Published online Oct 27, 2024. doi: 10.4254/wjh.v16.i10.1188
Revised: September 12, 2024
Accepted: September 19, 2024
Published online: October 27, 2024
Processing time: 175 Days and 20.6 Hours
Complement activation is recognized as an important factor in the progression of liver damage caused by acetaminophen (APAP). However, the role of the com
To explore C2-FH in protecting against APAP-induced liver injury by inhibiting complement activation.
A model of APAP-induced liver injury was used to study the protective effect of C2-FH on liver injury. C2-FH was administered through intraperitoneal injection 30 minutes after APAP treatment. We detected the effects of C2-FH on liver function, inflammatory response and complement activation. Additionally, RNA-sequencing (RNA-Seq) analysis was conducted to understand the mechanism through which C2-FH provides protection against APAP-induced liver injury.
C2-FH inhibited the increase in serum alanine aminotransferase activity, aspartate aminotransferase activity and lactate dehydrogenase, and reduced liver tissue necrosis caused by APAP. Moreover, it attenuated the inflammatory response and inhibited complement activation in APAP-induced liver injury. RNA-Seq analysis provided additional explanations for the protective role of C2-FH against APAP-induced liver injury.
C2-FH attenuates APAP-induced liver injury by inhibiting complement activation.
Core Tip: Acetaminophen (APAP) is frequently utilized as a non-prescription medication for reducing fever and relieving pain. However, excessive intake of APAP may result in acute liver injury, liver failure and even death. This study found that C2-FH improved APAP-induced liver injury by alleviating liver tissue injury, inflammation and reducing the accumulation of complement activated products. Our research provided evidence that C2-FH shows potential as a possible treatment for APAP-induced liver injury.
- Citation: Li CM, Sun T, Yang MJ, Yang Z, Li Q, Shi JL, Zhang C, Jin JF. Complement activation targeted inhibitor C2-FH ameliorates acetaminophen-induced liver injury in mice. World J Hepatol 2024; 16(10): 1188-1198
- URL: https://www.wjgnet.com/1948-5182/full/v16/i10/1188.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i10.1188
Acetaminophen (APAP), also known as paracetamol, is frequently utilized as a nonprescription medication for reducing fever and relieving pain. However, it is important to note that APAP carries a potential risk of overdose. Excessive intake may result in endoplasmic reticulum stress in hepatocytes, which can lead to oxidative stress, inflammation, apoptosis and necrosis of liver cells. These effects can ultimately result in acute liver injury, liver failure and even death[1,2]. Treating APAP-induced acute liver injury and its mechanism remains a challenge despite the abundance of research studies.
Three pathways, including the classical pathway, lectin pathway and alternative pathway, can activate the complement system. These routes come together at a shared final pathway that triggers the central element C3[3]. C3 is cleaved into C3a and C3b fragments by C3 convertase, while C5 is cleaved into C5a and C5b fragments by C5 convertase[4,5]. Complement regulatory factors, such as membrane-binding proteins and liquid phase proteins, are essential for protecting host cells from attacks. FH is a soluble complement regulatory protein and acts as an inhibitor of complement activation. FH binds to C3 fragments, recognizing specific polyanionic markers on host cells, thus inhibiting the activation and enhancement of complement on host cells and tissues[6,7].
Natural IgM antibodies have been shown to localize to injured tissues and play a role in activating the complement cascade[8,9]. IgM antibodies activate the classical pathway to eliminate apoptotic cells primarily by binding to C1q[10]. Elvington et al[11] successfully cloned and purified an IgM natural antibody, known as C2, which recognizes a subset of phospholipids as well as a neoepitope that emerges after injury. C2 can be used for targeted delivery of complement-inhibiting proteins and serves as a biomarker for specific apoptosis induction[12]. Complement receptor 1 related gene/protein y (Crry) is a mouse complement regulatory protein that inhibits C3 convertase, thus blocking complement activation[13]. Banda et al[12] engineered a fusion protein called C2-Crry by linking C2 scFv (single-chain antibody construct) with Crry, which includes a 6 × His tag. This fusion protein is capable of direct and specific delivery to the injury site through C2 natural antibody, targeting inflammation-related phospholipids produced during injury or inflammation. Directing C2-Crry to inflamed joints decreased arthritis in mice. According to Atkinson et al[14], C2 antibody alone can also inhibit cardiac ischemia–reperfusion injury by inhibiting the complement response. Research has demonstrated that the activation of complement is crucial in APAP-induced liver injury and that this activation results in a worsening of liver damage in the initial phase[15]. In this study, we synthesized a new bifunctional inhibitor called C2-FH, which is designed to target the injury site and inhibit complement activation. We showed that C2-FH inhibited APAP-induced liver injury by suppressing complement activation.
Male C57BL/6 mice (21-22 g) aged 6-7 weeks were acquired from Hunan SJA Laboratory Animal Co., Ltd, China [Certification No. SCXK (Xiang) 2019-0005; Changsha, China]. The mice were housed and bred under standard conditions with access to regular chow and water. All animal experiments were conducted following the guidelines for the care and use of animals set by the Guilin Medical College Animal Ethics Committee, with a focus on minimizing the number of animals used and reducing their discomfort.
APAP was dissolved in saline by heating at 55 °C for 15 minutes. Prior to injection, all mice were fasted for approximately 12 hours and then received a single intraperitoneal dose of 300 mg/kg APAP (Med Chem Express, China) or saline. Thirty minutes after APAP injection, the mice were intraperitoneally injected with C2-FH (20 mg/mouse). Fourteen mice were randomly assigned to three groups: Control (Ctrl, n = 4), APAP + PBS (APAP, n = 5) and APAP + C2-FH (C2-FH, n = 5). C2-FH was dissolved in PBS, and mice were intraperitoneally injected with an equivalent volume of PBS as a control for C2-FH[16]. Blood samples and liver tissues were obtained 1 day following APAP administration.
