Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1678
Peer-review started: January 28, 2022
First decision: March 25, 2022
Revised: April 4, 2022
Accepted: August 15, 2022
Article in press: August 15, 2022
Published online: August 27, 2022
Processing time: 210 Days and 7.9 Hours
The global coronavirus disease 2019 (COVID-19) pandemic has caused more than 5 million deaths. Multiorganic involvement is well described, including liver disease. In patients with critical COVID-19, a new entity called "post-COVID-19 cholangiopathy" has been described.
Here, we present three patients with severe COVID-19 that subsequently devel
Severe COVID-19 infection should be considered a potential risk factor for chronic liver disease and liver transplantation.
Core Tip: Coronavirus disease 2019 (COVID-19) multiorganic involvement is well described, including liver disease. In patients with critical COVID-19 requiring invasive mechanical ventilation and management in the intensive care unit, a new entity called “post-COVID-19 cholangiopathy” has been described. It is characterized by persistent cholestasis and chronic liver disease. Therefore, severe COVID-19 infection should be considered a potential risk factor for chronic liver disease probably requiring liver transplantation.
- Citation: Mayorquín-Aguilar JM, Lara-Reyes A, Revuelta-Rodríguez LA, Flores-García NC, Ruiz-Margáin A, Jiménez-Ferreira MA, Macías-Rodríguez RU. Secondary sclerosing cholangitis after critical COVID-19: Three case reports. World J Hepatol 2022; 14(8): 1678-1686
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1678.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1678
The global coronavirus disease 2019 (COVID-19) pandemic has caused more than 5100000 deaths worldwide, and as it grows, the knowledge of the disease as well as the discovery of new complications increases. Up to 30% of patients with COVID-19 present with abnormal liver chemistry during the course of the disease[1]; this can occur due to the expression of angiotensin-converting enzyme II in cholangiocytes, a shared mechanism responsible for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry into the cell. While most patients with COVID-19 develop mild and transient elevation of aminotransferases, in patients with critical disease requiring invasive mechanical ventilation and management in the intensive care unit (ICU), a new entity called “post-COVID-19 cholangiopathy” has been described, with only few cases reported to date[2].
Here, we present three patients with severe COVID-19, who subsequently developed persistent cholestasis and chronic liver disease.
Case 1: A 45-year-old male presented to the emergency department of our hospital complaining of malaise, cough, fever, and progressive dyspnea.
Case 2: A 52-year-old male presented to the emergency department of our hospital with severe dyspnea and a positive real-time PCR (RT-PCR) SARS-CoV-2 test.
Case 3: A 46-year-old woman presented to the emergency department of our hospital complaining of malaise, headache, cough, fever, and progressive dyspnea.
Case 1: Patient´s symptoms started 10 d before hospital admission, with dyspnea at rest as the main complaint at admission.
Case 2: Patient’s symptoms started 7 d before his admission, and included malaise, cough, fever, and progressive dyspnea. Two days before admission, the patient presented with nausea, emesis, non-inflammatory diarrhea, and dyspnea at rest.
Case 3: Patient´s symptoms started 13 d before admission, including cough, malaise and headache. During this time, a positive RT-PCR SARS-CoV-2 test was obtained and she received symptomatic treatment with acetaminophen. Forty-eight hours before admission, she presented with persistent fever and resting dyspnea.
Case 1: Patient’s history was relevant for longstanding type 2 diabetes mellitus, systemic arterial hypertension, and chronic kidney disease KDIGO III. No history of hepatic disease was reported.
Case 2: Patient’s history was relevant for chronic kidney disease on hemodialysis, type 2 diabetes, and hypertension. No history of hepatic disease was reported.
Case 3: Patient’s history was relevant for history of chronic kidney disease on hemodialysis, type 2 diabetes mellitus, and hypertension. No history of hepatic disease was reported.
No SARS-CoV-2 vaccine was available at the time of presentation. Family history was unremarkable in all three patients.
