Published online Sep 27, 2022. doi: 10.4254/wjh.v14.i9.1817
Peer-review started: May 18, 2022
First decision: June 23, 2022
Revised: July 4, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: September 27, 2022
Processing time: 119 Days and 9.8 Hours
Palliative care (PC) has been shown to be beneficial in end stage liver disease (ESLD), yet the hospitalization data for PC utilization is unknown.
To identify the trend of PC utilization for the special population of alcohol-associated ESLD patients, factors affecting its use and ascertain its impact on healthcare utilization.
We analyzed around 78 million discharges from the 2007-2014 national inpatient sample and 2010-2014 national readmission database including adult patients admitted for decompensated alcohol-associated cirrhosis. We identified patients with PC consultation as a secondary diagnosis. Odds ratios (OR) and means were adjusted for confounders using multivariate regression analysis models.
Out of the total 1421849 hospitalizations for decompensated liver cirrhosis, 62782 (4.4%) hospitalizations had a PC consult, which increased from 0.8% (1258) of all alcohol-associated ESLD hospitalizations in 2007 to 6.6% in 2014 (P < 0.01). Patient and hospital characteristics associated with increased odds of PC utilization were advanced age, lower income, Medicaid coverage, teaching institution, urban location, length of stay > 3 d, prolonged ventilation, and administration of total parenteral nutrition (all P < 0.01). Palliative encounters in alcohol-associated ESLD and acute-on-chronic liver failure (ACLF) score were associated with increased odds of discharge to a rehabilitation facility, but significantly lower odds of 30-d readmissions (aOR: 0.35, 95%CI: 0.31-0.41), lower total hospitalization charges and lower mean hospitalization days (all P < 0.01).
Inpatient PC is sparingly used for patients with decompensated alcohol related liver disease, however it has increased over the past decade. PC consultation is associated with lower 30-d readmission rates on multivariate analysis, and lower hospitalization cost and length of stay in patients with ACLF score ≥ 2.
Core Tip: Alcohol related end stage liver disease (ESLD) carries a poor prognosis and is associated with significant loss of quality of life and symptom burden. We found that inpatient palliative care is sparingly used for patients with decompensated alcohol related liver disease, however it has increased over the past decade. Palliative care referral is associated with decreased hospitalization cost and length of stay in acute-on-chronic liver failure Positive alcohol-associated ESLD patients, as well as decreased rehospitalization rates in all alcohol associated ESLD patients.
- Citation: Gupta K, Hans B, Khan A, Sohail SH, Kapuria D, Chang C. A retrospective study on use of palliative care for patients with alcohol related end stage liver disease in United States. World J Hepatol 2022; 14(9): 1817-1829
- URL: https://www.wjgnet.com/1948-5182/full/v14/i9/1817.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i9.1817
Cirrhosis represents advanced chronic progressive liver disease, which eventually may lead to end stage liver disease (ESLD)[1]. ESLD is defined as the manifestations of decompensated liver cirrhosis or liver failure such as variceal hemorrhage, ascites, spontaneous bacterial peritonitis, hepatocellular carcinoma (HCC), hepatorenal syndrome, or hepatopulmonary syndrome[2]. While treatments are available to prevent further fibrosis and liver damage, once the disease reaches the stage of cirrhosis, the only existing cure is liver transplantation.
Alcoholic liver disease (ALD) presents a significant burden to our healthcare system, and contributes to 48% of cirrhosis related deaths in the United States. ALD comprises a broad spectrum of disease, ranging from early ALD to alcohol-associated steatohepatitis and advanced ALD, requiring liver transplantation. While in recent years, the number of patients with ALD receiving a liver transplant has increased, it is still a miniscule percentage of the patients with ALD[3]. A study from the United Nation for Organ Sharing Database found that the number of transplants for ALD was stable between 2002 and 2012, but rose by approximately 177 transplants per year between 2013 and 2015[4]. Meanwhile, the prevalence of alcohol-associated cirrhosis is increasing in the United States. In a privately insured population, the alcohol-associated cirrhosis prevalence rate increased by 43% over the course of a 7-year period from 2009 to 2015[5].
Patients with ESLD often experience symptoms such as abdominal pain secondary to ascites, fatigue, anorexia, depression and confusion[6]. As a result of the physical and psychological effects of ESLD, quality of life is often severely impacted. In fact, patients with ESLD have been shown to have a quality of life similar to patients with end stage heart or lung disease, as well as a symptom burden similar to patients with colorectal cancer[7,8]. Palliative care (PC) has shown to be effective in improving quality of life, decreasing economic burden of disease as well as improving survival in oncology, however its use in advanced liver disease has been limited. A study by Barnes et al[9] showed an early palliative care referral of only 19% in 74 admitted patients. Only 17% of patients taken off the transplant list were actually referred to palliative care, and death occurred within 70 h of referral in half of these patients[10].
Palliative care is of special importance in patients with alcohol-associated liver disease as the life expectancy of patients with alcohol-associated cirrhosis is very low, 5-year and 10-year survival rates are 23 percent and 7 percent, respectively[11]. These rates are significantly worse than survival rates for patients whose cirrhosis was not caused by alcohol. These factors make early intervention by palliative care greatly beneficial to patients with alcohol-associated cirrhosis.
Due to the combination of disadvantageous positions for alcohol-dependent patients to secure a liver transplantation, poorer prognosis in this cohort, and the negative association of alcohol-associated liver disease patients to have a palliative care referral we aimed to study the implications of palliative care consult for this population[9]. In this study, we evaluate the use of palliative care for patients with decompensated alcohol-associated liver disease while they are admitted to the hospital for inpatient care in the United States.
We performed a retrospective, multicenter, observational study using data from two national databases, nationwide inpatient sample (NIS) from 2007 to 2014, and national readmission database (NRD) from 2010 to 2014. We utilized NIS until 2014 because the International Classification of Diseases (ICD) codes utilized by NIS have differed from the year 2015 to incorporate ICD-10 codes. We used different time periods for the two databases as NRD came into existence from the year 2010, unlike NIS which began from 1997. Both of these databases are a part of the healthcare cost and utilization project maintained by the agency for healthcare research and quality. The NIS is an administrative database consisting (until 2012) of all hospitalizations drawn from a sample of 20% of United States hospitals, and then weighted to be nationally representative of all United States hospitalizations[12]. NRD represents about half of all United States Hospitalizations, and provides a national estimate of readmission rates[13].