Plasmid pEE12.4-C2-FH was generously provided by Professor Stephen Tomlinson from the Medical University of South Carolina, United States. Expi293F™ cells density was adjusted to 3 × 106 viable cells/mL. Solvent A: Plasmid (pEE12.4-C2-FH) DNA (25 mg) was added to 1.5 mL Opti-MEM™ I Reduced Serum Medium, gently shaken and incubated at room temperature for 5 min. Solvent B: Dilution of 80 µL ExpiFectamine™ 293 Reagent with 1.4 mL Opti-MEM™ I Reduced Serum; mixed well and incubated at room temperature for 5 minutes. Solvent C: Mixed Solvents A and B and left stand at room temperature for 20 minutes. Solvent C was introduced to 3 × 106 viable cells/mL Expi293F™ cells. The cells were incubated overnight at 37 °C, 80 mL/L CO2 and 80%-90% relative humidity. On the next day, 150 µL ExpiFectamine™ 293 Transfection Enhancer 1 and 1.4 mL ExpiFectamine™ 293 Transfection Enhancer 2 were mixed and added to Expi293F™ cells. Cells were cultured for 5-7 days and the supernatant was collected. Gene transfection and expression were subsequently determined. Protein extraction and purification were performed using the magnetic beads His-tag protein purification kit (IDA-Ni).
Concentrations of C3, alanine aminotransferase (ALT) activity, aspartate aminotransferase (AST) activity and lactate dehydrogenase (LDH) were measured using a biochemical analyzer (cobas® 8000).
Liver tissue was fixed with formalin and dehydrated in continuous concentrations of ethanol and xylene. After embedding in paraffin, the tissue was sliced into 4-µm sections. Hematoxylin and eosin (HE) were used to stain the sections. The stained samples were examined with a LEICA DM40B microscope to assess the level of necrosis.
For the paraffin-embedded sections, xylene dewaxing, gradient alcohol dehydration and antigen repair were performed, followed by treatment with 1% H2O2. Following goat serum blocking, the sections were left to incubate at 4 °C overnight with primary antibodies against CD68 (ab283654, 1:3000; Abcam, Cambridge, MA, United States), S100A9 (ab97050, 1:3000; Abcam), C3d (AF2655-SPl, 3 mg/mL; R&D), and C5b (ab275931, 1:200; Abcam). Secondary antibodies were applied for 1 hour at 37 °C. Finally, horseradish peroxidase conjugates were detected using diaminobenzidine.
Total RNA was extracted using TRIzol reagent, and the purity and integrity of the extracted RNA were assessed using MagNA Pure 96 instrument (Roche, Switzerland). RNA-Seq analysis was conducted by Tsingke Biotechnology Co. Ltd. (Beijing, China). Genes that showed differential expression were identified by applying screening criteria of log2 FC ≥ 1 or log2 FC ≤ 1 and P < 0.05.
Data are expressed as means ± SD. Statistical significance was analyzed using ANOVA and two-tailed Student’s t-test. P < 0.05 was considered statistically significant.
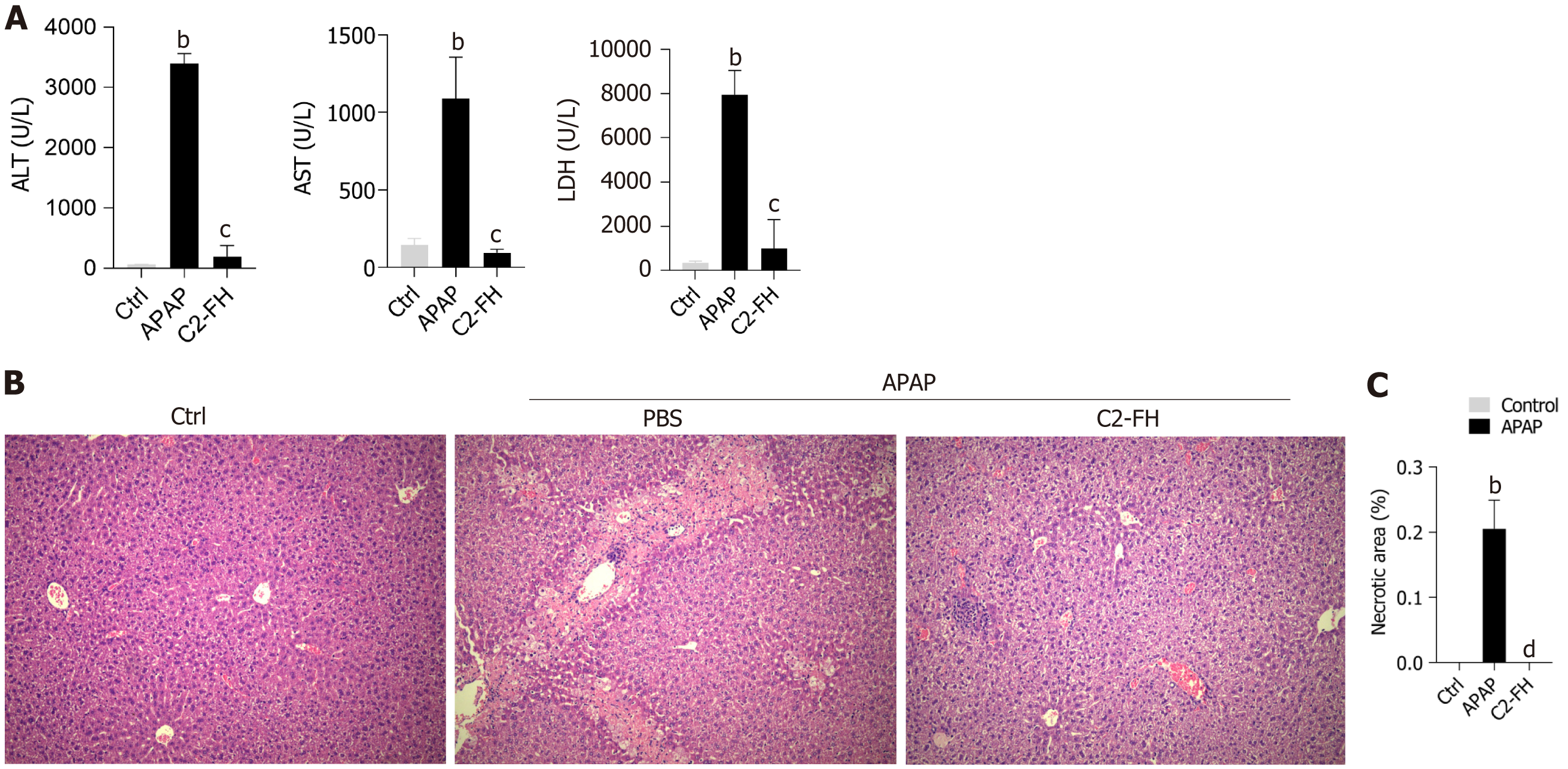
In order to assess the impact of C2-FH on APAP-induced liver injury, we analyzed the plasma concentrations of ALT, AST and LDH (Figure 1A). Following administration of a single dose of APAP, the activities of ALT, AST and LDH increased rapidly. However, C2-FH significantly reduced serum ALT, AST and LDH levels compared with the APAP group.