Case 1: Physical examination was relevant for oxygen saturation (SpO2) measured by pulse oximeter of 52%, tachypnea, respiratory distress, and crackles on chest auscultation.
Case 2: Physical examination was relevant for SpO2 measured by pulse oximeter of 50%, tachypnea, temperature of 37.8 °C, respiratory distress, and crackles on chest auscultation. Bilateral lower extremity edema was present.
Case 3: Physical examination was relevant for SpO2 measured by pulse oximeter of 80%, tachypnea, and crackles on chest auscultation.
Case 1: At admission, blood tests showed lymphopenia, D-dimer 1093 ng/mL, ferritin 1436 ng/mL, creatinine 8.8 mg/dL and normal liver chemistry. SARS-CoV-2 infection was subsequently confirmed by RT-PCR, and the patient required invasive mechanical ventilation due to respiratory failure type 1 [partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio of 80].
Case 2: At admission, blood tests showed lymphopenia and elevated inflammatory blood markers. Liver chemistry was normal.
Case 3: Initially, her liver chemistry was normal and elevated inflammatory blood markers were reported. After 72 h of admission, she developed severe hypoxemia (PaO2/FiO2 ratio of 91) requiring mechanical ventilation and admission to the ICU.
Chest computed tomography (CT) was performed in all cases, which showed peripheral, bilateral ground glass opacities consistent with severe pulmonary involvement (> 50%) secondary to SARS-CoV-2 infection.
