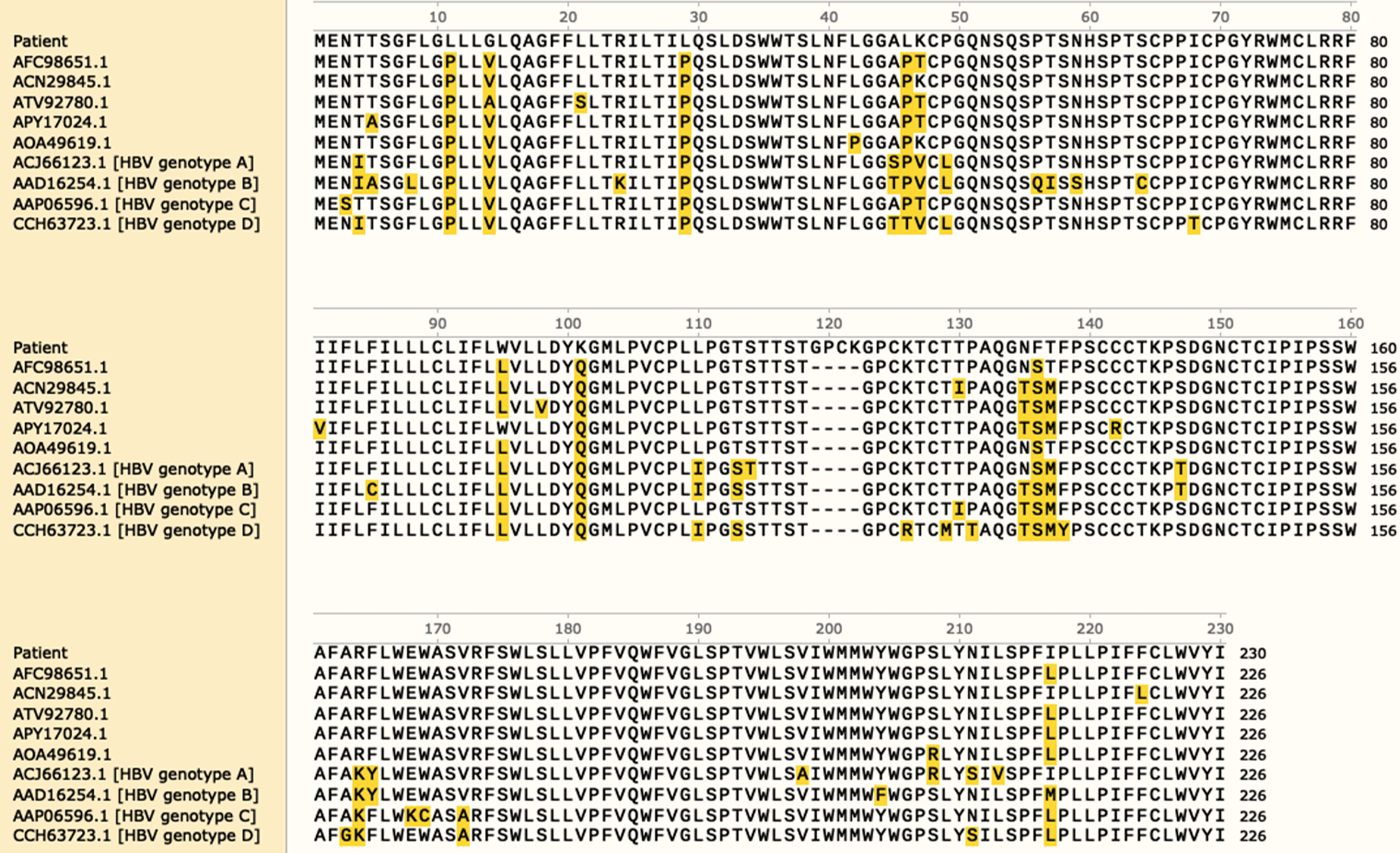
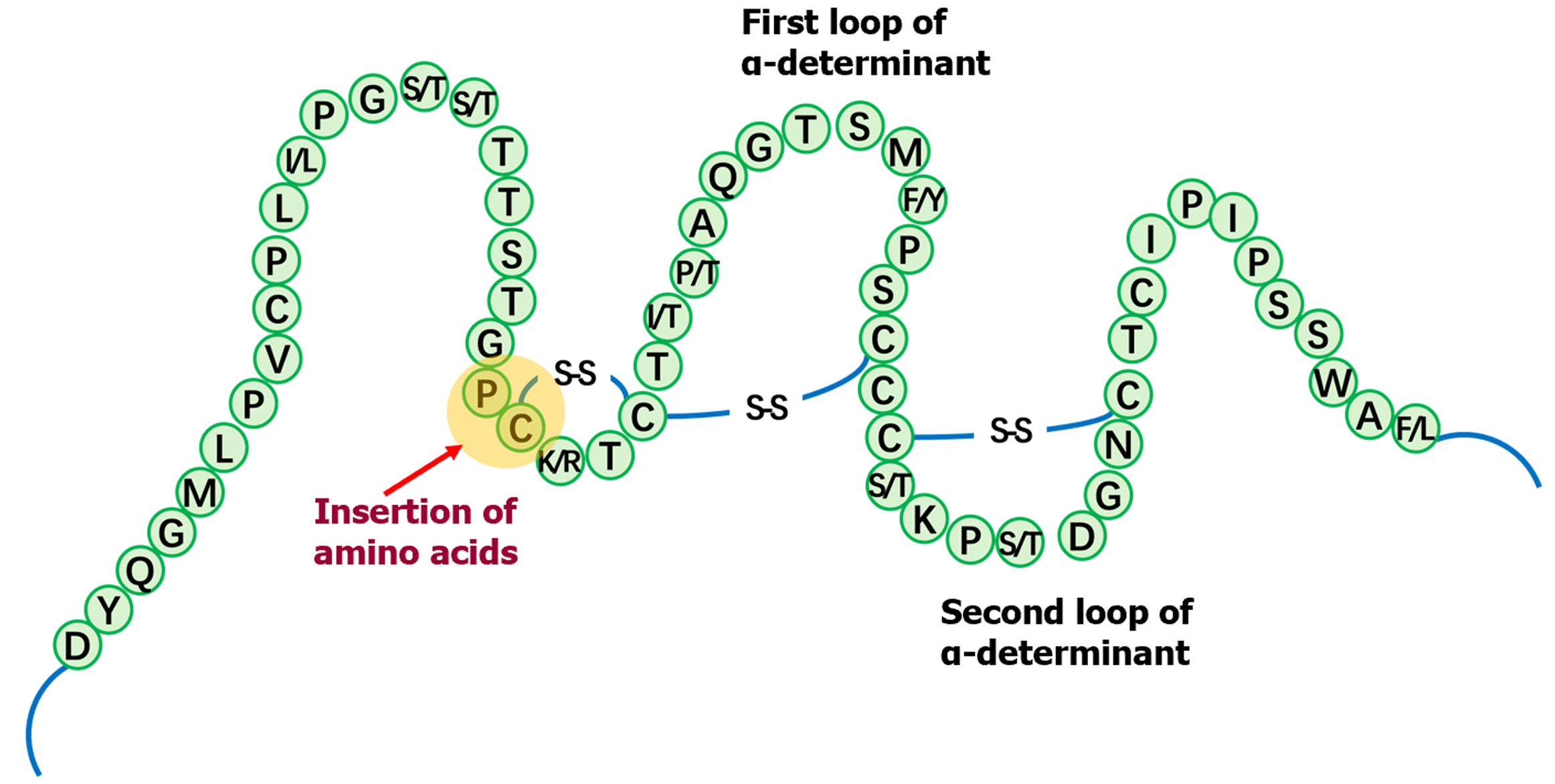
It was unclear why this OBI patient was positive for HBeAg. Sequence analysis revealed that there was an insertion of four amino acid residues in the S protein, which led to changes in the formation of a disulfide bond that is associated with the formation of a loop. In addition, the α determinant of HBsAg showed a unique immune escape mutant (Figure 1 and Figure 2).