HE staining revealed that the hepatic lobules were centered on the central vein, with hepatic sinusoids distributed radially around them in the Ctrl group (Figure 1B). The APAP group exhibited confluent necrotic areas around the central region of the hepatic lobules, as well as bridging necrosis between different lobules. Compared with the APAP group, the necrotic area in the C2-FH group was significantly reduced (Figure 1C). These findings suggest that C2-FH has a protective effect on APAP-induced liver injury.
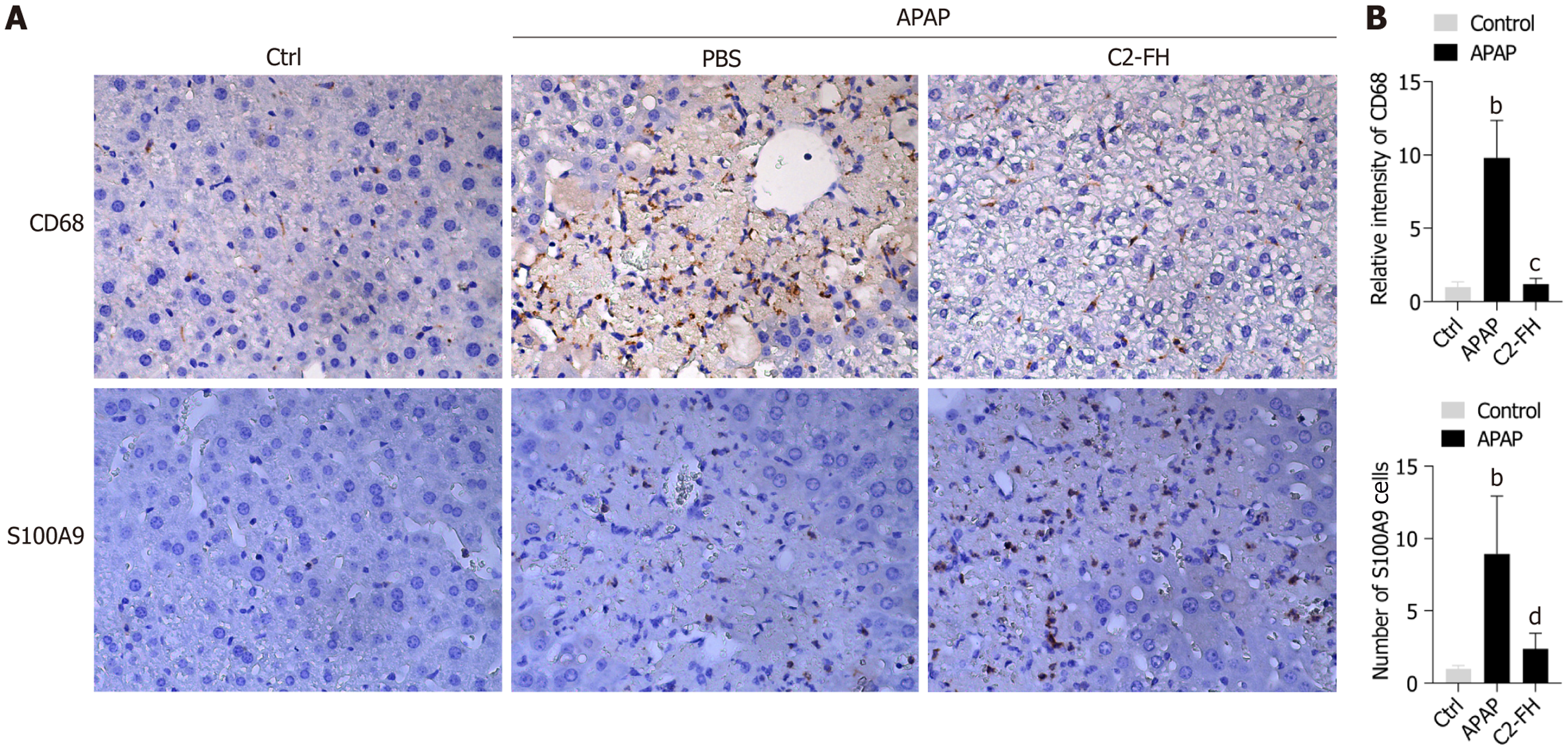
The impact of C2-FH on the infiltration of macrophages in APAP-induced liver injury was studied by analyzing the number of CD68+ macrophages in the liver tissues of each group. CD68+ cells increased rapidly in the APAP group. C2-FH markedly decreased the number of CD68+ macrophages compared with the APAP group (Figure 2A and B). S100A9, a member of the S100s protein family, has been found to activate signaling pathways related to inflammatory processes by binding to cell surface receptors[17,18]. As a result, S100A9 has been used as an indicator of inflammatory response. We found a significant increase in S100A9+ cells in the APAP group. However, expression of S100A9 was reduced in the C2-FH group compared with the APAP group (Figure 2A and B). The findings indicate that C2-FH helps reduce the inflammatory reaction in liver damage caused by APAP.