Case 1: During hospitalization after 33 d of stay in the ICU, the patient required sedation with midazolam, fentanyl, and ketamine, high positive end-expiratory pressure (up to 20 cm H2O) and use of norepinephrine (maximum dose of 0.45 µg/kg/min). In addition, he was treated with meropenem, vancomycin, ceftriaxone, and co-trimoxazole due to blood and tracheal aspirate cultures yielding Enterobacter cloacae, Stenotrophomonas maltophilia, and Klebsiella pneumoniae. Finally, the patient developed gastrointestinal bleeding caused by duodenal ulcers and required hemodialysis for acute renal failure and metabolic acidosis.
Interestingly, during his stay in the ICU, liver chemistry showed a cholestatic pattern (R factor of 0.7) with an isolated and persistent increase in alkaline phosphatase (ALP) levels. The initial diagnostic workup, included abdominal ultrasound and CT, which did not show bile duct dilatation. The patient eventually improved his clinical conditions, including liver chemistry showing a decrease in ALP levels, extubation on the 35th day, and discharged 42 d after his initial presentation at the endoscopy.
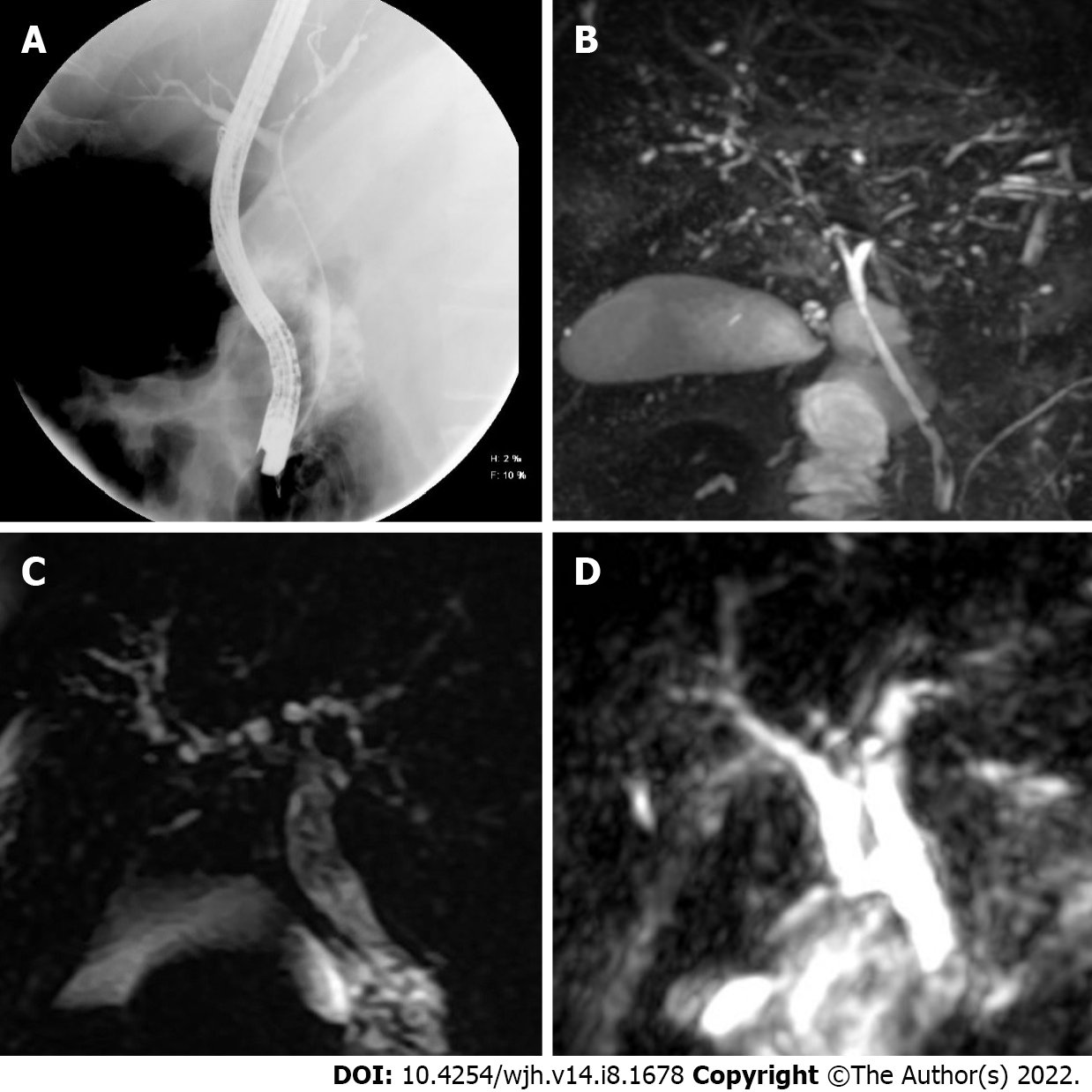
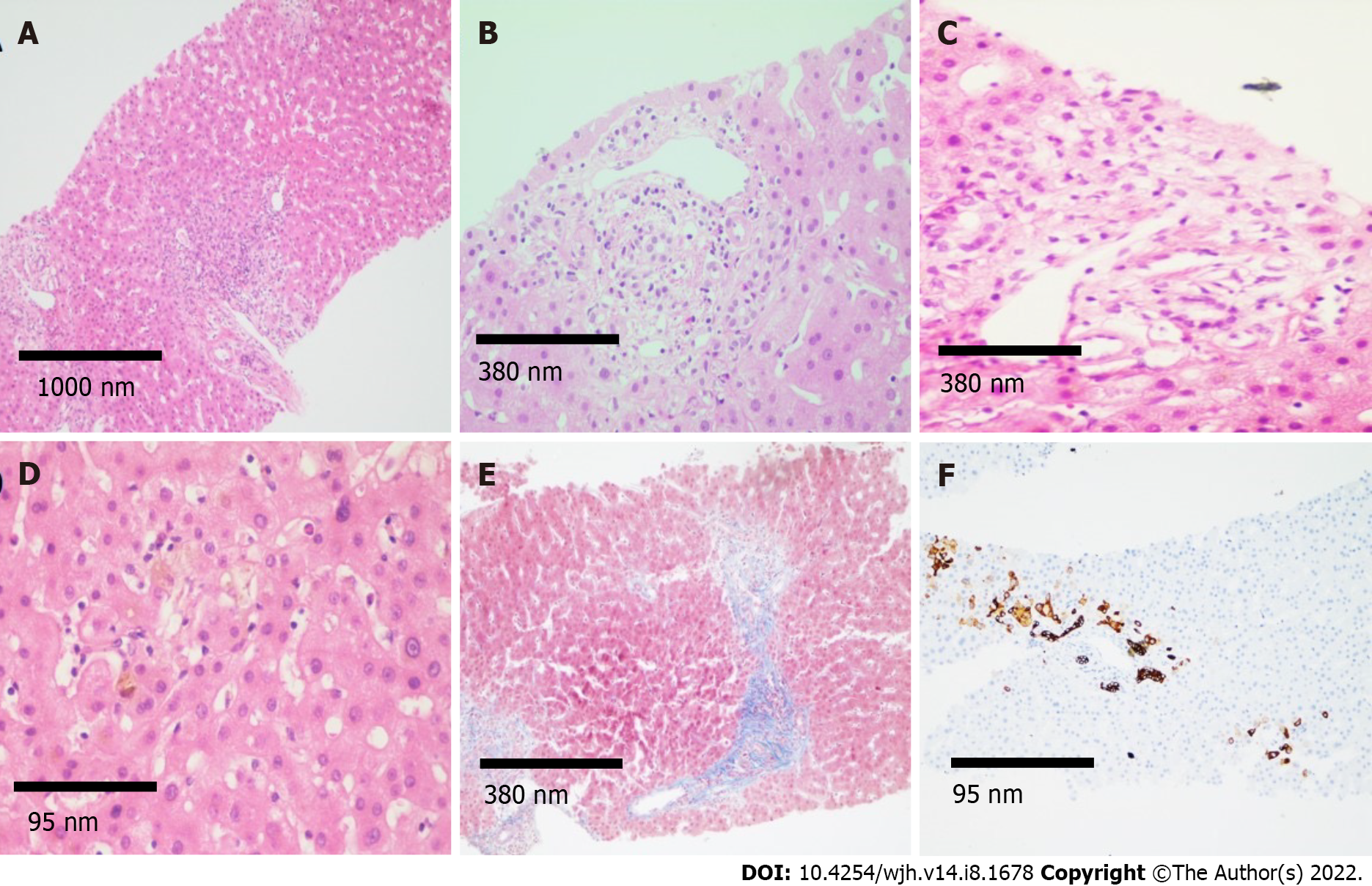