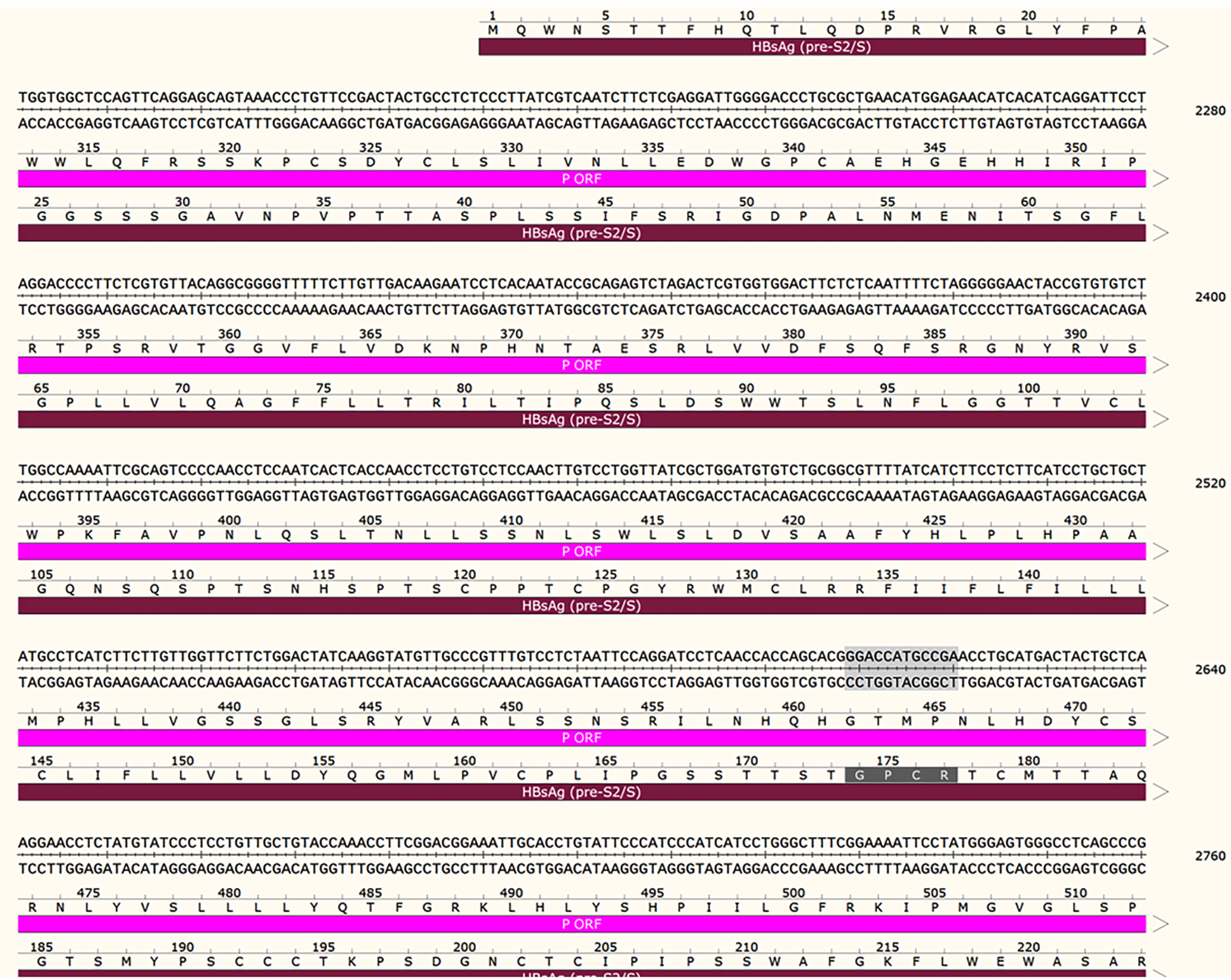
It is well documented that the gene coding sequences of S protein and Pol protein overlap. The polymerase/reverse transcriptase (RT) domain of HBV was defined by amino acid residues 336-679. In this patient, the insertion of the four amino acid residues in the S protein was also situated in the RT, indicating that changes in RT activity occurred during HBV infection (Figure 3).