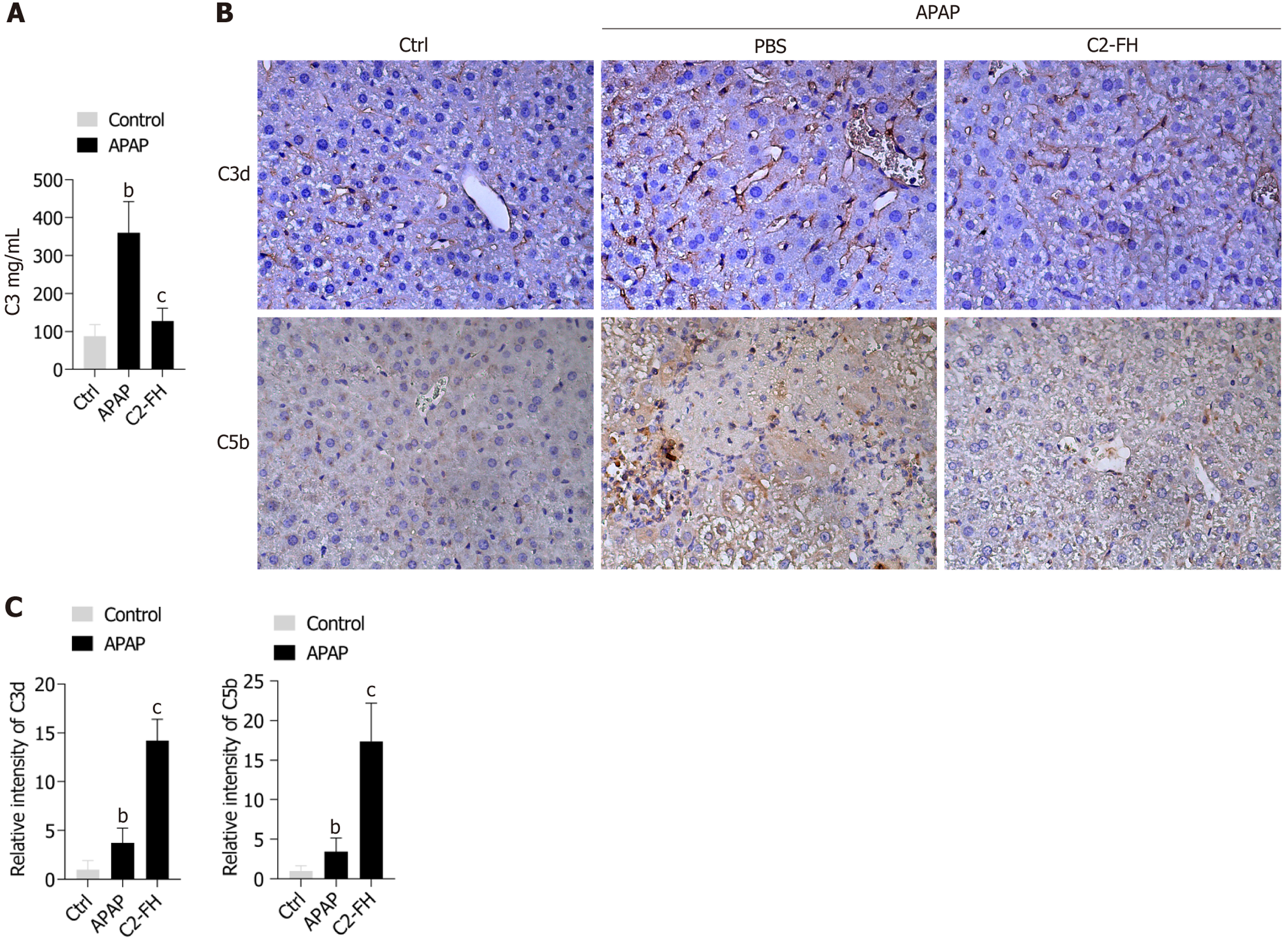
C3 is the central component activated by all complement pathways. Therefore, we assessed serum C3 levels in mice. As expected, C2-FH significantly reduced C3 levels compared with in the APAP group (Figure 3A). To investigate the connection between C2-FH and complement activation in APAP-induced liver injury, we evaluated the deposition of C3d. Compared with the Ctrl group, the APAP group showed a notable increase in C3d deposition. The C2-FH group exhibited significantly lower levels of C3d deposition compared with the APAP group (Figure 3B and C). To further establish the protective effect of C2-FH through complement inhibition, we also examined the impact of C2-FH on C5b deposition (Figure 3B and C). C2-FH significantly reduced C5b deposition compared with the APAP group. These findings indicate that C2-FH provides protection from APAP-induced liver injury by blocking complement activation.