Six weeks after discharge, he developed jaundice, pruritus, and sleep disturbances. New biochemical parameters reported a total bilirubin (TB) 5.8 mg/dL, direct bilirubin (DB) 3.4 mg/dL, and ALP 1328 U/L. Interestingly, hypercholesterolemia developed in the patient, with peak levels reaching 1920 mg/dL (normal < 200 mg/dL). A contrast-enhanced CT scan showed intrahepatic bile duct dilatation and a common bile duct diameter of 8 mm with biliary sludge. An endoscopic retrograde cholangiography (ERCP) was performed and cholangiography confirmed dilation of intrahepatic and extrahepatic bile ducts, a sphincterotomy and balloon sphincteroplasty were also performed, obtaining a bile duct stone, bile duct casts and dark bile, ultimately a biliary plastic stent was placed (Figure 1A). Despite this, there was no improvement in liver chemistry, showing a persistent elevation of ALP levels (> 15 × upper limit of normal); therefore, magnetic resonance cholangiography was performed, showing multiple areas of stenosis in the distal intrahepatic bile ducts (Figure 1B). Differential diagnosis of liver chemistry abnormalities included autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis (SSC), immunoglobulin G4-related disease, viral hepatitis, and drug-induced liver injury (DILI), all of which were ruled out by negative specific antibodies, Ig, and liver biopsy. Percutaneous liver biopsy showed findings consistent with intracanalicular cholestasis, portal inflammation, ductular reaction, and moderate portal fibrosis (Figure 2).