We performed separate analysis on both of the databases owing to their unique characteristics. The data cannot be merged from the two databases as the identifying information in both is encoded as different numbers. The NIS database provides information regarding the index hospital admission and includes patient demographic data, primary and secondary diagnosis, procedures, hospital characteristics, and inpatient and discharge mortality rates. Each record includes one primary and up to 24 discharge diagnoses, procedure codes, demographic data, hospitalized inpatient mortality indicator, payer status, total hospitalization charges and length of stay[10]. The NRD in addition to the information provided by NIS, also assigns a unique, unidentified patient association number to each patient, and tracks all patients at each hospital in each state throughout the calendar year.
We used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to identify primary and secondary diagnosis codes of interest. To identify patients with end stage alcohol-associated liver disease, an entry was required to have the following diagnosis: (1) Diagnosis code for alcohol-associated cirrhosis (571.2) along with a diagnosis code for a decompensating event [defined by ICD-9-CM code of bleeding esophageal varices (456.0, 456.21), ascites (789.5, 789.59), and hepatic encephalopathy (572.2)]; and (2) Diagnosis code for other cirrhosis (571.5) with an alcohol disorder/comorbidity (571.1, 291x, 303x, 305x, 790.3, 980x, E860), and an event of decompensation (as defined above). This combination of ICD-9-CM codes for cirrhosis and complications has a positive predictive value of 78%, a negative predictive value of 91% for cirrhosis, with a c-statistic of 0.71[14].
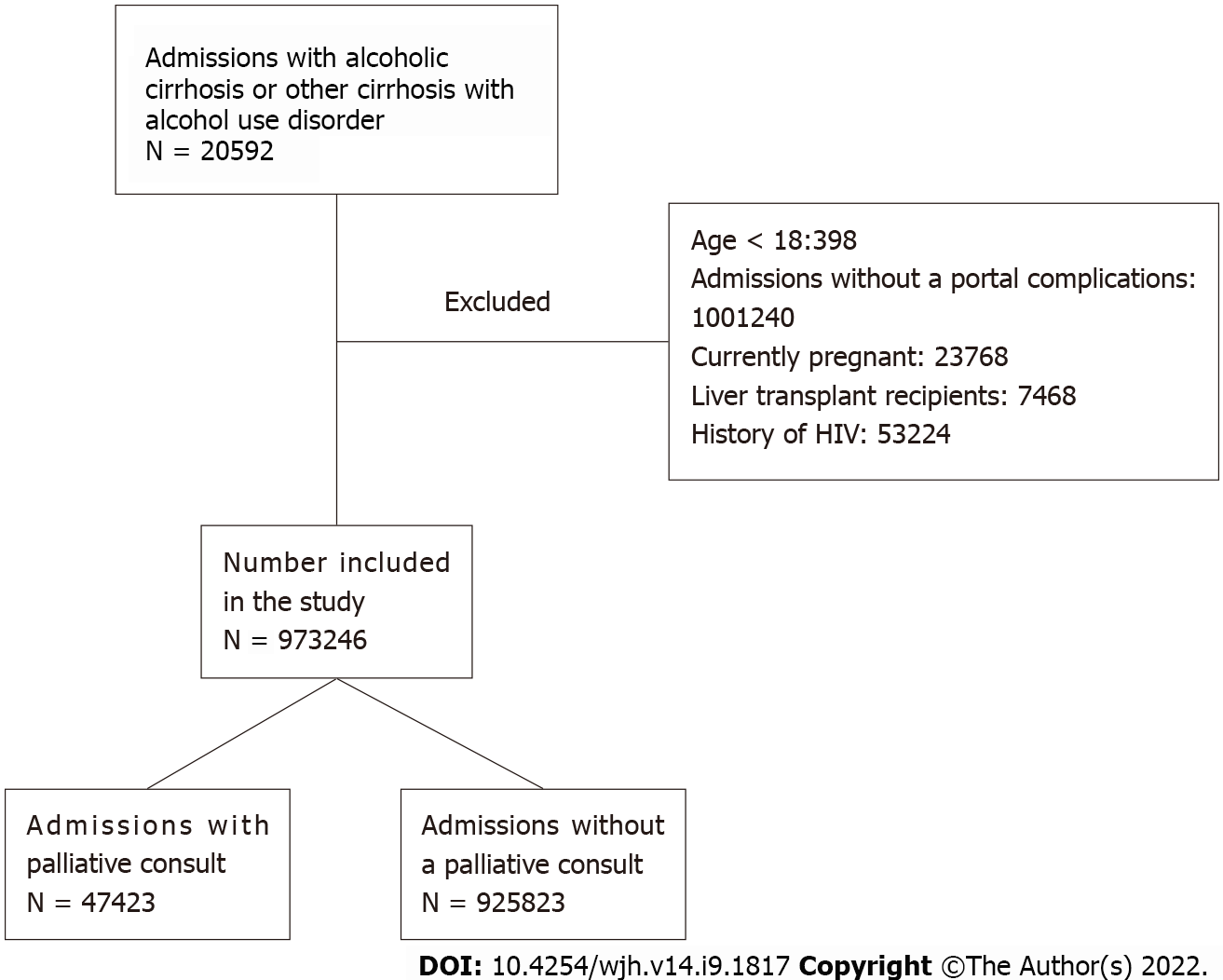
We excluded patients who were less than 18 years old at the time of admission or who were transferred from another health facility. In keeping with the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) exclusion criteria, we omitted patients with a history of prior liver transplant, human immunodeficiency virus or actively pregnant. Palliative care consultation was identified using the ICD codes (ICD 9: V66.7, ICD 10: Z51.5). Other factors such as cirrhosis complications, in-hospital death, medical complications, intensive care unit care, length of hospitalization and costs were examined as dependent variables. Independent variables included were age, sex, race, payer source (commercial or health maintenance organization, Medicare, Medicaid, self-pay, or other), comorbidity, nature of admission (emergent/urgent, or other), hospital bed-size, hospital location (rural or urban), geographic region and hospital teaching status. Figure 1 depicts the flow of the study cohort. The diagnostic codes associated with these diagnoses are shown in supporting Table 1.