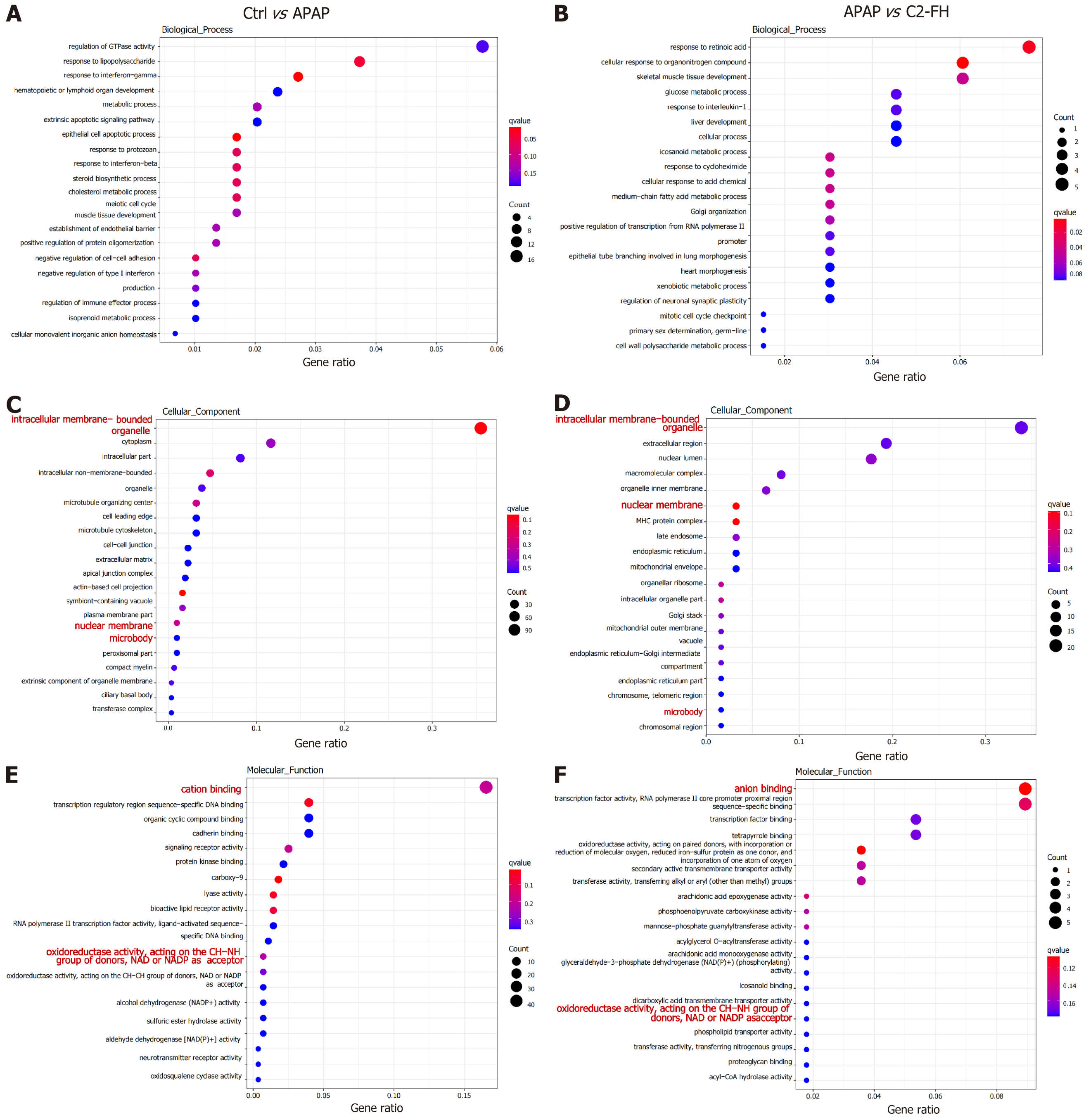
We explored by RNA-Seq how C2-FH affected gene expression in APAP-induced liver injury. The biological processes (BPs) associated with upregulated genes in APAP-induced liver injury were primarily enriched in the regulation of GTPase activity, hematopoietic or lymphoid organ development, extrinsic apoptotic signaling pathway, meiotic cell cycle, and epithelial cell apoptotic process (Figure 4A). The BPs related to downregulated genes in the C2-FH group were mainly enriched in cellular response to organonitrogen compounds, glucose metabolic process, glucose metabolism, and cellular process (Figure 4B). However, none of these BPs overlapped. Interestingly, cellular components (CCs) associated with upregulated genes in APAP-induced liver injury included regulation of intracellular membrane-bounded organelles, nuclear membranes, and microbodies (Figure 4C). The C2-FH group showed a significant decrease in these CCs (Figure 4D). The molecular functions (MFs) associated with upregulated genes in APAP-induced liver injury included regulation of cation binding and oxidoreductase activity, acting on the CH-NH group of donors, NAD or NADP as acceptor (Figure 4E). In contrast, the MF terms were notably decreased in the C2-FH group (Figure 4F). These findings suggest that the protective effect of C2-FH against APAP-induced liver injury may be linked to these CCs and MFs.
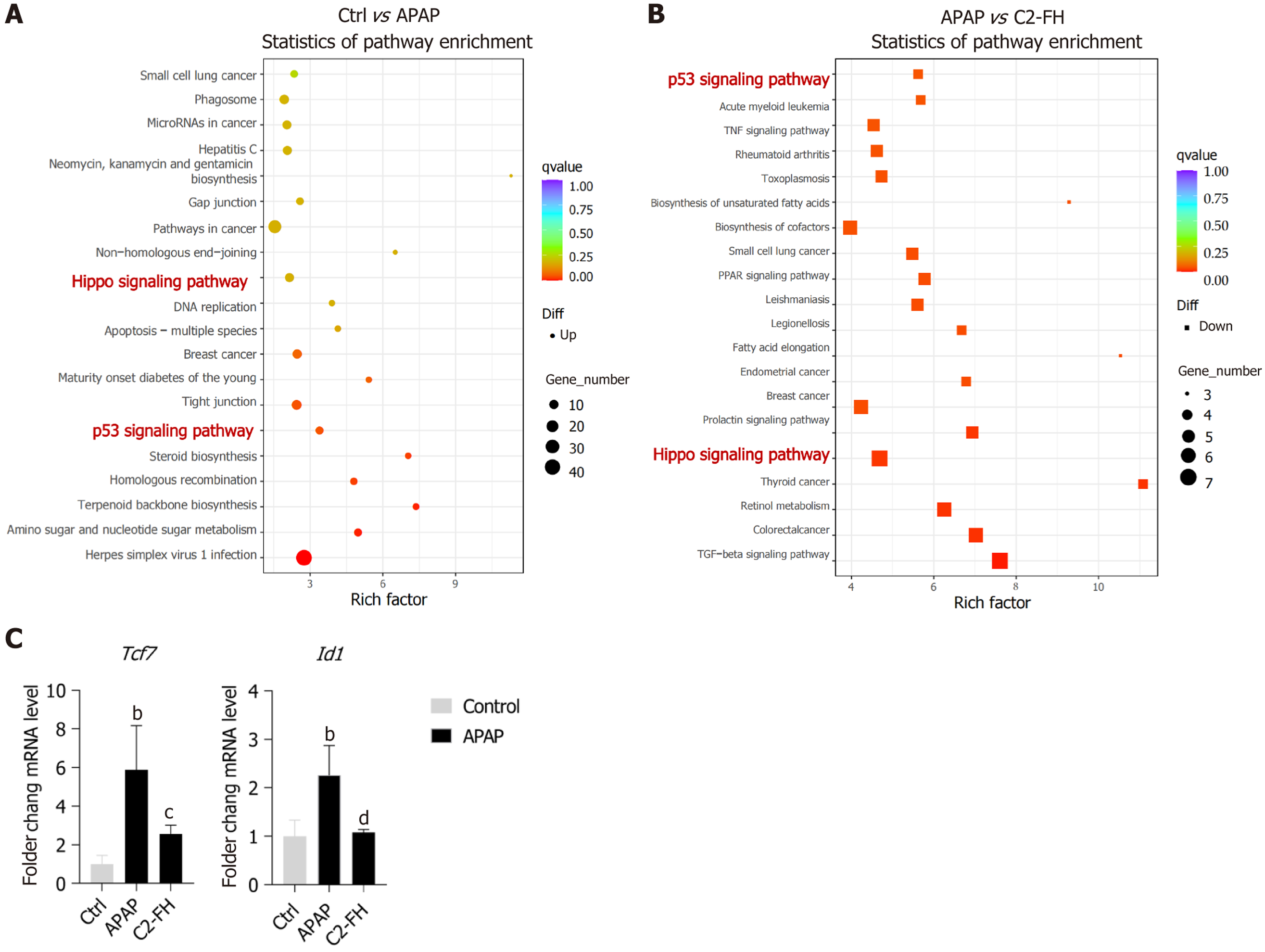