Case 2: He required invasive mechanical ventilation with intermittent prone positioning due to respiratory failure type 1 (PaO2/FiO2 ratio of 73). On day 28 of ICU stay, the patient required hemodialysis, red blood cell transfusions, high positive end-expiratory pressure (up to 20 cm H2O), and use of norepinephrine (maximum dose of 0.5 μg/kg/min). He received treatment with meropenem, vancomycin, moxifloxacin, co-trimoxazole and voriconazole due to Streptococcus pneumoniae and Staphylococcus aureus bacteremia (endocarditis was ruled out); ventilator-associated pneumonia due to S. maltophilia, E. cloacae, and Aspergillus fumigatus. Subsequently, liver chemistry showed a cholestatic pattern (R factor < 2) with a persistent increase in ALP and gamma-glutamyl transferase (GGT) levels. Initial diagnostic workup with abdominal ultrasound was negative.
The patient improved his general condition and was discharged 2 mo after admission. During follow-up, he presented with jaundice; liver chemistry reported TB 9.47 mg/dL, DB 5.62 mg/dL, and ALP of 1695 U/L. Viral hepatitis panel and autoimmune cholestatic disease-specific antibodies were negative. Magnetic resonance cholangiography was performed, showing multiple areas of short stenosis with a pattern of SSC (Figure 1C). ERCP was performed in which filling defects of the main bile duct were identified in cholangiography; after sphincterotomy, bile sludge and biliary casts were obtained. Despite the ERCP, there was no improvement in liver function test, showing persistent elevation of ALP levels and TB 22.7 mg/dL.
Case 3: During her 20-d ICU stay, the patient required hemodialysis, high positive end-expiratory pressure (up to 20 cm H2O), and use of norepinephrine (maximum dose of 0.13 µg/kg/min). She developed ventilator-associated pneumonia due to Pseudomonas aeruginosa and received treatment with imipenem, piperacillin/tazobactam, and moxifloxacin.
During her stay, she presented with progressive cholestasis (R factor of < 2) reaching TB up to 17.32 mg/dL, DB 11.59 mg/dL, GGT 211 U/L, and ALP 705 U/L. Abdominal CT scan showed intrahepatic and extrahepatic biliary dilation without evident cause of obstruction. Viral hepatitis panel and autoimmune cholestatic disease-specific antibodies were negative. Magnetic resonance cholangiography was performed, showing intrahepatic and extrahepatic bile ducts with irregular morphology, without evidence of obstruction and periportal edema (Figure 1D).
With these findings, including clinical course, ruling out other alternative diagnoses and a close and temporal relationship with SARS-CoV-2 infection, a diagnosis of secondary SSC due to severe COVID-19 was made.
Case 1: Treatment with ursodeoxycholic acid, cholestyramine, and sertraline was started, showing no clinical improvement on liver chemistry at 8 wk, with persistent elevation of ALP, TB, and GGT.
Case 2: Treatment with ursodeoxycholic acid was started, showing no clinical improvement on liver chemistry.
Case 3: Treatment with ursodeoxycholic acid was started, with persistent elevation of ALP, TB, and GGT.
Case 1: Currently, the patient remains under follow-up without cholestasis improvement and is being evaluated for liver transplantation at our center.
Case 2: A vibration-controlled transient elastography was performed 6 mo after severe COVID-19 admission showing a median of 20.2 KPa (interquartile range/med 17%; FibroScan Echosens™, M probe). Currently, the patient is under palliative care due to Fournier's gangrene and penile necrosis associated sepsis. Liver transplantation protocol was stopped.
Case 3: The clinical evolution of the patient was protracted, and 1 mo after admission, she presented with cardiorespiratory arrest that was not reversible after advanced cardiopulmonary resuscitation maneuvers.