| Variable | Overall | Patients with palliative consult | Patients without palliative consult | P value |
| Number of patients | 973246 | 47423 | 925823 | |
| Patient characteristics | ||||
| Female | 276401 (28.4%) | 14037 (29.6%) | 262933 (28.4%) | < 0.001 |
| Age in yr | 54.7 ± 004 | 57.2 ± 0.1 | 54.5 ± 0.04 | < 0.001 |
| Age divided into categories | ||||
| 18-35 | 36010 (3.7%) | 1327 (2.8%) | 33329 (3.6%) | < 0.001 |
| 36-45 | 108030 (11.1%) | 3604 (7.4%) | 103692 (11.2%) | < 0.001 |
| 46-65 | 625797 (64.3%) | 28975 (61.1%) | 595304 (64.3%) | < 0.001 |
| > 66 | 204381 (21%) | 13136 (27.7%) | 191645 (20.7%) | < 0.001 |
| Race | ||||
| White | 660834 (67.2%) | 33433 (70.5%) | 614746 (66.4%) | < 0.001 |
| Black | 96351 (9.9%) | 4647 (9.8%) | 95359 (10.3%) | < 0.001 |
| Hispanic | 160585 (16.5%) | 6544 (13.8%) | 161093 (17.4%) | < 0.001 |
| Other | 60341 (6.2%) | 2940 (6.2%) | 57401 (6.2%) | 0.532 |
| Charleston comorbidity index | ||||
| 0 or 1 | 118736 (12.2%) | 2371 (5%) | 116653 (12.6%) | < 0.001 |
| 2 | 46715 (4.1%) | 948 (2%) | 38884 (4.2%) | < 0.001 |
| 3 or more | 854509 (87.8%) | 45051 (95%) | 809169 (87.4%) | < 0.001 |
| Median income in patient zip code | ||||
| $1–$38999 | 297813 (30.6%) | 13894 (29.3%) | 283301 (30.6%) | 0.003 |
| $39000–$47999 | 255963 (26.3%) | 12946 (27.3%) | 245343 (26.5%) | 0.004 |
| $48000–$62999 | 231632 (23.8%) | 11144 (23.5%) | 220345 (23.8%) | 0.133 |
| > $63000 | 187836 (19.3%) | 9389 (19.8%) | 178683 (19.3%) | < 0.001 |
| Insurance provider | ||||
| Medicare | 341609 (35.1%) | 17878 (37.7%) | 328667 (35.5%) | < 0.001 |
| Medicaid | 260829 (26.8%) | 12045 (25.4%) | 249046 (26.9%) | < 0.001 |
| Private | 255963 (26.3%) | 12282 (25.9%) | 243491 (26.3%) | < 0.001 |
| Uninsured | 114843 (11.8%) | 5216 (11%) | 109247 (11.8%) | < 0.001 |
| Hospital characteristics | ||||
| Teaching hospital | 514847 (52.9%) | 28690 (60.5%) | 486982 (52.6%) | < 0.001 |
| Urban hospital | 891493 (91.6%) | 44387 (93.6%) | 847128 (91.5%) | < 0.001 |
| Hospital region | ||||
| Northeast | 177130 (18.2%) | 6828 (14.4%) | 170351 (18.4%) | < 0.001 |
| Midwest | 199515 (20.5%) | 9911 (20.9%) | 189793 (20.5%) | 0.832 |
| South | 345502 (35.5%) | 16692 (35.2%) | 328667 (35.5%) | 0.104 |
| West | 251097 (25.8%) | 13989 (29.5%) | 237010 (25.6%) | < 0.001 |
| Hospital size | ||||
| Small | 122628 (12.6%) | 4931 (10.4%) | 118505 (12.8%) | < 0.001 |
| Medium | 258883 (26.6%) | 12756 (26.9%) | 246268 (26.6%) | 0.002 |
| Large | 590760 (60.7%) | 29734 (62.7%) | 561048 (60.6%) | < 0.001 |
| Hospital complications | ||||
| Variceal bleed | 114843 (11.8%) | 4789 (10.1%) | 110428 (11.9%) | < 0.001 |
| HRS | 22619 (7.2%) | 14942 (23.8%) | 75917 (8.2%) | < 0.001 |
| Hepatic encephalopathy | 321171 (33%) | 24375 (51.4%) | 322186 (34.8%) | < 0.001 |
| Ascites | 605359 (62.2%) | 34191 (72.1%) | 565677 (61.1%) | < 0.001 |
| SBP | 23357 (2.4%) | 1659 (3.5%) | 21293 (2.3%) | < 0.001 |
| Hepatocellular carcinoma | 60341 (6.2%) | 5927 (12.5%) | 49994 (5.4%) | < 0.001 |
| NACSELD-ACLF score | ||||
| 0 | 495382 (50.9%) | 11191 (23.6%) | 482353 (52.1%) | < 0.001 |
| 1 | 389298 (40%) | 22810 (48.1%) | 366625 (39.6%) | < 0.001 |
| 2 | 64234 (6.6%) | 8536 (18%) | 56475 (6.1%) | < 0.001 |
| 3 | 22384 (2.3%) | 4505 (9.5%) | 18516 (2%) | < 0.001 |
| 4 | 973 (0.1%) | 426(0.9%) | 925 (0.1%) | < 0.001 |
| ACLF ≥ 2 | 89538 (9.2%) | 13420 (28.3%) | 76843 (8.3%) | < 0.001 |
| Mean ACLF score | 0.64 | 1.21 | 0.61 | < 0.001 |
Data were analyzed using Stata 15.0 (StataCorp, College Station, TX). Pearson χ2 test was used to compare proportions between the patients with PC and without PC. Associations between variables were analyzed using cross-tabulations and multivariate logistic regression modeling. Data were weighted and modified hospital and discharge weights to correct for changes in sampling over time were applied. Variance estimation was performed using procedures for survey data analysis with replacement. Strata with one sampling unit were centered at the population mean. Multivariable regression analysis models were used to adjust the results for potential confounders. Multivariable regression models were built by including all confounders that were significantly associated with the outcome of univariable analysis with a cutoff P-value of 0.05. The model controlled for age, sex, race, median household income of residents in the patient's zip code, insurance, charlson comorbidity index, hospital bedsize, academic status of hospital, hospital location, length of stay, acute-on-chronic liver failure (ACLF) score, history of hepatocellular carcinoma, acute infections, transjugular intrahepatic portosystemic shunt (TIPS) and total parenteral nutrition (TPN). Logistic regression was used for binary outcomes and linear regression was used for continuous outcomes.
Our primary outcome of interest was the proportion of decompensated liver cirrhosis patients who received a PC consult during their hospitalization and their trend over the study period. Secondary outcomes were: (1) All-cause in-hospital mortality; (2) Healthcare total hospital charge; (3) Duration of hospitalization [Length of stay (LOS) in days], which were all encoded in the data set as unique variables; and (4) Major in hospital procedures and portal hypertensive complications, and these were compared between the two groups. We further categorized patients according to the number of organ failures based upon the NACSELD-ACLF, a bedside tool to predict short-term mortality in ESLD patients. This score has been previously validated using the NIS. A positive ACLF score is deemed as ≥ 2. We also included other complications of cirrhosis such as portal hypertension, hepatorenal syndrome and spontaneous bacterial peritonitis. We also identified procedures commonly performed in ESLD hospitalizations such as total parenteral nutrition, TIPS and prolonged mechanical ventilation.
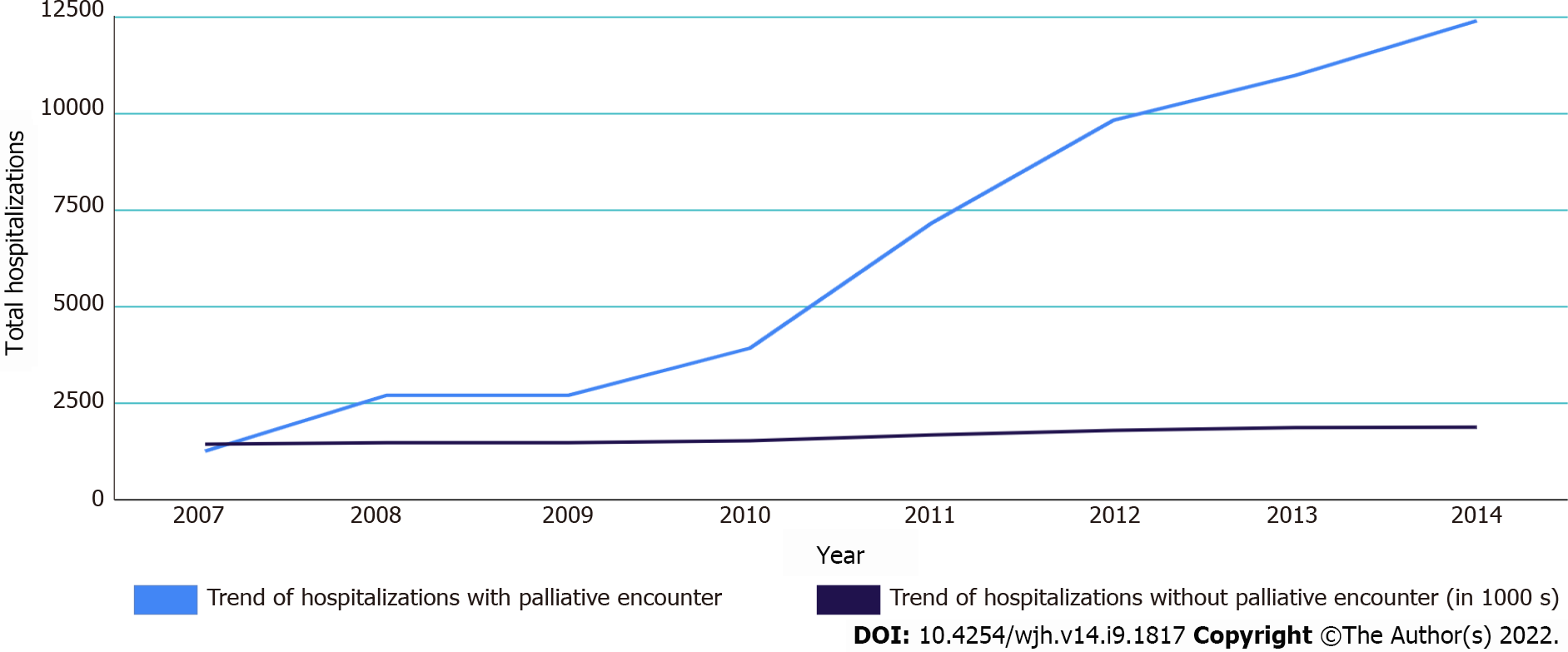
There was a total of 2059524 hospitalizations for alcohol-associated cirrhosis recorded, out of which 973246 met our inclusion criteria of presenting with a portal complication. The majority of the patients were male (73.1%), white (67%), had a mean Charlson comorbidity index < 3 (83.6%) and belonged to the age group 46-65 years (68%). The mean age was 54.7 years. A palliative care encounter was recorded in only 4.8% of cases (n = 47423). On trending the utilization of palliative care, it was observed to have increased from 0.8% (956) of all ESLD hospitalizations in 2007 to 6.6% (9430) in 2014. Figure 2 depicts the trend of hospitalizations in both groups.
A palliative care encounter was more likely in female patients (29.6% vs 28.4% P < 0.01), patients older than 65 years (27.7% vs 20.7%, P < 0.01), whites (70.5% vs 66.4%, P < 0.01), Charlson comorbidity score ≥ 3 (95% vs 87.4%, P < 0.01) and medicare patients (37.7% vs 35.5%, P < 0.01), but was less likely for hispanic patients (13.8% vs 17.4%, P < 0.01), patients belonging to the lowest quarter of mean income (29.3% vs 30.6%, P = 0.003) and patients with medicaid (25.4% vs 26.9%, P < 0.001) or no insurance at all (11% vs 11.8%, P < 0.01). With regards to hospital characteristics, a significantly higher proportion of patients receiving palliative care were treated at teaching hospitals (60.5% vs 52.6%, P < 0.01), in urban locations (93.6% vs 91.5%, P < 0.01), large hospitals (62.7% vs 60.6%, P < 0.01) and in western states (29.5% vs 25.6%, P < 0.001), whereas patients in northeastern states (14.4% vs 18.4%, P < 0.01) were less likely to receive an inpatient palliative care consult.