We also analyzed Kyoto encyclopedia of genes and genomes (KEGG) pathways that might be affected by C2-FH in APAP-induced liver injury. Significantly, enriched KEGG pathways associated with upregulated genes in the APAP group included Hippo and p53 signaling pathways. These pathways were also enriched in the downregulated genes of the C2-FH group (Figure 5A and B). Within the Hippo signaling pathway, we identified two intersecting genes: TCF7 and Id1. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) demonstrated that expression of Tcf7 and Id1 genes was significantly elevated in the APAP group, whereas treatment with C2-FH markedly reduced their expression (Figure 5C). These indicates that C2-FH may offer defense against APAP-induced liver injury through the regulation of both the Hippo and p53 signaling pathways.
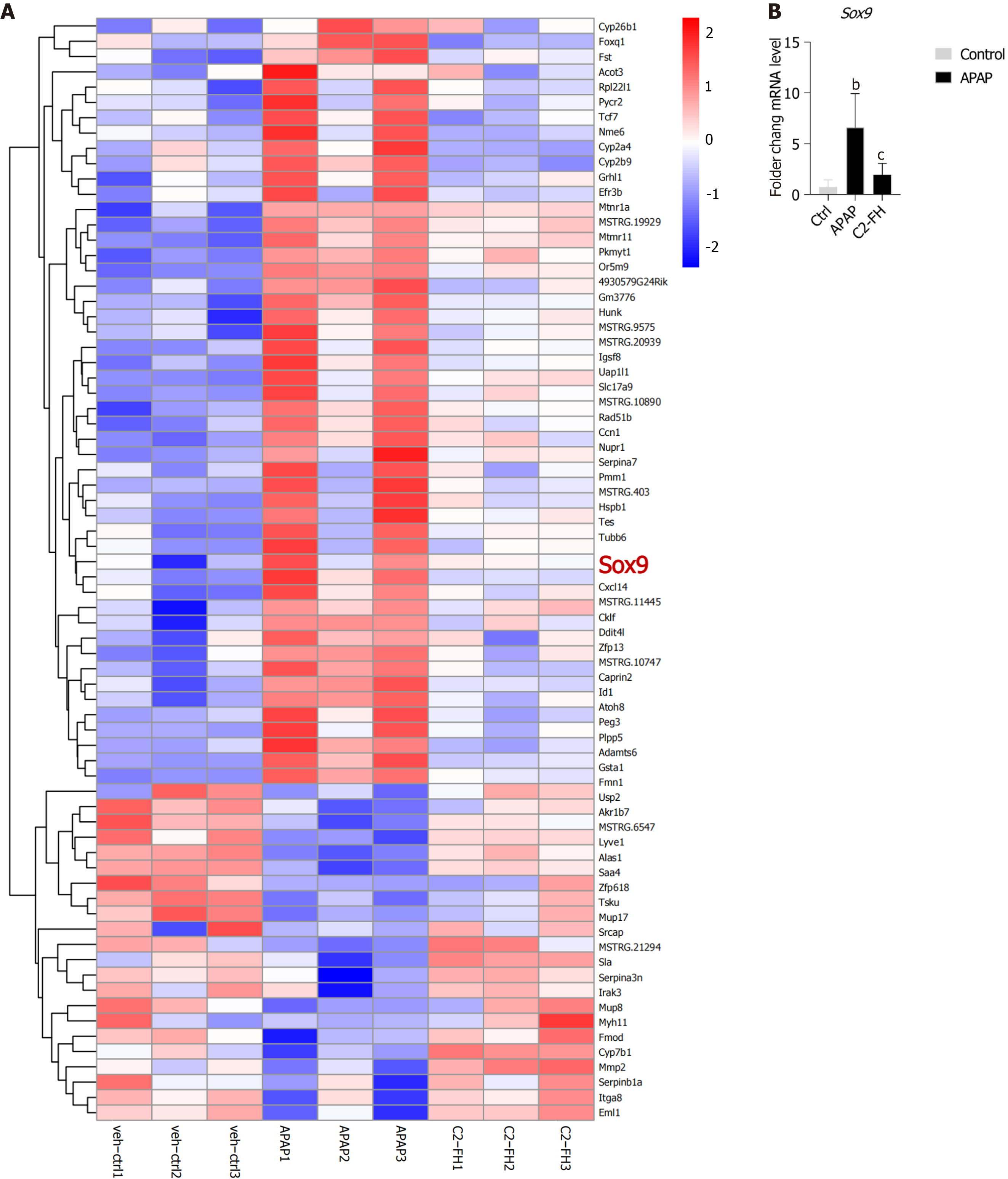
We conducted a thorough analysis of genes that exhibited differential expression in the APAP group and displayed an opposite pattern of expression in the C2-FH group. Among these genes, we identified an important gene called Sox9. It is worth noting that Sox9+ hepatocytes function as bipotent progenitor cells, capable of generating both hepatocytes and ductal cells following liver injury[19]. Our findings revealed upregulation of Sox9 in the APAP group, while it was downregulated in the C2-FH group (Figure 6A). RT-qPCR confirmed that the Sox9 gene was upregulated in the APAP group, while reversed under C2-FH treatment (Figure 6B). These suggests that liver regeneration may commence as early as 24 hours after APAP exposure, and the degree of liver injury is related to the extent of liver regeneration.
Complement activation is recognized as a factor in the progression of liver damage from APAP. In this study, our objective was to investigate the impact of a targeted inhibitor of complement activation, C2-FH, on APAP-induced liver injury. Our findings demonstrated that C2-FH treatment led to a reduction in liver tissue damage, inflammation, and accumulation of complement activation products. The findings indicate that focusing on complement inhibition with C2-FH shows potential as a possible treatment strategy for APAP-induced liver injury.
The complement pathway was activated following injury, which led to exacerbation of liver injury[20,21]. CR2-FH, a targeted inhibitor of the alternative complement pathway, prevents complement activation and provides defense against intestinal ischemia–reperfusion injury[22]. Several studies have reported the role of CR2-FH in various diseases[23,24]. In this study, we found that treatment with C2-FH significantly reduced ALT, AST and LDH and liver damage in mice with APAP-induced liver injury.
We investigated whether C2-FH attenuated the inflammatory response to APAP-induced liver injury. Complement activation, alongside the release of various proinflammatory substances, plays a role in phagocytosis and inflammation at the injury site[25]. In instances of APAP-induced liver injury, the innate immune response is activated, resulting in the recruitment of inflammatory cells to the damaged tissue and worsening tissue damage[26]. Evidence points to activation of the complement system playing a pivotal role in liver inflammation and injury following APAP treatment[15]. Tsuji et al[27] revealed a notable increase in the number of CD68+ cells 24 hours after APAP injection, indicating substantial hepatocyte injury or necrosis. Our results were consistent with this finding, as the number of CD68+ cells in liver tissue was markedly increased in the APAP group, while C2-FH significantly reduced CD68+ cells in APAP-induced liver injury. Additionally, S100A9 can be released from inflammatory or damaged cells and bind to cell surface receptors, triggering an inflammatory response[28,29]. In our study, we observed that C2-FH significantly reduced S100A9 ex
FH regulates complement activity by preventing the splitting of C3 into C3a and C3b, as well as deactivating C3b[30]. C3d serves as a downstream effector of complement pathways and is considered an indicator of complement activation. Previous research has demonstrated that C3d deposition significantly increases after hepatic ischemia reperfusion injury or 70% partial hepatectomy, indicating that liver injury triggers complement activation[16]. We observed less C3d deposition in the C2-FH group compared with the APAP group, suggesting that C2-FH inhibits complement activation. C5 converting enzyme is capable of cleaving C5 into C5a and C5b fragments. C5b can then form a membrane attack complex (C5b-9) with complement proteins C6-C9, which can directly dissolve targeted pathogens or damaged cells[4,5]. Our study found that C2-FH significantly reduced the deposition of C5b compared with in the APAP group. Overall, C2-FH provides protection against APAP-induced liver injury through the suppression of complement activation.
The KEGG results suggest that the p53 and Hippo signaling pathways could be involved in APAP-induced liver injury. In response to various stressors, p53 is activated and initiates different stress response programs, such as halting the cell cycle, repairing DNA damage, inducing apoptosis and potentially leading to cell death[31]. Prior research has demon
Sox9 is a marker that indicates the presence of bile duct lining cells and liver progenitor cells, and it is essential for repairing injuries and keeping the liver stable[35]. In carbon-tetrachloride-induced chronic liver injury, an increase in HOXB6 Levels resulted in decreased Sox9 expression. This ultimately hindered the proliferation and differentiation of liver progenitor cells that contain Sox9, consequently worsening liver injury[36]. Conversely, our observations revealed an increase in Sox9 expression in the APAP group, while a decrease was seen in the C2-FH group. This suggests that liver progenitor cells in the APAP group underwent rapid proliferation and differentiation, thereby promoting liver repair and regeneration. However, liver regeneration in C2-FH group was ameliorated compared with in the APAP group since liver damage was inhibited by C2-FH.
We have developed a novel inhibitor, C2-FH, that specifically targets complement activation. Our study demonstrated that C2-FH effectively mitigated APAP-induced liver injury by inhibiting complement activation. These findings highlight the potential of C2-FH administration as a promising therapy for APAP-induced liver injury.