SCC is a chronic cholestatic disease, derived from multiple insults to the biliary tract including chronic obstruction, infectious disease, autoimmune, and ischemic cholangiopathy. Similar to primary SSC, its manifestations include chronic cholestasis, radiologic evidence of stenosis and dilations of the biliary tract, and the potential to progress to liver cirrhosis.
In 2001, Scheppach et al[3] reported a series of 3 patients admitted to the ICU due to extrahepatic infections without preexisting biliary or hepatic disease. During their stay, all three developed progressive persistent cholestasis with radiologic (magnetic resonance imaging [MRI] and ERCP) evidence of biliary dilation and stenosis without mechanical obstruction, and eventually progression to liver cirrhosis. In recent years, many centers worldwide have reported SSC in a growing number of patients who have recovered from critical illnesses.
The key element in the pathophysiology of SCC in critically ill patients (SSC-CIP) seems to be isch
In 2020 with the emergence of COVID-19, many patients were admitted to the ICU, requiring prolonged mechanical ventilation and use of vasopressors due to shock and severe hypoxemia; which are factors associated with ischemic injury to the biliary tract. Since then, some centers have reported cases of progressive and persistent cholestasis in COVID-19, 16 patients (Table 1) with abnormal findings on MRI or ERCP (beading of intrahepatic ducts, bile duct wall thickening with enhancement, and peribiliary diffusion high signal) some associated with the use of Ketamine[2,5-10]. Roth et al[2] described 3 patients who developed prolonged and severe cholestasis during recovery from severe COVID-19. Clinical, histologic, and imaging features of these 3 patients were similar to those of SSC-CIP with few exceptions; no biliary casts were found during ERCP and biopsies revealed severe cholangiocyte injury and intrahepatic microangiopathy suggesting direct biliary injury from SARS-CoV-2. Only 1 of 3 biopsies was positive for SARS-CoV-2 in immunohistochemistry and in situ hybridization.
| Ref. | Patients | Underlying conditions | Drugs | ERCP | MR cholangiography | Liver biopsy | Follow-up |
| Knooihuizen et al[5] | Female, 54 yr | Diabetes, hypothyroidism, hypertension, and hyperlipidemia | Hydromorphone, midazolam, propofol and ketamine | No reported | Intrahepatic dilatationwith a beaded appearance and dilated common bile duct with distal narrowing | Biliary ductular reaction with lobular inflammation and one small non-necrotizing lobular granuloma without viral inclusions | Continued improvement |
| Edwards et al[6] | Male, 59 yr | None | Vancomycin and co-trimoxazole | Sclerosing cholangitis in the intrahepatic ducts | Hypointense filling defects within the common bile duct and intrahepatic bile ducts were also dilated and demonstrated some beading | Not reported | Not reported |
| Mallet et al[7] | 3 males and 2 females | Hypertension, diabetes, one with KT and one with HBV infection | Ketamine and no other drugs reported | Filling defects in CBD and rarefication of the intrahepatic biliary tract | Sclerosing cholangitis, with strictures and dilatations of intrahepatic bile ducts, peribiliary cysts and multiple biliary casts | Biliary obstructions, cholangiolar proliferation, biliary plugs, portal inflammation with neutrophil infiltrates, extensive biliary fibrosis and cirrhosis | 1 died SSC and cirrhosis, 1 died biliary sepsis, 1 pruritus without jaundice and 2 recurrent biliary sepsis |
| Sanders et al[8] | Male, 57 yr | Hypertension and diabetes | No reported | Bile duct stone cast and intrahepatic duct stenosis without dilation | No reported | No reported | No reported |
| Durazo et al[9] | Male, 47 yr | Obesity, OSA, hypertension, and hyperlipidemia | HCQ | Small pigment stone and diffuse intrahepatic biliary strictures | Mild intrahepatic biliary ductal dilatation with multifocal strictures or beading without extrahepatic biliary dilatation | Mononuclear inflammatory infiltration within the wall of the bile duct, bile lake associated with bile duct injury, microarteriopathy with endothelial cell swelling and obliteration of the lumen and obliterative portal venopathy | On day 108, the patient underwent an OLT |
