Published online Sep 27, 2022. doi: 10.4254/wjh.v14.i9.1804
Peer-review started: May 5, 2022
First decision: June 8, 2022
Revised: June 20, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: September 27, 2022
Processing time: 140 Days and 11.9 Hours
Hereditary hemochromatosis (HH) has an increased risk of hepatocellular cancer (HCC) both due to genetic risks and iron overload as iron overload can be carci
To evaluate HH yearly trends, patient demographics, symptoms, comorbidities, and hospital outcomes. The secondary aim sheds light on the risk of iron overload for developing HCC in HH patients, independent of liver cirrhosis complications. The study investigated HH (without cirrhosis) as an independent risk factor for HCC.
We analyzed data from National Inpatient Sample (NIS) Database, the largest national inpatient data collection in the United States, and selected HH and HCC cohorts. HH was first defined in 2011 International Classification of Disease - 9th edition (ICD-9) as a separate diagnosis; the HH cohort is extracted from January 2011 to December 2019 using 275.01 (ICD-9) and E83.110 (ICD-10) diagnosis codes of HH. Patients were excluded from the HH cohort if they had a primary or secondary diagnostic code of cirrhosis (alcoholic, non-alcoholic, and biliary), viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH). We removed these patients from the HH cohort to rule out bias or ICD-10 diagnostic errors. The HCC cohort is selected from January 2011 to December 2019 using the ICD-9 and ICD-10 codes of HCC. We selected a non-HCC cohort with the 1:1 fixed ratio nearest neighbor (greedy) propensity score method using the patients' age, gender, and race. We performed multivariate analysis for the risk factors of HCC in the HCC and non-HCC matched cohort. We further analyzed HH without cirrhosis (removing HH patients with a diagnosis of cirrhosis) as an independent risk factor of HCC after adjusting all known risk factors of HCC in the multivariate model.
During the 2011-2019 period, a total of 18031 hospitalizations with a primary or secondary diagnosis of HH (excluding liver diseases) were recorded in the NIS database. We analyzed different patients’ characteristics, and we found increments in inpatient population trend with a Ptrend < 0.001 and total hospital cost of care trend from $42957 in 2011 to $66152 in 2019 with a Ptrend < 0.001 despite no change in Length of Stay over the last decade. The multivariate analyses showed that HH without cirrhosis (aOR, 28.8; 95%CI, 10.4–80.1; P < 0.0001), biliary cirrhosis (aOR, 19.3; 95%CI, 13.4–27.6; P < 0.0001), non-alcoholic cirrhosis (aOR, 17.4; 95%CI, 16.5–18.4; P < 0.0001), alcoholic cirrhosis (aOR, 16.9; 95%CI, 15.9–17.9; P < 0.0001), hepatitis B (aOR, 12.1; 95%CI, 10.85–13.60; P < 0.0001), hepatitis C (aOR, 8.58; 95%CI, 8.20–8.98; P < 0.0001), Wilson disease (aOR, 4.27; 95%CI, 1.18–15.41; P < 0.0001), NAFLD or NASH (aOR, 2.96; 95%CI, 2.73–3.20; P < 0.0001), alpha1-antitrypsin deficiency (aOR, 2.10; 95%CI, 1.21–3.64; P < 0.0001), diabetes mellitus without chronic complications (aOR, 1.17; 95%CI, 1.13–1.21; P < 0.0001), and blood transfusion (aOR, 1.80; 95%CI, 1.69–1.92; P < 0.0001) are independent risk factor for liver cancer.
Our study showed an increasing trend of in-hospital admissions of HH patients in the last decade. These trends were likely related to advances in diagnostic approach, which can lead to increased hospital utilization and cost increments. Still, the length of stay remained the same, likely due to a big part of management being done in outpatient settings. Another vital part of our study is the significant result that HH without cirrhosis is an independent risk factor for HCC with adjusting all known risk factors. More prospective and retrospective large studies are needed to re-evaluate the HH independent risk in developing HCC.
Core Tip: Our study is a National Inpatient Sample -based study in which we aim to analyze hereditary hemochromatosis (HH) patients’ characteristics, temporal trends, and sociodemographic characteristics over the last decade, in addition to studying this disease as an independent risk factor for developing hepatocellular cancer (HCC) without the stage of liver cirrhosis. Our large and diverse sample of about 18000 HH hospitalizations showed an increasing trend of inpatient admissions and costs with a similar length of hospital stay over the last decade. It also showed HH as an independent risk factor for HCC with an aOR close to 29 on multivariate analysis. We believe this will open the door for further retrospective and prospective studies to address disease trends and the understudied and independent relationship between HH and HCC in a large patient cohort.
- Citation: Haider MB, Al Sbihi A, Chaudhary AJ, Haider SM, Edhi AI. Hereditary hemochromatosis: Temporal trends, sociodemographic characteristics, and independent risk factor of hepatocellular cancer – nationwide population-based study. World J Hepatol 2022; 14(9): 1804-1816
- URL: https://www.wjgnet.com/1948-5182/full/v14/i9/1804.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i9.1804
Hereditary hemochromatosis (HH) is a genetic disorder of iron metabolism that increases alimentary iron absorption. It is a common inherited iron metabolism disorder characterized by increasing iron deposition in body organs[1]. Organs mainly involved in this disease include the liver, skin, pancreas, heart, joints, pituitary gland, and testes resulting in these organ dysfunctions[2]. As hepatocytes store the most considerable portion of the excess iron, the liver is the most affected organ leading to liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[3].
HCC is the most common type of primary liver cancer and is one of the leading causes of cancer-related death worldwide. It mainly affects patients suffering from chronic liver diseases such as viral, alcoholic, metabolic, or autoimmune hepatitis and cirrhosis[4]. Per one study, HCC incidence rates have been rising in the past three decades, and by the year 2025, more than a million individuals are expected to be affected annually[5].
HH has an increased risk of HCC both due to genetic risks and iron overload as iron overload can be carcinogenic; HH impacts the increasing risk of HCC, not only through the development of cirrhosis but concerning hepatic iron deposition, which has been studied further recently. The role of iron in carcinogenesis is presumed mainly through reactive oxygen species generation, which is studied in many disorders such as asbestos fibers exposure and endometriosis[6-8]. Interestingly, some studies have shown that excess body iron may be complicated by developing HCC independently of any underlying liver disease due to the carcinogenic effects of iron, which can promote direct malignant transformation in hepatocytes[9-11].
According to the Centers for Disease Control and Prevention, the prevalence of HH is one case in three hundred in the United States[12]. Given the rarity of the disease, in the past, only a few studies have been done to evaluate HH patients, their comorbidities, and the disease’s effect on developing HCC.