We would like to thank Professor Stephen Tomlinson (the Medical University of South Carolina, United States) for providing Plasmid pEE12.4-C2-FH.
| 1. | Cai C, Huang H, Whelan S, Liu L, Kautza B, Luciano J, Wang G, Chen G, Stratimirovic S, Tsung A, Billiar TR, Zuckerbraun BS. Benzyl alcohol attenuates acetaminophen-induced acute liver injury in a Toll-like receptor-4-dependent pattern in mice. Hepatology. 2014;60:990-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Neag MA, Catinean A, Muntean DM, Pop MR, Bocsan CI, Botan EC, Buzoianu AD. Probiotic Bacillus Spores Protect Against Acetaminophen Induced Acute Liver Injury in Rats. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 733] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 4. | Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol. 2015;6:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 1194] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 5. | Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement System Part II: Role in Immunity. Front Immunol. 2015;6:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 769] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 6. | Moore SR, Menon SS, Cortes C, Ferreira VP. Hijacking Factor H for Complement Immune Evasion. Front Immunol. 2021;12:602277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (7)] |
| 7. | Haque A, Cortes C, Alam MN, Sreedhar M, Ferreira VP, Pangburn MK. Characterization of Binding Properties of Individual Functional Sites of Human Complement Factor H. Front Immunol. 2020;11:1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GC, Holers VM. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363-5373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Elvington A, Atkinson C, Kulik L, Zhu H, Yu J, Kindy MS, Holers VM, Tomlinson S. Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J Immunol. 2012;188:1460-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Banda NK, Tomlinson S, Scheinman RI, Ho N, Ramirez JR, Mehta G, Wang G, Vu VP, Simberg D, Kulik L, Holers VM. C2 IgM Natural Antibody Enhances Inflammation and Its Use in the Recombinant Single Chain Antibody-Fused Complement Inhibitor C2-Crry to Target Therapeutics to Joints Attenuates Arthritis in Mice. Front Immunol. 2020;11:575154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Kang HJ, Bao L, Xu Y, Quigg RJ, Giclas PC, Holers VM. Increased serum C3 levels in Crry transgenic mice partially abrogates its complement inhibitory effects. Clin Exp Immunol. 2004;136:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Atkinson C, Qiao F, Yang X, Zhu P, Reaves N, Kulik L, Goddard M, Holers VM, Tomlinson S. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation. 2015;131:1171-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Singhal R, Ganey PE, Roth RA. Complement activation in acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2012;341:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Marshall K, Jin J, Atkinson C, Alawieh A, Qiao F, Lei B, Chavin KD, He S, Tomlinson S. Natural immunoglobulin M initiates an inflammatory response important for both hepatic ischemia reperfusion injury and regeneration in mice. Hepatology. 2018;67:721-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Xu Z, Cheng C, Kong R, Liu Y, Wang S, Ma Y, Xing X. S100A8 and S100A9, both transcriptionally regulated by PU.1, promote epithelial-mesenchymal transformation (EMT) and invasive growth of dermal keratinocytes during scar formation post burn. Aging (Albany NY). 2021;13:15523-15537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Shabani F, Farasat A, Mahdavi M, Gheibi N. Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer. Inflamm Res. 2018;67:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 19. | Liu S, Qin D, Yan Y, Wu J, Meng L, Huang W, Wang L, Chen X, Zhang L. Metabolic nuclear receptors coordinate energy metabolism to regulate Sox9(+) hepatocyte fate. iScience. 2021;24:103003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Markiewski MM, Mastellos D, Tudoran R, DeAngelis RA, Strey CW, Franchini S, Wetsel RA, Erdei A, Lambris JD. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J Immunol. 2004;173:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181:8068-8076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Rohrer B, Coughlin B, Bandyopadhyay M, Holers VM. Systemic human CR2-targeted complement alternative pathway inhibitor ameliorates mouse laser-induced choroidal neovascularization. J Ocul Pharmacol Ther. 2012;28:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Annamalai B, Parsons N, Brandon C, Rohrer B. The use of Matrigel combined with encapsulated cell technology to deliver a complement inhibitor in a mouse model of choroidal neovascularization. Mol Vis. 2020;26:370-377. [PubMed] |
| 25. | Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 696] [Cited by in RCA: 644] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 26. | Guo H, Sun J, Li D, Hu Y, Yu X, Hua H, Jing X, Chen F, Jia Z, Xu J. Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother. 2019;112:108704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 27. | Tsuji Y, Kuramochi M, Golbar HM, Izawa T, Kuwamura M, Yamate J. Acetaminophen-Induced Rat Hepatotoxicity Based on M1/M2-Macrophage Polarization, in Possible Relation to Damage-Associated Molecular Patterns and Autophagy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Vogl T, Eisenblätter M, Völler T, Zenker S, Hermann S, van Lent P, Faust A, Geyer C, Petersen B, Roebrock K, Schäfers M, Bremer C, Roth J. Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nat Commun. 2014;5:4593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Gebhardt C, Németh J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 566] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 30. | Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3144] [Article Influence: 149.7] [Reference Citation Analysis (0)] |
| 31. | Lahav G. Oscillations by the p53-Mdm2 feedback loop. Adv Exp Med Biol. 2008;641:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Fan X, Chen P, Tan H, Zeng H, Jiang Y, Wang Y, Wang Y, Hou X, Bi H, Huang M. Dynamic and coordinated regulation of KEAP1-NRF2-ARE and p53/p21 signaling pathways is associated with acetaminophen injury responsive liver regeneration. Drug Metab Dispos. 2014;42:1532-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Liu Y, Lu T, Zhang C, Xu J, Xue Z, Busuttil RW, Xu N, Xia Q, Kupiec-Weglinski JW, Ji H. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J Hepatol. 2019;71:719-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 34. | Alarcón C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, Sapkota G, Pan D, Massagué J. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 623] [Article Influence: 36.6] [Reference Citation Analysis (1)] |
| 35. | Liu Y, Zhuo S, Zhou Y, Ma L, Sun Z, Wu X, Wang XW, Gao B, Yang Y. Yap-Sox9 signaling determines hepatocyte plasticity and lineage-specific hepatocarcinogenesis. J Hepatol. 2022;76:652-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 36. | Yan Y, Wang R, Hu X, Wang S, Zhang L, Hou C, Zhang L. MiR-126 Regulates Properties of SOX9(+) Liver Progenitor Cells during Liver Repair by Targeting Hoxb6. Stem Cell Reports. 2020;15:706-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/