On analyzing complications related with cirrhosis, patients receiving palliative care as inpatients had a significantly higher proportion of hepatic encephalopathy (51% vs 34 %, P < 0.001), ascites (72% vs 61%, P < 0.001), hepatorenal syndrome (23% vs 8%, P < 0.001), spontaneous bacterial peritonitis (3.5% vs 2.3%, P < 0.001) and HCC (12% vs 5%, P < 0.001), whereas patients with variceal bleeding (10% vs 12%, P < 0.001) were less likely to receive a palliative care. Palliative care consults were more common in all patients with North American Consortium for the Study of End-Stage Liver Disease's definition of acute-on-chronic liver failure (NACSELD-ACLF) score ≥ 1, with the proportion increasing by each grade (Grade 1: 48.1% vs 39.6%; Grade 2: 18% vs 6.1%; Grade 3: 9.5% vs 2%; Grade 4: 0.9% vs 0.1%, all P < 0.001).
In the palliative care cohort, more people received total parenteral nutrition, or TPN (2.9% vs 1.4%, P < 0.001) however, a lower number were liver transplant recipients (0.6% vs 1.8%, P < 0.001). There was no difference in receiving a transjugular intrahepatic systemic shunt, or TIPS (1.2% vs 1.3%, P = 0.359) between the two groups.
Multivariate logistic regression analysis of predictor variables for palliative consultation associated with alcohol related ESLD is shown in Table 2. After controlling for all other variables, hepatorenal syndrome (aOR: 3.4, 95%CI: 3.04-3.81, P < 0.001), ascites (aOR: 1.13, 95%CI: 1.03-1.24, P = 0.007), spontaneous bacterial peritonitis (SBP) (aOR: 3.32, 95%CI: 2.65-3.86, P < 0.001) and HCC (aOR: 1.78, 95%CI: 1.58-2.00, P < 0.001) were associated with higher odds of palliative care encounter than alcohol related ESLD patients. Patients with ACLF scores ≥ 2 were associated with higher odds of palliative care consult (aOR: 1.02 95%CI: 1.00-1.04, P < 0.001). Patient and hospital characteristics associated with increased palliative care utilization on multivariate regression were advanced age (aOR: 1.02, 95%CI: 1.00-1.04, P < 0.001), female sex (aOR: 1.07, 95%CI: 1.00-1.14, P < 0.001), uninsured (aOR: 1.52, 95%CI: 1.36-1.7, P < 0.001), teaching institution (aOR: 1.4, 95%CI: 1.28-1.53, P < 0.001), hospital bedsize > 400 beds (aOR: 1.25, 95%CI: 1.1-1.41, P < 0.001), and length of stay > 5 d (aOR: 1.18, 95%CI: 1.10-1.26, P < 0.001). Major infections during the hospitalization, as described above, had higher odds of palliative care use (aOR: 1.58, 95%CI: 1.48-1.69, P < 0.001). Other patient characteristics with increased odds of palliative consult included mechanical ventilation (OR: 3.32 95%CI: 3.1-3.54, P < 0.01), and administration of TPN (OR: 2.02, 95%CI: 1.8-2.27, P < 0.01).
| Variable | Adjusted odds ratio | 95%CI | P value |
| Age | 1.02 | 1.00-1.04 | < 0.001 |
| Median income in patient zip code | |||
| $1-$38999 | Reference | ||
| $39000-$47999 | 1.07 | 0.98-1.16 | 0.117 |
| $48000-$62999 | 0.99 | 0.9-1.08 | 0.983 |
| > $63000 | 0.95 | 0.85-1.02 | 0.141 |
| Female sex | 1.07 | 1.00-1.14 | 0.023 |
| Race | |||
| Caucasian | Reference | ||
| African Americans | 0.81 | 0.72-0.9 | < 0.001 |
| Hispanic | 0.71 | 0.64-0.78 | < 0.001 |
| Others | 0.89 | 0.78-1.00 | 0.069 |
| Charleson comorbidity index | 1.1 | 1.08-1.12 | < 0.001 |
| Insurance status | |||
| Medicare | Reference | ||
| Medicaid | 1.34 | 1.19-1.51 | < 0.001 |
| Private insurance | 1.18 | 1.08-1.29 | < 0.001 |
| Uninsured | 1.52 | 1.36-1.7 | < 0.001 |
| Teaching hospital | 1.4 | 1.28-1.53 | < 0.001 |
| Bed size | |||
| < 250 beds | Reference | ||
| 251-400 beds | 1.24 | 1.09-1.42 | 0.001 |
| > 400 Beds | 1.25 | 1.1-1.41 | < 0.001 |
| Urban location | 0.96 | 0.83-1.19 | 0.625 |
| Length of stay by groups | |||
| < 3 d | Reference | ||
| 3-5 d | 0.92 | 0.84-1.00 | 0.065 |
| > 5 d | 1.18 | 1.10-1.26 | < 0.001 |
| Rehab transfer | 4.07 | 3.29-5.04 | < 0.001 |
| ACLF Score | |||
| 1 | 2.53 | 2.35-2.71 | < 0.001 |
| 2 | 6.05 | 5.5-6.66 | < 0.001 |
| 3 | 9.86 | 8.75-11.1 | < 0.001 |
| 4 | 10.61 | 7.32-15.42 | < 0.001 |
| ACLF ≥ 2 | 3.47 | 3.21-3.74 | < 0.001 |
| Hepatocellular carcinoma | 1.78 | 1.58-2.00 | < 0.001 |
| Infection | 1.58 | 1.48-1.69 | < 0.001 |
| Mechanical ventilation | 3.32 | 3.1-3.54 | < 0.001 |
| Total parenteral nutrition | 2.02 | 1.8-2.27 | < 0.001 |
| Hepatorenal syndrome | 3.4 | 3.04-3.81 | < 0.001 |
| Ascites | 1.13 | 1.03-1.24 | < 0.001 |
| Spontaneous bacterial peritonitis | 3.34 | 2.65-3.86 | < 0.001 |
| Age | 1.02 | 1.00-1.04 | < 0.001 |
| Female sex | 1.07 | 1.00-1.14 | 0.023 |
| Race | |||
| Caucasian | Reference | ||
| African Americans | 0.81 | 0.72-0.9 | < 0.001 |
| Hispanic | 0.71 | 0.64-0.78 | < 0.001 |
| Others | 0.89 | 0.78-1.00 | 0.069 |
| Charleson comorbidity index | 1.1 | 1.08-1.12 | < 0.001 |
| Insurance status | |||
| Medicare | Reference | ||
| Medicaid | 1.34 | 1.19-1.51 | < 0.001 |
| Private insurance | 1.18 | 1.08-1.29 | < 0.001 |
| Uninsured | 1.52 | 1.36-1.7 | < 0.001 |
| Teaching hospital | 1.4 | 1.28-1.53 | < 0.001 |
| Bed size | |||
| < 250 beds | Reference | ||
| 251-400 beds | 1.24 | 1.09-1.42 | 0.001 |
| > 400 Beds | 1.25 | 1.1-1.41 | < 0.001 |
| Urban location | 0.96 | 0.83-1.19 | 0.625 |
| Length of stay by groups | |||
| < 3 d | Reference | ||
| 3-5 d | 0.92 | 0.84-1.00 | 0.065 |
| > 5 d | 1.18 | 1.10-1.26 | < 0.001 |
| Rehab transfer | 4.07 | 3.29-5.04 | < 0.001 |
| ACLF ≥ 2 | 3.47 | 3.21-3.74 | < 0.001 |
On multivariate analysis, total hospitalization charges (regression coefficient: $1813, 95%CI: -1106 to 4734, P = 0.224) and length of stay (regression coefficient: 0.342, 95%CI: -1.031 to 1.71, P = 0.625) were unchanged in patients with PC. Looking at patients who were NACSELD ACLF positive (ACLF ≥ 2), we saw that palliative care was associated with significantly reduced total hospitalization charges (regression coefficient: -$8405, 95%CI: -16721 to -90, P = 0.048) and length of stay (regression coefficient: -2.34 d, 95%CI: -2.88 to -1.81 d, P < 0.001).
Utilizing the NRD 2010-2014, a total of 356215 patients with alcohol related ESLD met the inclusion criteria, out of which 164940 patients were readmitted, leading to a 30-d readmission rate of 46.3%. Table 3 shows the factors associated with readmission rates. On univariate analysis, we found palliative care, age, charlson comorbidity index, hospital location, teaching status, ACLF score and infection had a statistically significant association with readmission rates. We used these factors to analyze the association of PC with 30-d readmission rate with cox multivariate regression model. PC consult was associated with significantly lower odds of 30-d readmissions (aOR: 0.35, 95%CI: 0.31-0.41, P < 0.001). Other factors found to be associated were age (aOR: 0.99, 95%CI: 0.99-0.99, P < 0.001), charlson comorbidity index (aOR: 1.06, 95%CI: 1.05-1.06, P < 0.001), positive ACLF (aOR: 0.86, 95%CI: 0.81-0.91, P < 0.001), infection (aOR: 1.09, 95%CI: 1.07-1.13, P < 0.001) and hospital located in rural area (aOR: 0.86, 95%CI: 0.81-0.91, P < 0.001).
| Variable | Unadjusted odds ratio | 95%CI | P value | Adjusted odds ratio | 95%CI | P value |
| Palliative care | 0.36 | 0.31-0.41 | < 0.001 | 0.35 | 0.31-0.41 | < 0.001 |
| Patient characteristics | ||||||
| Age | 0.99 | 0.99-0.99 | < 0.001 | 0.99 | 0.99-0.99 | < 0.001 |
| Median income in patient zip code | ||||||
| $1-$38999 | Reference | |||||
| $39000-$47999 | 0.97 | 0.94-1.01 | 0.236 | |||
| $48000-$62999 | 1 | 0.97-1.04 | 0.655 | |||
| > $63000 | 1.02 | 0.99-1.06 | 0.133 | |||
| Female sex | 1.01 | 0.99-1.04 | 0.188 | |||
| Race | ||||||
| Caucasian | Reference | |||||
| African-Americans | 0.77 | 0.52-1.12 | 0.175 | |||
| Hispanic | 0.93 | 0.65-1.34 | 0.721 | |||
| Others | 0.76 | 0.47-1.24 | 0.282 | |||
| Mean charlson comorbidity index | 1.04 | 1.03-1.04 | < 0.001 | 1.06 | 1.05-1.06 | < 0.001 |
| Insurance status | ||||||
| Medicare | Reference | |||||
| Medicaid | 1.14 | 1.11-1.18 | < 0.001 | |||
| Private insurance | 0.89 | 0.86-0.93 | < 0.001 | |||
| Uninsured | 0.75 | 0.71-0.78 | < 0.001 | |||
| ACLF ≥ 2 | 0.89 | 0.84-0.95 | 0.001 | 0.86 | 0.81-0.91 | < 0.001 |
| Infection | 1.07 | 1.04-1.10 | < 0.001 | 1.09 | 1.07-1.13 | < 0.001 |
| Hospital characteristics | ||||||
| Teaching hospital | 1.05 | 1.02-1.05 | < 0.001 | 1.02 | 0.99-1.05 | 0.208 |
| Bed size | ||||||
| < 250 beds | Reference | |||||
| 251-400 beds | 1 | 0.95-1.06 | 0.723 | |||
| > 400 Beds | 1.02 | 0.97-1.07 | 0.37 | |||
| Rural location | 0.85 | 0.83-0.88 | < 0.001 | 0.86 | 0.81-0.91 | < 0.001 |
In this large, nationally representative analysis of patients with alcohol-assocaited ESLD, only a small proportion of patients (4.4%) received palliative care. The rate is lower as compared to PC consultations for advanced cancers which was recorded at 9.9% using NIS[15]. While still low, there has been an encouraging increase in the utilization of palliative care from less than 1% in 2007 to almost 7 % of all inpatient encounters in 2014. This is comparable to an increase in PC consults in inpatients with all-cause ESLD, reported by Rush et al[16] over a similar time period, and can be attributed to the increased recognition of the role PC plays in improving quality of life and reducing disease burden.
We identified geographical, socioeconomic as well as racial disparities in PC referrals. This may be due to an incomplete understanding of the concept of palliative care amongst some patient populations, such as the hispanic population. In addition, access to healthcare services was also a significant factor as PC referrals were more common in large and urban hospitals. This follows the trend oncologists have reported amongst minorities and low-income groups[17].
As expected, sicker patients were more likely to receive palliative consults. Patients with ≥ 4 ACLF score had ten times higher odds of a palliative consult as compared to patients with a score of zero. The ACLF score has been shown to have better predictive value. Patients who presented with variceal bleeding as a symptom of decompensated alcohol related ESLD were less likely to receive PC consults, this could be because of effective endoscopic interventions available compared to the more insidious and perhaps more advanced illness indicated by ascites and hepatorenal syndrome. Similarly, patients with HCC were more likely to receive PC consults, the oncological nature of their disease perhaps facilitating recognition of the need for palliative care. Hudson et al[18] have introduced a model to identify patients at high risk of impending death in patients with decompensated cirrhosis, which included patients with presence of 3 or more factors on admission to the emergency department, a history of 2 or more admissions in the prior 6 mo, ongoing alcohol use in the context of known alcohol-related liver disease, unsuitability for liver transplant, and World Health Organization Performance status 3 or 4 predicted 1-year mortality with a sensitivity of 72%.
Increased PC use seen in patients with prolonged ventilation and TPN suggest a delayed referral to PC, occurring after significant progression of disease. The timing of palliative care is important, and early PC has been shown to improve quality of life and prolong survival in other patient populations[19] and may help avoid aggressive and futile treatments[20]. Previous data has shown that alcohol-associated ESLD patients are more likely to have a delayed PC referral, with young age and recent alcohol use found to be predictors of late hospice referrals[21]. We saw that inpatients with positive ACLF score, length of stay and total hospitalization charges were significantly reduced with the use of PC services. This is likely as patients with positive ACLF score have a 6-mo mortality rate of 90%, thus meeting the criteria for hospice care as per medicare rules and are likely to have PC involved[22,23]. On analyzing the national readmission database, we found that PC was associated with a significantly reduced odds of 30-d readmission in alcohol associated ESLD patients, with an adjusted odds ratio of 0.35 on multivariate analysis, accounting for several factors found to be associated with readmission rates such as age and ACLF score. We found that the rate of readmissions in our cohort of alcohol-associated ESLD patients with C use was lower than that of all ESLD patients which has been studied before[24,25]. Also, utilization of PC was lower than all-ESLD patients, where it was 5.3% vs 4.4% for our cohort of patients.
Our study has several limitations. First, inherent to the nature of our retrospective discharge database, our analysis is limited by the errors in coding as well as missing data. Additionally, we were unable to identify interventions performed to alleviate decompensating events, and whether successful interventions reduced the referral to PC. The period over which our data has been collected has also witnessed changes in the management of alcohol related ESLD, with a significant increase in ALD liver transplantation. While survival in alcohol-associated cirrhosis remains low, increased transplantation potentially reduces the number of alcohol related ESLD patients. Our study does not account for this increase in liver transplantation.
Despite these limitations, the study has many advantages. To date, this is the largest study that measures the utilization of palliative care and its impact on the care of patients with alcohol associated liver disease. We utilized the largest and most inclusive readmission database in the United States. These data are collected from all hospitals in 22 states, so these data are reasonably generalizable, and we hope they will increase the validity of our study. We provided the first national estimate of 30-d readmission risk specifically for alcohol-associated ESLD which are known to have poorer access to healthcare in general and liver transplantation in particular. Also, we were able to grade patients using ACLF scoring to better ascertain the referral rate for palliative care depending on the clinical condition of these patients.
The increase in adoption of PC for alcohol related ESLD suggests an increasing recognition of the role PC plays in mitigating symptom burden and improving quality of life in these patients. Early palliative care referrals[26], and easier access to high quality palliative care should be an integral part of managing patients with alcohol related ESLD, and special attention needs to be paid to ensure inclusion of ethnic minorities and patients of low socioeconomic status.
Inpatient palliative care is sparingly used for patients with decompensated alcohol related liver disease, however it has increased over the past decade. Palliative care referral is associated with decreased hospitalization cost and length of stay in ACLF positive alcohol-associated ESLD patients, as well as decreased rehospitalization rates in all alcohol-associated ESLD patients.
Use of palliative care (PC) consultation has been steadily increasing, especially in the field of cirrhosis.
Alcohol-associated end stage liver disease (ESLD) patients are at a disadvantage for being referred to palliative care as they are younger and are more likely to belong to lower socioeconomic strata. The use of palliative care is especially important for this subgroup as the only definite treatment is liver transplant which is often not an option for these patients.
To assess the trend of PC use in patients hospitalized with alcohol associated ESLD as the primary diagnosis, study the baseline characteristics of these patients, evaluate the factors associated with increased PC use, study the impact of PC use on hospitalization outcomes and 30-d readmission rates.
We used the national inpatient sample from 2007 to 2014, and the national readmission database from 2010 to 2014. We identified the patients admitted with alcoholic cirrhosis and at least one cirrhosis decompensation event. We identified patients with PC consultation as a secondary diagnosis. Baseline characteristics between the groups were compared with linear regression, and multivariate regression analysis model was used to assess the impact that PC use has on the hospitalization outcomes.
PC use has increased over 8 times during the study period and was used in 6.6% of alcohol-associated ESLD hospitalizations in 2014. PC use was more common in patients with ascites, hepatic encephalopathy and hepatocelluluar carcinoma. Other factors associated with increased PC use were females, whites, uninsured patients, teaching hospitals and patients with a higher North American Consortium for the Study of End-Stage Liver Disease's definition of acute-on-chronic liver failure score. The length of stay and total hospitalization costs were lower in patients with acute-on-chronic liver failure score ≥ 2 and receiving PC, but not significantly different in the overall cohort. PC use was associated with significantly lower 30-d readmission rates, with odds ratios of 0.35.
PC use has been increasing over the years, however is still underutilized especially in select population and in rural areas. We show that PC use is associated with decreased length of stay in patients with more complications, and also leads to decreased 30-d readmission rates.
This study calls for further research to assess the point during the disease course in which patients with alcohol-associated ESLD would benefit from PC use. Further research should also be conducted to assess for the reasons for decreased PC use in select disadvantaged population.
| 1. | Kelly EM, James PD, Murthy S, Antonova L, Wong F, Shaw-Stiffel T, Chalifoux M, Salim M, Tanuseputro P. Health Care Utilization and Costs for Patients With End-Stage Liver Disease Are Significantly Higher at the End of Life Compared to Those of Other Decedents. Clin Gastroenterol Hepatol. 2019;17:2339-2346.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology. 2012;55:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Kling CE, Perkins JD, Carithers RL, Donovan DM, Sibulesky L. Recent trends in liver transplantation for alcoholic liver disease in the United States. World J Hepatol. 2017;9:1315-1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, Volk ML, Blow FC, Lok ASF. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68:872-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 6. | Potosek J, Curry M, Buss M, Chittenden E. Integration of palliative care in end-stage liver disease and liver transplantation. J Palliat Med. 2014;17:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Díaz-Domínguez R, Pérez-Bernal J, Pérez-San-Gregorio MA, Martín-Rodríguez A. Quality of life in patients with kidney, liver or heart failure during the waiting list period. Transplant Proc. 2006;38:2459-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96:2199-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 236] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Barnes A, Woodman RJ, Kleinig P, Briffa M, To T, Wigg AJ. Early palliative care referral in patients with end stage liver disease is associated with reduced resource utilisation. J Gastroenterol Hepatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Kathpalia P, Smith A, Lai JC. Underutilization of palliative care services in the liver transplant population. World J Transplant. 2016;6:594-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 11. | Huang Y, Joseph J, de Boer WB, Cheng W, Adams LA, MacQuillan G, Garas G, Raftopoulos S, Jeffrey GP. Long-term Liver-related Outcomes of Patients With Chronic Liver Diseases in Australia. Clin Gastroenterol Hepatol. 2020;18:496-504.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Gupta K, Khan A, Goyal H, Cal N, Hans B, Martins T, Ghaoui R. Weekend admissions with ascites are associated with delayed paracentesis: A nationwide analysis of the 'weekend effect'. Ann Hepatol. 2020;19:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Gupta K, Khan A, Kumar M, Sawalha K, Abozenah M, Singhania R. Readmissions Rates After Myocardial Infarction for Gastrointestinal Bleeding: A National Perspective. Dig Dis Sci. 2021;66:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 15. | Rubens M, Ramamoorthy V, Saxena A, Das S, Appunni S, Rana S, Puebla B, Suarez DT, Khawand-Azoulai M, Medina S, Viamonte-Ros A. Palliative Care Consultation Trends Among Hospitalized Patients With Advanced Cancer in the United States, 2005 to 2014. Am J Hosp Palliat Care. 2019;36:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Rush B, Walley KR, Celi LA, Rajoriya N, Brahmania M. Palliative care access for hospitalized patients with end-stage liver disease across the United States. Hepatology. 2017;66:1585-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Currow DC, Allingham S, Bird S, Yates P, Lewis J, Dawber J, Eagar K. Referral patterns and proximity to palliative care inpatient services by level of socio-economic disadvantage. A national study using spatial analysis. BMC Health Serv Res. 2012;12:424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Hudson BE, Ameneshoa K, Gopfert A, Goddard R, Forbes K, Verne J, Collins P, Gordon F, Portal AJ, Reid C, McCune CA. Integration of palliative and supportive care in the management of advanced liver disease: development and evaluation of a prognostic screening tool and supportive care intervention. Frontline Gastroenterol. 2017;8:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Baumann AJ, Wheeler DS, James M, Turner R, Siegel A, Navarro VJ. Benefit of Early Palliative Care Intervention in End-Stage Liver Disease Patients Awaiting Liver Transplantation. J Pain Symptom Manage. 2015;50:882-6.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Kelly SG, Campbell TC, Hillman L, Said A, Lucey MR, Agarwal PD. The Utilization of Palliative Care Services in Patients with Cirrhosis who have been Denied Liver Transplantation: A Single Center Retrospective Review. Ann Hepatol. 2017;16:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 21. | Chen BH, Tseng HJ, Chen WT, Chen PC, Ho YP, Huang CH, Lin CY. Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Kelley AS, Morrison RS. Palliative Care for the Seriously Ill. N Engl J Med. 2015;373:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 537] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 23. | Ufere NN, Donlan J, Waldman L, Dienstag JL, Friedman LS, Corey KE, Hashemi N, Carolan P, Mullen AC, Thiim M, Bhan I, Nipp R, Greer JA, Temel JS, Chung RT, El-Jawahri A. Barriers to Use of Palliative Care and Advance Care Planning Discussions for Patients With End-Stage Liver Disease. Clin Gastroenterol Hepatol. 2019;17:2592-2599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Rush B, Fruhstofer C, Walley KR, Celi LA, Brahmania M. Palliative medicine and hospital readmissions in end-stage liver disease. BMJ Support Palliat Care. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Adejumo AC, Kim D, Iqbal U, Yoo ER, Boursiquot BC, Cholankeril G, Wong RJ, Kwo PY, Ahmed A. Suboptimal Use of Inpatient Palliative Care Consultation May Lead to Higher Readmissions and Costs in End-Stage Liver Disease. J Palliat Med. 2020;23:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Haun MW, Estel S, Rücker G, Friederich HC, Villalobos M, Thomas M, Hartmann M. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev. 2017;6:CD011129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta L, Indonesia; Singh A, India S-Editor: Wang DM L-Editor: A P-Editor: Wang DM