We analyzed data from NIS Database, the largest national inpatient data collection in the United States. The NIS is a part of the healthcare and cost utilization project (HCUP), developed through a federal-state industry partnership and maintained by the agency of healthcare research quality (AHRQ). The NIS is a weighted dataset containing over 7 million admissions per year, representing a 20% stratified sample of all discharges[13]. The NIS includes discharge weights to produce national or regional estimates. The discharge weights were calculated for NIS data by first stratifying the NIS hospitals on the same variables used for creating samples. These variables were census division, urban/rural location, etc. The discharge weights are applied to the unweighted data; the result estimates the number of discharges for the entire community hospitals in the US. The dataset comprises patients’ demographic, clinical and economic data. All medical procedures and diagnoses are coded using the International Classification of Disease - 9th edition (ICD-9) until 2015 Q3 and upgraded to ICD-10 in September 2015 with redesigned sampling techniques and weights[14-16]. For analyzing trends for multiple years, we followed HCUP guidelines and used trend weight (Trendwt) for years before 2012 and discharge weights (discwt) with 2012 onward[17]. As the dataset is publicly available and lacks patients’ identification, Institutional Review Board (IRB) approval or informed consent was not required under the Health Insurance Portability and Accountability Act[18].
We worked on two separate data populations in this study, HH and HCC cohorts. HH was first defined in 2011 ICD-9 as a separate diagnosis in the NIS dataset; the HH cohort is extracted from January 2011 to December 2019 using 275.01 (ICD-9) and E83.110 (ICD-10) diagnosis codes of HH. Patients were excluded from the HH cohort if they had a primary or secondary diagnostic code of cirrhosis (alcoholic, non-alcoholic, and biliary), viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH). We removed these patients from the HH cohort to rule out bias or ICD-10 diagnostic errors. The HCC cohort is also extracted from January 2011 to December 2019 using ICD-9 and ICD-10 codes. The primary outcomes of the HH cohort included HH yearly trends, demographics, symptoms, comorbidities, and hospital outcomes. The secondary outcomes on the HCC cohort investigated HH without cirrhosis as an independent risk factor for HCC after accounting for all known other risk factors of HCC.
We performed data and statistical analyses using R Studio 1.4 and SAS statistical software (SAS Institute Inc., Cary, NC, United States). Categorical variables are presented using the frequency distribution, and continuous variables such as age and total charges were presented using the mean ± standard deviation (SD). We performed weighted analysis to improve national estimates and followed year-specific AHRQ recommendations[13]. Group comparisons were performed using the student's t-test for continuous variables and the χ2 test for categorical variables. We divided age into five categories for group-level comparison (< 18; 18-44; 45-64; 65-84; and ≥ 85 years of age). The age group categories were selected from HCUPnet, an online tool for identifying, tracking, and analyzing healthcare statistics[19]. We computed established risk factors for HCC in HCC patients and compared them against matched non-HCC patients. We selected a non-HCC cohort with the 1:1 fixed ratio nearest neighbor (greedy) propensity score method using the patients' age, gender, and race. We performed hypothesis testing using a two-tailed P value with the significance level set at 0.05. Due to the limited number of HH patients, we did not remove patients with missing data on race, primary payer, median household income, discharge status, and hospital variables. The missing data was labeled as "other."
We performed a univariate analysis of risk factors for HCC from logistic regression models. We selected all significant variables in the univariate analysis in the first step. Next, we analyzed the variables for multicollinearity by creating a correlation matrix using Pearson correlation coefficients and removing highly correlated regressors. Then, the model is selected from the remaining regressors with lasso selection (shrinkage and regularization), choosing the external 10-fold cross-validation criteria. Finally, the multivariate analysis is performed on the selected model using multi-level mixed effect models with SAS’s GLIMMIX procedure with hospital identifier as a random effect.
During the 2011-2019 period, a total of 18031 hospitalizations with a primary or secondary diagnosis of HH (excluding liver diseases) were recorded in the NIS database. Baseline demographic and hospital characteristics, comorbidities, clinical symptoms, substance use, and family history of HH patients are shown in Table 1 and Supplementary Table 1. HH patients in the United States were mostly male (62%), with a mean age of 62 years (SD ± 15); the majority were aged between 65–84 years old (44%; 95%CI; 42.2–45.7), predominantly white race (88%; 95%CI; 86.6–89.2) with Medicare insurance (52%; 95%CI; 49.9–53.6) and have highest estimated median household income in quartile 4 (29%; 95%CI; 27.2–30.5). In terms of hospital characteristics, the majority of HH patients were admitted to large (55%; 95%CI; 53.9–56.3) urban teaching (70%; 95%CI; 68.9–70.8) hospitals in the south region (30%; 95%CI; 29.4–31.6). The most common comorbidities included Hypertension (44%;95%CI; 42.0–45.5), lipid metabolism disorders (38%; 95%CI; 35.9–39.3), arrhythmias (24%; 95%CI; 22.1–25.1), coronary artery disease (19%; 95%CI; 17.2–20.2), thyroid disorders (18%;95%CI; 16.9–19.6), heart failure (16%; 95%CI; 14.5–17.1), obesity (16%; 95%CI; 15.1–17.9), and depression (15%; 95%CI; 13.5–16.1). Musculoskeletal pain (5.6; 95%CI; 4.79–6.42) was the most common clinical symptom, followed by malaise and fatigue (3.1%; 95%CI; 2.49–3.68), arthropathy (2.1%; 95%CI; 1.65–2.61), disorientation (2.1%; 95%CI; 1.61–2.54), weight loss (1.2%; 95%CI; 0.81–1.641), and hypogonadism (1.1%; 95%CI; 0.66–1.49).