| Roth et al[2] | 2 males and 1 female | None | Multiple antibiotics | 2 sludge and stone extracted | Beading, with multiple short segmental strictures | Ductal reaction, bile duct paucity, cholangiocyte swelling, cholangiocyte regenerative change, portal tract inflammation, endothelial swelling, focal endophlebitis portal veins, cholestasis hepatocanalicular and fibrosis | No reported |
| Bütikofer et al[10] | 3 males and 1 female | Diabetes | Ketamine | No reported | Diffuse irregularities of the bile ducts with dilatations and strictures | Portal edema, mixed portal inflammation and pronounced bile duct damage with ductular reaction as well as lobular bile infarcts and severe hepatocellular, canalicular, focally ductular cholestasis and pericellular fibrosis around portal tracts and central veins | 1 cirrhosis Child B, MELD 17, 2 died pulmonary infection and 1 persistently increased ALP |
The 3 cases described here (Table 2), could also represent a confluence between SSC-CIP and direct hepatic injury from COVID-19. Our patients were admitted to the ICU due to severe COVID-19 requiring prolonged mechanical ventilation and vasopressors and developed cholestasis after admission, which was progressive and persisted even after resolution of choledocholithiasis and long after cardiopulmonary recovery. Characteristic imaging changes were found in MRI in our patients such as intrahepatic bile ducts stenosis and histopathologic changes were identical to those reported by Roth et al[2], suggesting a direct biliary injury from SARS-CoV-2. We did not perform immunohistochemistry and in situ hybridization for SARS-CoV-2 due to lack of availability in our center.
| Patient 1 | Patient 2 | Patient 3 | |
| Demographics | |||
| Age (yr) | 45 | 52 | 46 |
| Sex | Male | Male | Female |
| Comorbidities | T2D, HT, CKD KDIGO III | T2D, HT, CKD KDIGO V | T2D, HT, CKD KDIGO V |
| COVID-19 infection | |||
| ICU admission | Yes | Yes | Yes |
| Mechanical ventilation | Yes | Yes | Yes |
| Vasopressor support | Yes | Yes | Yes |
| Renal replacement therapy | Yes | Yes (on hemodialysis before admission) | Yes (on hemodialysis before admission) |
| Secondary infections | Ventilator-associated pneumonia due to Enterobacter cloacae, Stenotrophomonas maltophilia and Klebsiella pneumoniae | Streptococcus pneumoniae and Staphylococcus aureus bacteremia. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia, Enterobacter cloacae and Aspergillus fumigatus | Ventilator associated pneumonia due to Pseudomonas aeruginosa |
| Antibiotics | Meropenem, vancomycin, ceftriaxone and co-trimoxazole | Meropenem, vancomycin, moxifloxacin, co-trimoxazole and voriconazole | Imipenem, piperacillin/tazobactam and moxifloxacin |
| COVID-19 specific therapy | Dexamethasone | Dexamethasone | Dexamethasone |
| Liver chemistries on admission | |||
| TB (mg/dL) | 0.36 | 0.37 | 0.47 |
| ALT (U/L) | 37 | 20 | 11.8 |
| AST (U/L) | 33 | 46 | 35.9 |
| ALP (U/L) | 89 | 128 | 91 |
| Peak liver chemistries | |||
| TB (mg/dL) | 11.72 | 22.7 | 17.32 |
| ALT (U/L) | 63 | 62.7 | 7.9 |
| AST (U/L) | 119 | 184.1 | 46.4 |
| ALP (U/L) | 2146 | 2370 | 705 |
| Last liver chemistries | |||
| TB (mg/dL) | 6.41 | 8.82 | |
| ALT (U/L) | 48 | 9.3 | |
| AST (U/L) | 129 | 52.6 | |
| ALP (U/L) | 3250 | 1870 | |
| Sclerosing cholangitis imaging findings (CT, ERCP, MRI) | Yes | Yes | Yes |
| Histology | Intracanalicular cholestasis, portal inflammation, ductular reaction and moderate portal fibrosis | None | None |
| Evidence of liver fibrosis | Yes (histology) | Yes (VCTE) | No |
| Death | No | No | Yes |
Nevertheless, we must take into consideration that the differential diagnosis of cholestasis in the ICU is broad, and one important diagnosis to consider is DILI. Bile duct injury due to DILI has emerged as a distinct entity, causing persistent cholestasis and cholangiographic changes consistent with SSC. Our patients received antibiotics and ketamine, both associated with bile duct injury due to DILI. However, the Council for International Organizations of Medical Sciences/Roussel Uclaf Causality Assessment Method Score discarded causality in all cases, mostly because other causes of cholestasis could not be ruled out.