Taking the overt HBV infection and S-escape variants into account, we finally proved that his patient had a false OBI after reviewing the literature (Table 2). It was uncovered by the following post hoc analysis that the previous physicians ignored some very important information when diagnosing the patient. For example, noninfectious disease physicians had not paid attention to the patient’s HBV DNA levels nor the conflicting results of a positive HBeAg test coexisting with a negative HBsAg test, both of which are unusual in OBI. Furthermore, the use of insensitive commercial kits for detecting HBsAg also contributed to a misleading diagnosis.
| Ref. | Case | Biochemical and virological characteristics | Serological characteristics | History of HBV infection history and treatment | Sequencing of the S gene |
| Han et al[4] 2015, China | Case 1: A 56-year-old man | ALT (U/L): Normal; AST (U/L): Normal; HBV DNA (IU/mL): 234-567 | HBsAg: - | Assumed to have resolved HBV infection, treatment naive | Double mutations (A1762T and G1764A) in BCP; no mutations in the preC/C gene; the α determinant sequences were all mutant type |
| HBsAb: + | |||||
| HBeAg: + | |||||
| HBeAb: - | |||||
| HBcAb: + | |||||
| Case 2: An 81-year-old man | ALT (U/L): Normal; AST (U/L): Normal; HBV DNA (IU/mL): 1130-135 | HBsAg: - | Diagnosed with HCC with no known HBV infection history | Double mutations (A1762T and G1764A) in BCP; no mutations in the preC/C gene; three residue substitutions in the α determinant | |
| HBsAb: + | |||||
| HBeAg: + | |||||
| HBeAb: - | |||||
| HBcAb: + | |||||
| Zhou et al[5] 2009, China | Case 1: A 50-year-old man | ALT (U/L): Normal; AST (U/L): Not done; HBV DNA: 370-491 copies/mL | HBsAg: - | Not known | The insertion is a perfect repeat of the preceding 15 nts. This insert resulted in an insertion of 5 amino acids between residues 128 and 129 in HBsAg, which is located in the α determinant. HBV variant had mutations both in preS2 and S proteins |
| HBsAb: + | |||||
| HBeAg: + | |||||
| HBeAb: - | |||||
| HBcAb: + | |||||
| Paparella et al[6] 2010, Italy | Case 1: A 76-year-old man | ALT(U/L): Normal; HBV DNA: < 40 IU/mL | HBsAg: - | Peritoneal dialysis since 2004 and regarded as naturally immune to HBV in 2007 | Serotype adw2, one immune escape mutation (G145R), and no drug resistance mutations |
| HBsAb: + | |||||
| HBeAg: + | |||||
| HBeAb: - | |||||
| HBcAb: - |
False OBI.
The patient had never received treatment with interferons or anti-HBV nucleoside/nucleotide analogs. She did not receive specialized management due to undetectable HBsAg and normal ALT and AST levels.
The levels of ALT, AST, and total bilirubin remained in normal ranges in the subsequent follow-up. The patient’s HBV DNA level was measured last time in July 2021, and it was 2.72 × 104 IU/mL, which is similar to the level found originally in 2017.
The case presented here is a patient who was negative for HBsAg, but positive for HBeAg and HBV DNA. At the beginning, since noninfectious disease physicians believed that HBsAg-/HBV DNA+ defines OBI, they diagnosed the patient as having OBI. Since the prevalence of OBI is only 0.82% in the world, the physicians display an unfamiliarity with this condition[3]. Even, the 2018 American Association for the Study of Liver Diseases chronic hepatitis B (CHB) guidelines[4] do not include a particular part to deal with OBI. In the 2017 EASL[5] and 2019 China CHB guidelines[6], only HBsAg-/HBV DNA+ is mentioned for the definition of OBI, and there is no requirement of HBV DNA < 200 IU/mL. Only the 2008/2019 Taormina statements on OBI emphasize HBV DNA levels as the only reliable diagnostic marker for OBI and explain that the patients’ HBV DNA levels should be < 200 IU/mL. The molecular basis for OBI can be accounted for by the low levels of transcriptionally active covalently closed circular DNA (cccDNA). This may cause low or undetectable HBV RNA transcription and the following protein translation and expression. The low viral load indicates that HBV is in effective control by the immune system in most patients with OBI. False OBI patients may lose chances to receive the best treatment if they are not correctly classified. HBV DNA levels play a crucial role in deciding treatment and follow-up. Active CHB is possible in cases of HBsAg-/HBV DNA+ when HBV DNA levels are considered. The fact that noninfectious disease physicians may not realize that HBV DNA levels are very important for the diagnosis of OBI promotes us to report the present case.
The concept of false OBI is also mentioned in the 2008/2019 Taormina statements on OBI. Patients whose serum HBV DNA levels are similar to those in the different stages of serologically evident (overt) HBV infection should be suspected to have false OBI due to infections caused by HBV variants with mutations in the S gene (escape mutants). These variants result in a modified HBsAg which some or all commercially available detection assays may not identify[1]. In the present case, due to the belief that a false negative HBsAg result might take place because the conventional HBsAg assays might be not sensitive enough to or be unable to identify HBV S variants, we cloned the gene encoding the α determinant of HBsAg. The sequence analysis revealed a unique insertion of amino acid residues at positions 120-124 in the S protein, which affects the formation of a disulfide bond that is associated with the formation of a loop. It is well-known that the gene coding sequences of S protein and Pol protein overlap. Here, we found that our newly identified insertion site was located in the RT, indic
Next, we sought to understand why HBeAg remained persistently positive in this patient. HBeAg is related to active replication and it emerges often together with HBsAg seroconversion and/or a rise in serum HBV DNA levels, which is representative of HBV reactivation in OBI patients. A literature search revealed that seven out of ten articles described the reactivation of HBV[7-13], and only three articles reported four confirmed OBI cases that were HBeAg-positive. Besides, all patients possessed S gene escape mutants, while only two cases reported HBV DNA levels < 200 IU/mL. In the first case reported in 2009, Zhou et al[14] concluded that the HBeAg test may identify HBV carriers with mutant HBsAg. In the second case reported in 2010, Paparella et al[15] thought that reactivation of a latent HBV infection, associated with escape mutants, or an infection sustained by an HBV strain carrying one significant mutation in the “S” gene, occurred in the patient. For these two patients, the weak replication competence of this mutant or the neutralizing effect, albeit incomplete, of the pre-existing anti-HBs antibodies, was responsible for the occult pattern. Besides, an article published by Han et al[16] in 2015 reported that there were two patients whose HBV DNA levels were more than 200 IU/mL. Their study showed that true OBI may occur due to infection with HBV with S gene escape mutants. This suggests that it is possible that a unique subtype of OBI may exist for patients negative for HBsAg but positive for HBeAg. They proposed that testing for HBeAg is critical for identifying this type of OBI.
In the current study, we sought to determine whether HBeAg positivity represents a unique type of OBI. According to a study including data from plasma samples of 1261 HBsAg-/HBV DNA+ blood donors in China, which were transported to the National Center for Clinical Laboratories (NCCL) between January 2010 and December 2013[17], samples identified as HBsAg-/HBV DNA+ (with HBV DNA load < 200 IU/mL) and either anti-HBc+ or anti-HBs+ were divided into the OBI group. Since they were not able to rule out a pre-seroconversion window period for the lack of follow-up, they did not include samples that were both anti-HBc-negative and anti-HBs-negative. Among the 1261 blood donors screened in the beginning, 918 (72.8%) were OBI carriers. Host and viral markers were available for 906 OBI donors; nevertheless, none of the samples were reported as HBeAg+ or characterized as unique OBI cases with HBeAg+. We believe that this represents the largest and most up-to-date OBI database and that the NCCL unified testing method is more reliable than earlier case reports; therefore, we used this method. To conclude, we believe that HBeAg+ with HBsAg-/HBV DNA+ is a key indicator of possible active CHB instead of representing a distinct form of OBI. However, more data is required to confirm our hypothesis. Finally, it is critical for physicians to understand how to treat these true or false OBI patients. For true OBI cases, current guidelines and the 2019 Taormina statement update on OBI state that antiviral therapy is not recommended for individuals with OBI, and that drugs to eradicate cccDNA and integrated HBV DNA are not available.
Discussions on false OBI cases are limited. Notarnicola et al[18] reported the development of false OBI in a patient with psoriatic arthritis undergoing infliximab and methotrexate therapy. The definition of false OBI is predicated on the occurrence of HBV infection in the absence of seroconversion and with very high levels of serum HBV DNA, but undetectable levels of HBsAg. Interestingly, HBV DNA levels were not measured in this patient when she was first treated with infliximab plus methotrexate in 2005 or when referred to our department in December 2010. After half a year, it was identified that the patient had false OBI, with HBsAg being still negative.
Here, we have discussed a case of false OBI, which was quite different from the above-mentioned cases. We hope to remind clinical physicians to be cautious with “false” OBI, i.e., the possibility of infectious CHB instead of OBI, in which the DNA levels indicate that it is overt infection and HBsAg is not identified by some commercially available HBsAg assays. Recently, Yuan et al[19] reported an OBI with HBeAg+ and HBsAg-/HBV DNA+ (2.32 × 103 IU/mL). They administered entecavir treatment to the patient. After 4 wk, the patient’s HBV DNA level was less than 100 IU/mL, which was maintained for half a year. Later, the patient voluntarily stopped entecavir for half a year. In this period, his HBV DNA level increased to 2.79 × 103 IU/mL. As a result, the patient was recommended to receive entecavir treatment again, which resulted in undetectable HBV DNA levels once more. Unfortunately, the patient again voluntarily stopped entecavir therapy and finally developed chronic HBV infection with drug resistance. It is not clear why the patient repeatedly stopped the treatment, although it is possible that the patient had difficulties in managing long-term compliance.
There are several limitations to the current study. First, it was a single case report. Second, Since this is the first report of a unique insertion of amino acid residues at positions 120 to 124 in the S protein, the effects of the insertion on HBsAg production patterns require further validation.
This case report highlights two points: (1) An insertion of amino acid residues at positions 120-124 affects the formation of a disulfide bond and subsequent formation of immunodominant epitopes. And this insertion results in negative HBsAg levels; and (2) The possibility of false OBI should be considered in patients who are simultaneously negative for HBsAg and positive for HBeAg.
We thank Professor Liu KC for help in HBV molecular analysis. We also thank Fan W for help in HBV testing.
| 1. | Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta GB, Gerlich WH, Levrero M, Locarnini S, Michalak T, Mondelli MU, Pawlotsky JM, Pollicino T, Prati D, Puoti M, Samuel D, Shouval D, Smedile A, Squadrito G, Trépo C, Villa E, Will H, Zanetti AR, Zoulim F. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 619] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 2. | Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 400] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 3. | Ji DZ, Pang XY, Shen DT, Liu SN, Goyal H, Xu HG. Global prevalence of occult hepatitis B: A systematic review and meta-analysis. J Viral Hepat. 2022;29:317-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3101] [Article Influence: 387.6] [Reference Citation Analysis (1)] |
| 5. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4014] [Article Influence: 446.0] [Reference Citation Analysis (1)] |
| 6. | Zhang WH. [Guidelines for the prevention and treatment of chronic hepatitis B (2019 edition)]. Zhonghua Chuanranbing Zazhi. 2019;37. [DOI] [Full Text] |
| 7. | Fabbri G, Mastrorosa I, Vergori A, Mazzotta V, Pinnetti C, Grisetti S, Zaccarelli M, Ammassari A, Antinori A. Reactivation of occult HBV infection in an HIV/HCV Co-infected patient successfully treated with sofosbuvir/ledipasvir: a case report and review of the literature. BMC Infect Dis. 2017;17:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Muraishi J, Shibata M, Honma Y, Hiura M, Abe S, Harada M. Reactivation of Occult Hepatitis B Virus Infection 27 Months after the End of Chemotherapy Including Rituximab for Malignant Lymphoma. Intern Med. 2017;56:1967-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Talotta R, Atzeni F, Sarzi Puttini P. Reactivation of occult hepatitis B virus infection under treatment with abatacept: a case report. BMC Pharmacol Toxicol. 2016;17:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Kamitsukasa H, Iri M, Tanaka A, Nagashima S, Takahashi M, Nishizawa T, Okamoto H. Spontaneous reactivation of hepatitis B virus (HBV) infection in patients with resolved or occult HBV infection. J Med Virol. 2015;87:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Du W, Zheng Z, Han S, Ma S, Chen S. HBV reactivation in an occult HBV infection patient treated with prednisone for nephrotic syndrome: case report and literature review. BMC Infect Dis. 2013;13:394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 12. | Feeney SA, McCaughey C, Watt AP, Agnaf MR, McDougall N, Wend UC, Gerlich WH, Coyle PV. Reactivation of occult hepatitis B virus infection following cytotoxic lymphoma therapy in an anti-HBc negative patient. J Med Virol. 2013;85:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Imamura T, Yokosuka O, Chiba T, Kanda T, Kojima H, Fukai K, Imazeki F, Nishimura M, Saito Y, Saisho H. Lamivudine treatment in a patient with hepatitis B virus reactivation after allogenic peripheral bone marrow transplantation. Leuk Lymphoma. 2005;46:915-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Zhou YH, Zhou J, Li L, Bi Y, Liu Y, Pan J, Wu C. A novel hepatitis B virus mutant coexisting with wild type virus in a carrier with negative HBsAg yet positive HBeAg and anti-HBs. J Clin Virol. 2009;46:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Paparella C, De Rosa F, Longo R, Cappiello G, Ursitti A, Rosa M, Morosetti M, Spanò A. Appearance of HbeAg in an occult persistent hepatitis B virus infection. Intervirology. 2010;53:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Han Z, Liu Y, Pan J, Bi Y, Liu J, Zhou YH. Occult hepatitis B virus infection with positive hepatitis B e antigen. Clin Chim Acta. 2015;438:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Zhang L, Chang L, Laperche S, Ji H, Zhao J, Jiang X, Wang L, Candotti D. Occult HBV infection in Chinese blood donors: role of N-glycosylation mutations and amino acid substitutions in S protein transmembrane domains. Emerg Microbes Infect. 2019;8:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Notarnicola A, Iannone F, Lopalco G, Covelli M, Lapadula G. A false occult hepatitis B virus infection developed in a patient with psoriatic arthritis under infliximab and methotrexate therapy. Reumatismo. 2014;65:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Yuan C, Peng J, Xia R, He J, Qiu T, Yao Y. Reactivation of Occult Hepatitis B Virus Infection During Long-Term Entecavir Antiviral Therapy. Front Microbiol. 2022;13:865124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/