| Variables | Weighted, n | Weighted, % (95%Cl) |
| Total, n | 18031 | |
| Sex | ||
| Female | 6903 | 38.27 (36.59–39.9) |
| Male | 11123 | 61.69 (59.9–63.4) |
| Age (y), mean (SD) | 62.19 ± 34.36 | |
| Age groups (yr) | ||
| < 18 | 158 | 0.88 (0.54–1.20) |
| 18-44 | 2046 | 11.37 (10.2–12.5) |
| 45-64 | 7007 | 38.90 (37.15–40.6) |
| 65-84 | 7933 | 43.93 (42.2–45.7) |
| 85+ | 887 | 4.91 (4.15–5.69) |
| Race/Ethnicity | ||
| White | 15850 | 87.87 (86.6–89.2) |
| Black | 315 | 1.76 (1.30–2.19) |
| Hispanic | 545 | 3.01 (2.42–3.62) |
| Asian or Pacific Islander | 105 | 0.58 (0.33–0.83) |
| Native American | 30 | 0.17 (0.03–0.30) |
| Other | 1186 | 6.60 (5.52–7.63) |
| Comorbidities | ||
| Coronary artery disease | 3357 | 18.61 (17.24–20.0) |
| Cardiomyopathy | 900 | 4.99 (4.19–5.79) |
| Arrythmias | 4250 | 23.57 (22.1–25.1) |
| Heart failure | 2848 | 15.79 (14.5–17.1) |
| Liver cancer | 209 | 1.16 (0.78–1.53) |
| HIV | 35 | 0.19 (0.05–0.34) |
| Alpha1-antitrypsin deficiency | 55 | 0.31 (0.11–0.49) |
| Disorders of porphyrin metabolism | 105 | 0.58 (0.30–0.86) |
| Hepatomegaly | 123 | 0.68 (0.38–0.98) |
| Splenomegaly | 169 | 0.93 (0.56–1.32) |
| Hypertension | 7892 | 43.76 (42.0–45.5) |
| Diabetes mellitus with chronic complications | 2323 | 12.88 (11.7–14.1) |
| Diabetes mellitus without chronic complications | 2485 | 13.78 (12.6–15.0) |
| Lipid metabolism disorders | 6781 | 37.60 (35.9–39.3) |
| Thyroid disorders | 3285 | 18.21 (16.9–15.6) |
| Pancreatitis | 484 | 2.69 (2.13–3.23) |
| Pituitary disorders | 259 | 1.43 (0.99–1.87) |
| Obesity | 2971 | 16.48 (15.1–17.9) |
| Depression | 2673 | 14.82 (13.5–16.1) |
| Symptoms | ||
| Musculoskeletal pain | 1011 | 5.61 (4.79–6.42) |
| Malaise and fatigue | 557 | 3.09 (2.49–3.68) |
| Arthropathy | 384 | 2.13 (1.65–2.61) |
| Weight Loss | 219 | 1.22 (0.81–1.61) |
| Loss of appetite | 87 | 0.49 (0.26–0.70) |
| Hypogonadism | 193 | 1.07 (0.66–1.49) |
| Erectile dysfunction | 140 | 0.78 (0.45–1.10) |
| jaundice | 184 | 1.02 (0.67–1.38) |
| Disorientation | 374 | 2.07 (1.61–2.54) |
| Family history | ||
| Family history of diseases of the circulatory system | 1109 | 6.15 (5.26–7.04) |
| Family history of diabetes mellitus | 500 | 2.77 (2.17–3.37) |
| Family history of malignant neoplasms | 814 | 4.51 (3.77–5.25) |
| Substance use | ||
| Smoking | 7577 | 42.02 (40.2–43.8) |
| Alcohol | 1536 | 8.52 (7.49–9.54) |
| Cannabis use | 219 | 1.22 (0.83–1.60) |
| Opioids | 315 | 1.74 (1.29–2.20) |
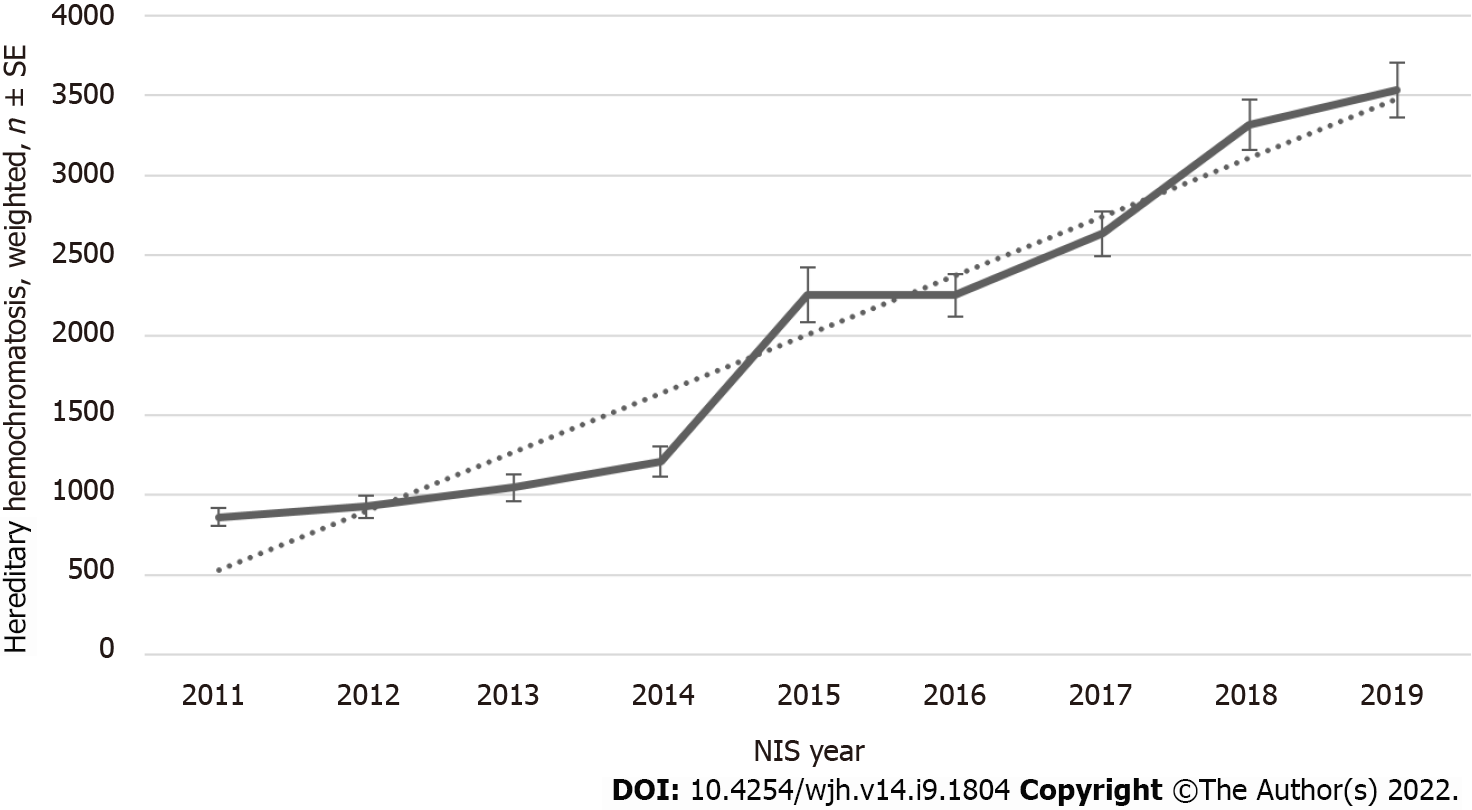
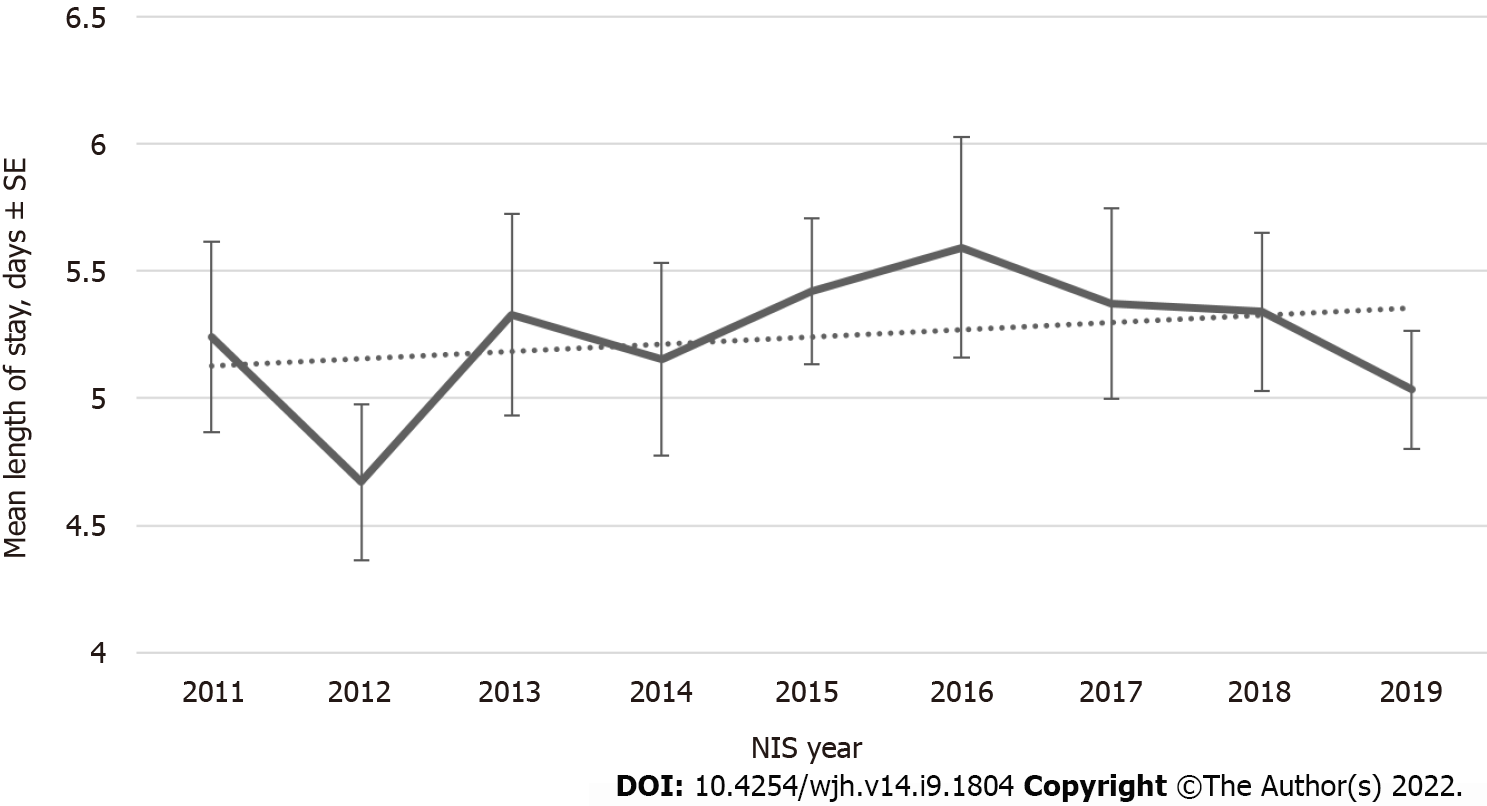
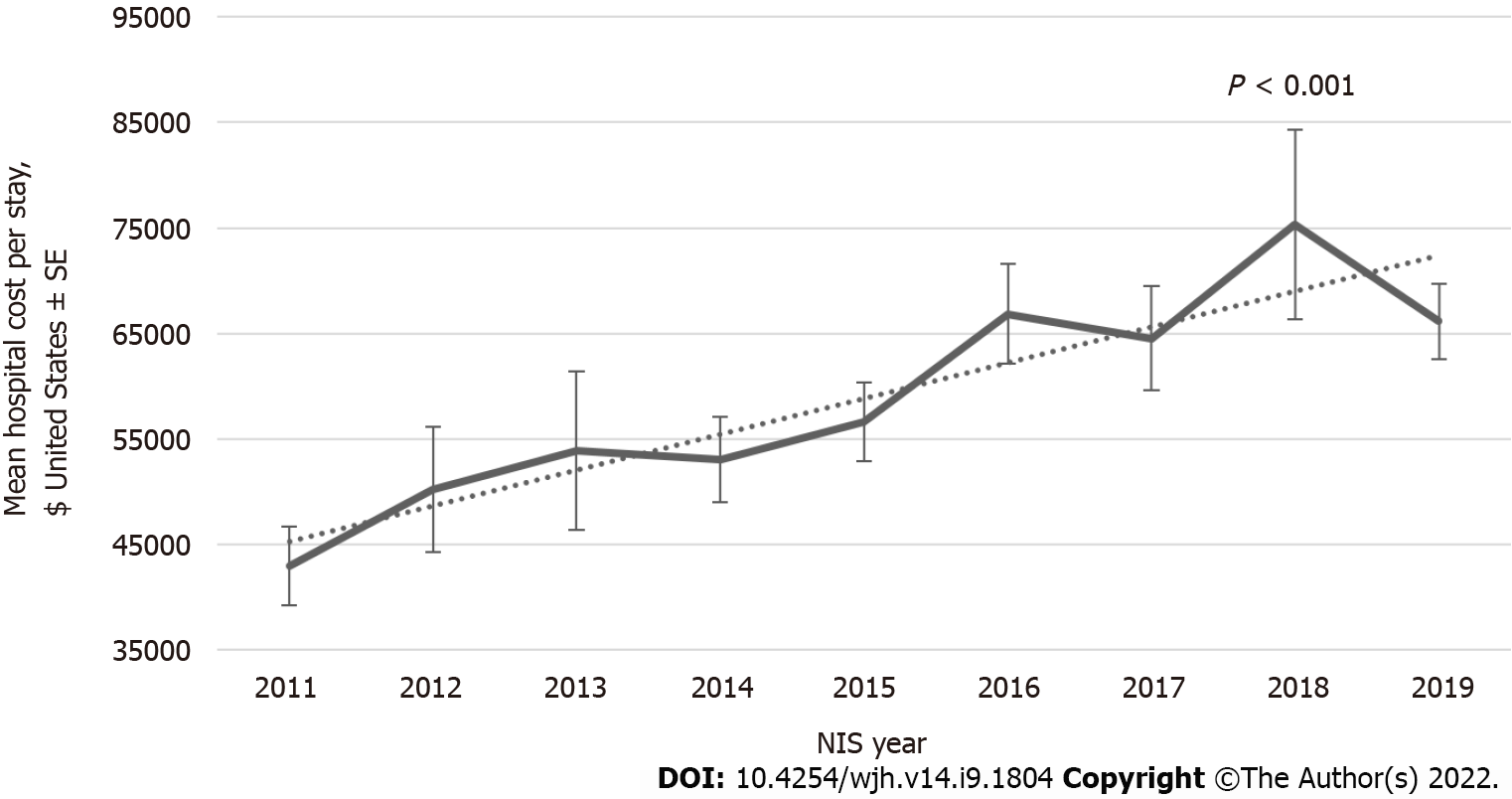
Figure 1 shows the annual trends in hospital admissions for HH patients from 2011 to 2019. An increasing inpatient population trend) was observed in NIS from 861 patients in 2011 to 3535 patients in the weighted sample with Ptrend < 0.001. There has been no change in Length of Stay (LOS) over the last decade (Figure 2). Although LOS stays the same, an increasing trend is noted for the total hospital cost of care from $42957 in 2011 to $66152 in 2019 with Ptrend < 0.001 (Figure 3).
In our study, the incidence of liver cancer in HH patients is 1.2% (95%CI; 0.78–1.53), unrelated to cirrhosis, viral hepatitis, alcoholic liver disease, NAFLD, and NASH. We performed multivariate analysis for the risk factors of HCC in the HCC and non-HCC matched cohort. We analyzed HH without cirrhosis (removing HH patients with a diagnosis of cirrhosis) as an independent risk factor of HCC after adjusting all known risk factors of HCC in the multivariate model. Table 2 compared HCC risk factors in the HCC population (cases) vs age-, sex-, race- matched non-HCC (controls) cohort. HH with cirrhosis is 0.12% for cases vs 0.01% for controls with a P value of < 0.0001, and HH without cirrhosis is 0.05% for cases vs 0.005% for controls with a P value of < 0.0001.
| Variables | HCC = No, n, %Weighted, N = 542280, % | HCC = Yes, n, %Weighted, N = 548773, % | P value1 |
| Hereditary hemochromatosis (without cirrhosis) | 25 (0.005) | 273 (0.05) | < 0.0001 |
| Hereditary hemochromatosis (with cirrhosis) | 55 (0.01) | 651 (0.12) | < 0.0001 |
| Alcoholic cirrhosis | 8577 (1.58) | 102871 (18.73) | < 0.0001 |
| Non-alcoholic cirrhosis | 9578 (1.75) | 204717 (37.24) | < 0.0001 |
| Biliary cirrhosis | 193 (0.04) | 2363 (0.43) | < 0.0001 |
| Alcoholic liver disease | 11551 (2.12) | 105799 (19.27) | < 0.0001 |
| NASH/NAFLD | 6756 (1.24%) | 46648 (8.52) | < 0.0001 |
| Hepatitis B | 2105 (0.39) | 33358 (6.07) | < 0.0001 |
| Hepatitis C | 16857 (3.10) | 210661 (38.40) | < 0.0001 |
| Other viral hepatitis | 4498 (0.83) | 13317 (2.42) | < 0.0001 |
| HIV | 4679 (0.86) | 7793 (1.42) | < 0.0001 |
| HIV - Hepatitis B | 246 (0.05) | 1649 (0.30) | < 0.0001 |
| HIV - Hepatitis C | 1082 (0.20) | 4834 (0.88) | < 0.0001 |
| Obesity | 76894 (14.12) | 47330 (8.58) | < 0.0001 |
| Diabetes mellitus with chronic complications | 80055 (14.61) | 69848 (12.64) | < 0.0001 |
| Diabetes mellitus without chronic complications | 110966 (20.51) | 126860 (23.17) | < 0.0001 |
| Hypertension | 244715 (45.14) | 216621 (39.45) | < 0.0001 |
| Blood transfusion | 14644 (2.65) | 33381 (6.03) | < 0.0001 |
| Alpha1-antitrypsin deficiency | 163 (0.03) | 641 (0.12) | < 0.0001 |
| Disorders of porphyrin metabolism | 68 (0.01) | 353 (0.06) | < 0.0001 |
| Glycogen storage diseases | 50 (0.01) | 105 (0.02) | 0.0482 |
| Wilson disease | 33 (0.01) | 143 (0.03) | 0.0002 |
| Smoking | 193145 (35.40) | 218897 (39.76) | < 0.0001 |
| Alcohol | 44983 (8.26) | 119704 (21.79) | < 0.0001 |
| Cannabis | 8334 (1.52) | 8775 (1.60) | 0.1650 |
| opioids | 11534 (2.11) | 16261 (2.95) | < 0.0001 |
We investigated multicollinearity among known risk factors for HCC by generating a correlation matrix. Supplementary Table 2 shows the correlation matrix using Pearson’s correlation coefficients between variables. Multicollinearity occurs when independent variables in a regression model are correlated. However, this correlation is problematic as the regression model investigates associations, and multicollinearity among the predictor variables can obscure the computations[20]. Therefore, we excluded HH with Cirrhosis (correlation of 0.65 with HH without Cirrhosis), alcoholic liver disease (correlation of 0.97 with alcoholic cirrhosis), and alcohol (correlation of 0.69 with alcoholic cirrhosis) from the prediction model.
Table 3 showed the univariate and multivariate analyses of HCC risk factors in weighted case-control cohort. We included known risk factors in the prediction model to ensure the completeness of the model. The multivariate analyses showed that HH without cirrhosis (aOR, 28.8; 95%CI, 10.4–80.1; P < 0.0001), biliary cirrhosis (aOR, 19.3; 95%CI, 13.4–27.6; P < 0.0001), non-alcoholic cirrhosis (aOR, 17.4; 95%CI, 16.5–18.4; P < 0.0001) alcoholic cirrhosis (aOR, 16.9; 95%CI, 15.9–17.9; P < 0.0001), hepatitis B (aOR, 12.1; 95%CI, 10.85–13.60; P < 0.0001), hepatitis C (aOR, 8.58; 95%CI, 8.20–8.98; P < 0.0001), Wilson disease (aOR, 4.27; 95%CI, 1.18–15.41; P < 0.0001), NAFLD or NASH (aOR, 2.96; 95%CI, 2.73–3.20; P < 0.0001), alpha1-antitrypsin deficiency (aOR, 2.10; 95%CI, 1.21–3.64; P < 0.0001), diabetes mellitus without chronic complications (aOR, 1.17; 95%CI, 1.13–1.21; P < 0.0001), and blood transfusion (aOR, 1.80; 95%CI, 1.69–1.92; P < 0.0001) are independent risk factors for liver cancer.
| Variables | Univariate analysis | Multivariate analysis | ||
| Unadjusted odds ratio1 (95%CI) | P value | Adjusted odds ratio2 (95%CI) | P value | |
| Hereditary hemochromatosis (without cirrhosis) | 10.887 (4.376-27.087) | < 0.0001 | 28.838 (10.387-80.068) | < 0.0001 |
| Alcoholic cirrhosis | 14.398 (13.701-15.131) | < 0.0001 | 16.881 (15.949-17.868) | < 0.0001 |
| Non-alcoholic cirrhosis | 33.241 (31.732-34.822) | < 0.0001 | 17.446 (16.507-18.439) | < 0.0001 |
| Biliary cirrhosis | 12.278 (8.858-17.019) | < 0.0001 | 19.245 (13.439-27.557) | < 0.0001 |
| NASH/NAFLD | 7.393 (6.982-7.828) | < 0.0001 | 2.955 (2.732-3.196) | < 0.0001 |
| Hepatitis B | 16.711 (15.149-18.434) | < 0.0001 | 12.145 (10.848-13.597) | < 0.0001 |
| Hepatitis C | 19.488 (18.798-20.203) | < 0.0001 | 8.576 (8.195-8.975) | < 0.0001 |
| Other viral hepatitis | 2.978 (2.762-3.211) | < 0.0001 | 1.357 (1.216-1.514) | < 0.0001 |
| HIV | 1.666 (1.537-1.807) | < 0.0001 | 0.649 (0.571-0.737) | < 0.0001 |
| Obesity | 0.571 (0.556-0.587) | < 0.0001 | 0.614 (0.590-0.640) | < 0.0001 |
| Diabetes mellitus with chronic complications | 0.845 (0.825-0.866) | < 0.0001 | 0.855 (0.824-0.887) | < 0.0001 |
| Diabetes mellitus without chronic complications | 1.168 (1.145-1.192) | < 0.0001 | 1.168 (1.132-1.205) | < 0.0001 |
| Hypertension | 0.792 (0.779-0.806) | < 0.0001 | 0.850 (0.828-0.872) | < 0.0001 |
| Blood transfusion | 2.356 (2.255-2.463) | < 0.0001 | 1.801 (1.689-1.919) | < 0.0001 |
| Alpha1-antitrypsin deficiency | 3.912 (2.669-5.735) | < 0.0001 | 2.100 (1.210-3.643) | 0.0087 |
| Disorders of porphyrin metabolism | 5.145 (2.902-9.120) | < 0.0001 | 2.428 (1.163-5.066) | 0.0187 |
| Wilson disease | 4.099 (1.800-9.332) | 0.0008 | 4.269 (1.182-15.414) | 0.0279 |
| Smoking | 1.204 (1.184-1.225) | < 0.0001 | 0.874 (0.851-0.897) | < 0.0001 |
| opioids | 1.412 (1.338-1.490) | < 0.0001 | 0.687 (0.631-0.749) | < 0.0001 |
Current literature lacks enough studies that have assessed admitted HH patients’ characteristics, which makes our case-control study unique, especially with its large sample size. Whether or not hepatic iron overload in HH patients is an independent risk factor for HCC without cirrhosis remains an understudied topic yet of important significance. Our study evaluates HH yearly trends, patient demographics, symptoms, comorbidities, and hospital outcomes. It sheds light on the risk of iron overload for developing HCC in HH patients, independent of liver cirrhosis complications. The study investigated HH without cirrhosis as an independent risk factor for HCC. Our study performed HCC risk factor analysis and found that HH without cirrhosis is about 29 times more likely to develop HCC. Thus, HH without cirrhosis is an independent risk factor for HCC. Previous studies showed that it could be from iron deposition and its carcinogenic effects or the HFE gene causing mutation[10,21].
A study published in March 2001 used the National Hospital Discharge Survey and census data to evaluate hemochromatosis hospitalization rates for adult patients admitted between 1979-1997. Total records were 79580, and the study concluded that the average age of studied patients was 62 years; 92% were white, and 62% were males. There was an increase in hospitalization from 1979 to 1997, mostly in males > 60 years. However, authors reported that sample sizes for each year were outside the range of reliability; this caused limitations in calculating the confidence intervals for the upward trend significance assessment, which was over 60% for HH-related hospitalizations increased rate (from 5.4 per 100000 US residents during 1979–1982 to 8.0 during 1993–1997). In this study, the most frequent co-diagnoses in HH-related hospitalizations were in order; heart, liver, joints, and diabetes diseases[22]. Patient demographics of our study showed a similar picture with 88% white patients and 62% male with a mean age of 62 years. Our study demonstrates a significant increase in the trend of hospital admissions of HH from 2011 to 2019, i.e., 861 patients in 2011 to 3535 patients in 2019 in the weighted sample. Another study published in 2019 in New Jersey has evaluated the healthcare utilization and economic burdens of hemochromatosis in the United States by comparing costs 12 mo following the first hemochromatosis diagnosis. It showed an increase in the total health care costs ($20023 vs $16905; P < 0.0001) per patient, health care costs were 2%, 8%, 23%, and 43% higher for inpatient admissions, emergency visits, outpatient visits, and pharmaceutical prescriptions respectively compared with one year before diagnosis. Hemochromatosis patients plotted about $2732 more in total unadjusted costs and $1370 for inpatient services than controls. The annual health care costs among type 2 diabetes, hypertension, arthritis, and Chronic Kidney Disease patients with hemochromatosis were $6968, $7424, $2967, and $43847, respectively, higher than all these comorbidities without hemochromatosis; all these results were statistically significant[23]. When compared, our study supports these findings as inpatient hospital charges significantly increased over a decade (2011-2019) from $42957 to $66152. However, LOS remained unchanged throughout the study period.
Animal model studies have attested to the idea of hepatocarcinogenic effects of iron. Increased dietary iron in a rats model study is an example that showed a direct role of hepatic iron accumulation for HCC pathogenesis, and the study showed that preneoplastic nodules and HCC developed in the absence of cirrhosis or fibrosis[24]. Another study was performed on sixty Wistar albino rats that were fed an iron supplemented diet, confirmed an etiological association between heavy dietary iron intake and HCC also in the absence of fibrosis or cirrhosis by histologically examining some rats’ livers which showed grade 4 iron overload comparable to advanced HH patients[25].
One study observed that drinking groundwater with a high iron content was associated with an increased incidence of HCC in southwestern coastal areas of Taiwan[26]. Iron concentrations in drinking water usually are less than 0.3 mg/L, whereas groundwater in these regions had significantly higher content with reported concentrations of 1.04 ± 0.20 mg/L[27]. The high rate of HCC among the residents of those regions has raised the possibility that iron has direct carcinogen effects on the hepatocytes based on these two studies.
As per a study done in 2004, HCC in non-cirrhotic patients was just mentioned in case reports[28], with only 10 cases in the English literature until 2007[29-31]. However, before that, a prospective study performed in 2001 had compared 230 patients with HH with the same number of matched patients of histologically proven non–iron-related chronic liver disease to assess the cancer development rate in both groups showed that HCC developed only in the cirrhotic patients[32].
In 2019, a comprehensive review study reported that 20% of HCC cases developed in a non-cirrhotic liver owing to multiple risk factors, these patients’ presentations are usually in advanced stages due to lack of surveillance imaging in non-cirrhotic patients, in addition to a higher hepatic reserve in this patients’ population[33].
Interestingly, a study that assessed livers in HCC without cirrhosis found a high percentage of an 83-90% range of mild iron overload detectable histologically and biochemically[11]. Other studies have also studied mutations in the HFE gene and reported mutations in the HFE gene in cases of iron overload and increased frequency of C282Y heterozygotes in HCC livers without cirrhosis[10,21]. So, based on these results, a study in Germany concluded that 15%-20% of HCC cases occur in non-cirrhotic livers, and the conclusion included that likely the level of hepatocellular toxicity necessary to reach cirrhosis level is not achieved. However, the carcinogenic effect is strong enough to induce HCC, possibly due to the influence of additional carcinogens like iron and gene mutations mentioned above and other disorders that are more associated with HCC risks without cirrhosis, including alpha-1-antitrypsin deficiency and NAFLD[34].
Currently, the American College of Gastroenterology guidelines from 2019 suggest against the routine surveillance of HCC in HH patients until they have more than stage 3 fibrosis[35].
Our study is unique as no such extensive study highlights sociodemographic and HH trends over the last decade. In addition, this study includes a large and diverse cohort of patients from all over the United States compared to local or regional studies done in the past. There are several limitations to our research. First, the NIS administrative database could be prone to selection bias and lacks disease process-specific variables and coding errors without formal validation. NIS entry is equivalent to one hospitalization. If a patient is admitted more than once, one patient may contribute multiple entries. Second, we lacked some of the patients' information like iron levels and other lab values. We also did not have information like fibrosis staging, hemochromatosis duration, serum ferritin level, or hepatic iron (siderosis grade). Third, although our study showed an increasing trend of hemochromatosis over the decade, it includes only inpatient admissions; outpatient encounters are not included in this study. In addition, this study’s hemochromatosis treatment was not assessed, nor does the database have HFE gene mutation. Lastly, HH was identified by ICD-10 and ICD-9 diagnosis codes, and there was no ICD-9 code for HH before 2010, which may have affected the incidence of HH.
Our study showed an increasing trend of in-hospital admissions of HH patients and care costs in the last decade. These trends were likely related to advances in the diagnostic approach, which can increase hospital utilization and costs. Still, the LOS remained the same, which we think can be related to the that most management strategies can be done in outpatient settings. Another vital part of our study showed HH without cirrhosis as an independent risk factor for HCC. More retrospective studies are needed to re-evaluate HH risk in developing HCC.
Hereditary hemochromatosis (HH) is an inherited genetic iron metabolism disorder characterized by high iron deposition in body organs due to elevated alimentary iron absorption. Because the liver is one of the most affected organs, HH is a risk factor for hepatocellular carcinoma (HCC) due to genetics and iron carcinogenic effects. HH as an independent risk factor of HCC and trends of admitted HH patients’ characteristics and admission demographics are understudied research topics.
Current large cohort studies on temporal trends, length of stay (LOS), costs, and sociodemographic characteristics of admitted HH patients, in addition to HH being an independent risk factor of HCC, are limited.
We aim to evaluate patient characteristics, admission trends, LOS, and costs for admitted HH in the United States over the last decade. We also consider HH an independent risk factor for developing HCC without cirrhosis.
We used the national inpatient sample database for our study. We identified a sample of 18031 hospital admissions of primary or secondary HH. We selected HH and HCC cohorts. HH was first defined in 2011 as ICD-9 as a separate diagnosis. The HH cohort was extracted from January 2011 to December 2019 using 275.01 (ICD-9) and E83.110 (ICD-10) diagnosis codes. We excluded patients with cirrhosis of different etiologies. The HCC cohort was selected from January 2011 to December 2019 using ICD-9 and ICD-10 codes for HCC. A non-HCC cohort was selected with the 1:1 fixed ratio nearest neighbor propensity score using patients' age, gender, and race. Multivariate analysis was performed for the risk factors of HCC in the HCC and non-HCC matched cohorts. We further analyzed HH without cirrhosis as an independent risk factor of HCC after adjusting all known risk factors of HCC in the multivariate model.
Most admitted HH patients were white males with a mean age of 62 years. Increments in HH inpatient population trend with a Ptrend < 0.001 and total hospital cost of care trend from $42957 in 2011 to $66152 in 2019 with a Ptrend < 0.001 were found despite no change in LOS over the last decade. The incidence of liver cancer in HH patients is 1.2% (95%CI: 0.78-1.53). HH without cirrhosis had 28.8 higher odds of developing HCC.
There were increments in the trend of HH admissions and costs over the last decade with no changes in LOS. HH without cirrhosis is an independent risk factor for HCC.
These trends could be related to advances in diagnostic approaches, which increase hospital admissions and costs. Still, outpatient-based management could be a related factor to the unchanged LOS.
| 1. | Crawford DHG. Hereditary hemochromatosis types 1, 2, and 3. Clin Liver Dis (Hoboken). 2014;3:96-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Porter JL, Rawla P. Hemochromatosis. 2022 Jun 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] |
| 3. | Golfeyz S, Lewis S, Weisberg IS. Hemochromatosis: pathophysiology, evaluation, and management of hepatic iron overload with a focus on MRI. Expert Rev Gastroenterol Hepatol. 2018;12:767-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular Carcinoma: Risk Factors, Diagnosis and Treatment. Open Access Maced J Med Sci. 2015;3:732-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Renne SL, Sarcognato S, Sacchi D, Guido M, Roncalli M, Terracciano L, Di Tommaso L. Hepatocellular carcinoma: a clinical and pathological overview. Pathologica. 2021;113:203-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Yamaguchi K, Mandai M, Toyokuni S, Hamanishi J, Higuchi T, Takakura K, Fujii S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin Cancer Res. 2008;14:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 388] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | McDonald AD, McDonald JC, Pooley FD. Mineral fibre content of lung in mesothelial tumours in North America. Ann Occup Hyg. 1982;26:417-422. [PubMed] |
| 9. | Selby JV, Friedman GD. Epidemiologic evidence of an association between body iron stores and risk of cancer. Int J Cancer. 1988;41:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Blanc JF, De Ledinghen V, Bernard PH, de Verneuil H, Winnock M, Le Bail B, Carles J, Saric J, Balabaud C, Bioulac-Sage P. Increased incidence of HFE C282Y mutations in patients with iron overload and hepatocellular carcinoma developed in non-cirrhotic liver. J Hepatol. 2000;32:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Turlin B, Juguet F, Moirand R, Le Quilleuc D, Loréal O, Campion JP, Launois B, Ramée MP, Brissot P, Deugnier Y. Increased liver iron stores in patients with hepatocellular carcinoma developed on a noncirrhotic liver. Hepatology. 1995;22:446-450. [PubMed] |
| 12. | Office of Science (OS), Office of Genomics and Precision Public Health. Hereditary Hemochromatosis. May 20, 2022. Accessed June 05, 2022. Available from: https://www.cdc.gov/genomics/disease/hemochromatosis.htm. |
| 13. | HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) 2012. Agency for Healthcare Research and Quality, Rockville, MD. Accessed February 03, 2022. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp. |
| 14. | Introduction to the NIS. Healthcare Cost and Utilization Project (HCUP). February 2018. Agency for Healthcare Research and Quality, Rockville, MD. Available from: https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2015.jsp. |
| 15. | HCUP Clinical Classifications Software Refined (CCSR) for ICD-10-CM diagnoses, v2021.2. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. Accessed April 4, 2021. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp. |
| 16. | HCUP Clinical Classifications Software Refined (CCSR) for ICD-10-PCS procedures, v2021.1. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. Accessed January 15, 2021. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/prccsr.jsp. |
| 17. | HCUP NIS Trend Weights. Healthcare Cost and Utilization Project (HCUP). October 2021. Agency for Healthcare Research and Quality, Rockville, MD. Available from: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. |
| 18. | DUA Training - Accessible Version. Healthcare Cost and Utilization Project (HCUP). April 2020. Agency for Healthcare Research and Quality, Rockville, MD. Accessed February 03, 2022. Available from: https://www.hcup-us.ahrq.gov/DUA/dua_508/DUA508version.jsp. |
| 19. | HCUPnet. Healthcare Cost and Utilization Project (HCUP). 2006-2009. Agency for Healthcare Research and Quality, Rockville, MD. Accessed May 5, 2022. Available from: https://hcupnet.ahrq.gov/#setup. |
| 20. | Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72:558-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 1132] [Article Influence: 161.7] [Reference Citation Analysis (0)] |
| 21. | Bralet MP, Degott C, Belghiti J, Terris B. Prevalence of the haemochromatosis gene mutation in non-cirrhotic liver with hepatocellular carcinoma. J Hepatol. 1998;28:740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Brown AS, Gwinn M, Cogswell ME, Khoury MJ. Hemochromatosis-associated morbidity in the United States: an analysis of the National Hospital Discharge Survey, 1979-1997. Genet Med. 2001;3:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Elsaid MI, John T, Li Y, Koduru S, Ali SZ, Catalano C, Narayanan N, Rustgi VK. Health Care Utilization and Economic Burdens of Hemochromatosis in the United States: A Population-Based Claims Study. J Manag Care Spec Pharm. 2019;25:1377-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 594] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 25. | Asare GA, Paterson AC, Kew MC, Khan S, Mossanda KS. Iron-free neoplastic nodules and hepatocellular carcinoma without cirrhosis in Wistar rats fed a diet high in iron. J Pathol. 2006;208:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Shyu HJ, Lung CC, Ho CC, Sun YH, Ko PC, Huang JY, Pan CC, Chiang YC, Chen SC, Liaw YP. Geographic patterns of hepatocellular carcinoma mortality with exposure to iron in groundwater in Taiwanese population: an ecological study. BMC Public Health. 2013;13:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | World Health Organization (WHO) Iron in drinking water. Background document for development of WHO Guidelines for Drinking Water Quality. World Health Organization; 1996 . [DOI] [Full Text] |
| 28. | Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127:S79-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 283] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 29. | Britto MR, Thomas LA, Balaratnam N, Griffiths AP, Duane PD. Hepatocellular carcinoma arising in non-cirrhotic liver in genetic haemochromatosis. Scand J Gastroenterol. 2000;35:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Hiatt T, Trotter JF, Kam I. Hepatocellular carcinoma in a noncirrhotic patient with hereditary hemochromatosis. Am J Med Sci. 2007;334:228-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Köhler HH, Höhler T, Küsel U, Kirkpatrick CJ, Schirmacher P. Hepatocellular carcinoma in a patient with hereditary hemochromatosis and noncirrhotic liver. A case report. Pathol Res Pract. 1999;195:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Fracanzani AL, Conte D, Fraquelli M, Taioli E, Mattioli M, Losco A, Fargion S. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology. 2001;33:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 166] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019;11:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (7)] |
| 34. | Evert M, Dombrowski F. “Hepatozelluläre Karzinome in der nichtzirrhotischen Leber” [Hepatocellular carcinoma in the non-cirrhotic liver]. Pathologe. 2008;29:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Kowdley, Kris V. MD, FACG1; Brown, Kyle E. MD, MSc2,3,4; Ahn, Joseph MD, MS, MBA, FACG (GRADE Methodologist)5; Sundaram, Vinay MD, MSc6 ACG Clinical Guideline: Hereditary Hemochromatosis. Am J Gastroenterol. 2019;114:1202-1218. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Association for the Study of Liver Diseases.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Inmutto N, Thailand; Ji G, China; Subhani M, United Kingdom; Villela-Nogueira CA, Brazil S-Editor: Liu JH L-Editor: A P-Editor: Liu JH