Prognosis in patients with SSC-CIP is poor, with a median transplant-free survival of 13-44 mo; significantly lower than other causes of SSC. Transplant-free survival at 1 year is 55% and 14% at 6 years[4]. In patients with COVID-19 cholangiopathy, prognosis is not well known; to our knowledge, there is one reported case of a 47-year-old man with a successful orthotopic liver transplantation post COVID-19 and is doing well with normal liver tests for 7 mo[9].
We believe that our diagnosis is consistent with post-COVID-19 cholangiopathy, although elements of the clinical course, histopathology and radiologic findings may be shared with SSC-CIP, severe COVID-19 is the common element in these patients, and seems to be associated with unique histopathologic features not previously observed in SSC-CIP. Further investigation into treatment and prognosis is required, mostly because persistent cholestasis may lead to liver cirrhosis. Therefore, we propose that severe COVID-19 infection should be considered a potential risk factor for chronic liver disease and liver transplantation.
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30478] [Article Influence: 5079.7] [Reference Citation Analysis (13)] |
| 2. | Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 3. | Scheppach W, Druge G, Wittenberg G, Mueller JG, Gassel AM, Gassel HJ, Richter F. Sclerosing cholangitis and liver cirrhosis after extrabiliary infections: report on three cases. Crit Care Med. 2001;29:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Martins P, Verdelho Machado M. Secondary Sclerosing Cholangitis in Critically Ill Patients: An Underdiagnosed Entity. GE Port J Gastroenterol. 2020;27:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Knooihuizen SAI, Aday A, Lee WM. Ketamine-Induced Sclerosing Cholangitis (KISC) in a Critically Ill Patient With COVID-19. Hepatology. 2021;74:519-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Keta-Cov research group. Intravenous ketamine and progressive cholangiopathy in COVID-19 patients. J Hepatol. 2021;74:1243-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Sanders D, Bomman S, Irani S. COVID-19-Induced Bile Duct Casts and Cholangitis: A Case Report. Cureus. 2021;13:e14560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Durazo FA, Nicholas AA, Mahaffey JJ, Sova S, Evans JJ, Trivella JP, Loy V, Kim J, Zimmerman MA, Hong JC. Post-Covid-19 Cholangiopathy-A New Indication for Liver Transplantation: A Case Report. Transplant Proc. 2021;53:1132-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Bütikofer S, Lenggenhager D, Wendel Garcia PD, Maggio EM, Haberecker M, Reiner CS, Brüllmann G, Buehler PK, Gubler C, Müllhaupt B, Jüngst C, Morell B. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41:2404-2417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver; American Association for the Study of Liver Diseases; Asociacion Mexicana de Hepatologia; Asociacion Mexicana de Endoscopia Gastrointestinal; Asociacion Mexicana de Gastroenterologia.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sood M, India; Zandi M, Iran S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP