Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1667
Peer-review started: February 15, 2022
First decision: March 9, 2022
Revised: April 12, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: August 27, 2022
Processing time: 191 Days and 11 Hours
Bacterial translocation exacerbates the hyperdynamic circulation observed in cirrhosis and contributes to a more severe disease course. Probiotics may reduce bacterial translocation and may therefore be useful to redress the circulatory imbalance.
To investigate the effect of probiotics on hemodynamic parameters, systemic inflammation, and complications of cirrhosis in this randomized placebo-controlled trial.
This single-blind randomized placebo-controlled study included 40 patients with Child-Pugh class B and C cirrhosis; 24 patients received probiotics (Saccharomyces boulardii) for 3 mo, and 16 patients received a placebo over the same period. Liver function and the systemic hemodynamic status were evaluated pre- and post-intervention. Echocardiography and simultaneous blood pressure and heart rate monitoring were performed to evaluate systemic hemodynamic indicators. Cardiac output and systemic vascular resistance were calculated.
Following a 3-mo course of probiotics in comparison to the control group, we observed amelioration of hyperdynamic circulation [a decrease in cardiac output (P = 0.026) and an increase in systemic vascular resistance (P = 0.026)] and systemic inflammation [a decrease in serum C-reactive protein levels (P = 0.044)], with improved liver function [an increase in serum albumin (P = 0.001) and a decrease in the value of Child-Pugh score (P = 0.001)] as well as a reduction in the severity of ascites (P = 0.022), hepatic encephalopathy (P = 0.048), and cholestasis [a decrease in serum alkaline phosphatase (P = 0.016) and serum gamma-glutamyl transpeptidase (P = 0.039) activity] and an increase in platelet counts (P < 0.001) and serum sodium level (P = 0.048).
Probiotic administration was associated with amelioration of hyperdynamic circulation and the associated complications of cirrhosis.
Core Tip: Bacterial translocation exacerbates the hyperdynamic circulation observed in cirrhosis and contributes to a more severe disease course. Probiotics may reduce bacterial translocation and may therefore be useful to redress the circulatory imbalance. The aim of the study was to investigate the effect of probiotics on hemodynamic parameters, systemic inflammation, and complications of cirrhosis in this randomized placebo-controlled trial. Following a 3-mo course of probiotics, we observed amelioration of hyperdynamic circulation and systemic inflammation, improvement liver function, regression of ascites and hepatic encephalopathy, and an increase in serum sodium level.
- Citation: Maslennikov R, Efremova I, Ivashkin V, Zharkova M, Poluektova E, Shirokova E, Ivashkin K. Effect of probiotics on hemodynamic changes and complications associated with cirrhosis: A pilot randomized controlled trial. World J Hepatol 2022; 14(8): 1667-1677
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1667.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1667
Cirrhosis, which represents the culmination of chronic liver disease[1], is characterized by changes in liver morphology, reduced liver function, and the onset of portal hypertension. However, in addition to the liver, the intestine and its microbiota are affected by the pathophysiological derangements in cirrhosis. Cirrhosis is known to be associated with disturbances in the composition of the gut microbiota (gut dysbiosis[2-16]), expansion of the microbiota of the small intestine (small intestine bacterial overgrowth[17]), and increased permeability of the intestinal barrier[18], all of which result in bacterial translocation, which refers to the entry of bacteria and their components from the intestinal contents through the intestinal wall into the lymph, blood, and body tissues[19,20]. Bacterial translocation leads to systemic inflammation, which precipitates hemodynamic alterations [hyperdynamic circulation indicated by increased cardiac output and decreased systemic vascular resistance (SVR)] that contribute to liver decompensation[21-25]. This bidirectional association between the gut along with its microbiota and the liver is referred to as the gut-liver axis[26] or the gut-heart-liver axis[25]. Studies have shown that certain drugs that affect this axis can redress the hemodynamic imbalance and improve the clinical course in patients with cirrhosis. Among these drugs, probiotics are live microorganisms, which when administered in adequate amounts confer several health benefits on the host[27]. Although evidence-based research supports the role of probiotics in cases of hepatic encephalopathy, their effects on other symptoms and manifestations of cirrhosis remain unclear[28]. A non-controlled study reported that a 6-wk course of probiotics reduced the cardiac output and heart rate and increased the SVR and serum sodium levels in the study population[29].
Saccharomyces boulardii (S. boulardii), a probiotic yeast, has shown significant effectiveness for the treatment or prevention of diarrhea, inflammatory bowel disease, irritable bowel syndrome, Helicobacter pylori infection, and dyslipidemia, among other such conditions[30,31]. S. boulardii produces pleiotropic effects; it reestablishes the gut microbiome after dysbiosis[32], strengthens the intestinal immune barrier[33], improves the trophic function of gut microbiota[34], restores the impaired gut barrier, and protects against bacterial translocation[35] in experimental models and in patients with gut diseases. S. boulardii administration in an experimental mouse model of cirrhosis led to correction of gut dysbiosis, decreased intestinal permeability, as well as reduced severity of liver inflammation and fibrosis[36]. However, the role of this probiotic is not known in humans with cirrhosis.
In this randomized placebo-controlled trial, we investigated the effect of probiotic administration (S. boulardii) on hemodynamic parameters, systemic inflammation, and complications of cirrhosis.
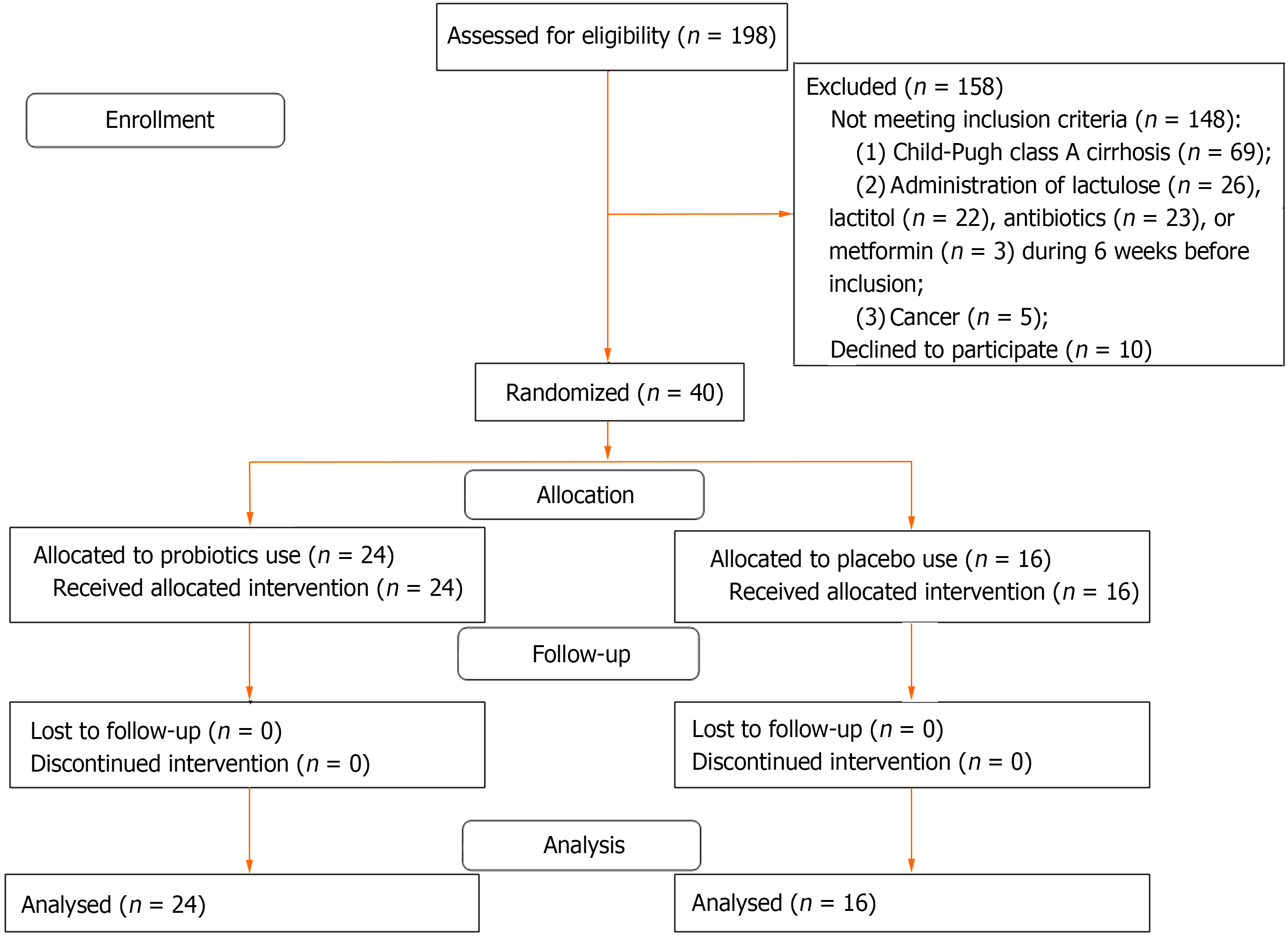
In this single-blind randomized placebo-controlled trial, 198 consecutive patients with cirrhosis who underwent health check-ups at the Department of Hepatology’s Clinic for Internal Diseases, Gastroenterology, and Hepatology at Sechenov University were screened for inclusion. The study procedures were explained to potential participants, and written informed consent was obtained before enrollment. The study was approved by the Ethics Committee of Sechenov University and was registered at clinicaltrials.gov (NCT05231772). The research protocol can be accessed from this website.
Inclusion criteria were as follows: (1) Diagnosis of cirrhosis based on histopathological, or clinical, biochemical, and ultrasonographic findings; (2) Child-Pugh class B or C cirrhosis; (3) Age between 18 years and 70 years; and (4) Signed informed consent. Exclusion criteria were as follows: (1) Administration of lactulose, lactitol, or other prebiotics, probiotics, antibiotics, or metformin during the 6 wk preceding study commencement; (2) Alcohol consumption 6 wk preceding study commencement; or (3) Diagnosis of inflammatory bowel disease, cancer, or any other serious disease.
There are no data to calculate the required sample size.
Of the 198 patients initially screened for inclusion, 40 met the inclusion criteria and were enrolled in the study (Figure 1). Patients included in the study were randomized into the test and control arms (ratio 1.5:1). The Excel function RANDBETWEEN (1:5) was used as a random number generator; for numbers 1 to 3, patients were assigned to the test arm and for numbers 4 or 5, patients were assigned to the placebo group. Patients who prematurely discontinued ingestion of the experimental probiotic/ placebo or were administered antibacterial drugs, other probiotics, or prebiotics during the follow-up period were excluded from the study.
Patients in the test arm received S. boulardii at a dose of 250 mg twice a day for 3 mo and those in the control group received a placebo preparation at the same dose over the same period. Patients were not aware whether they were administered a placebo or the experimental drug. Additionally, all patients received standard of care treatment for cirrhosis. Drugs administered did not significantly differ between patient groups (Table 1). Patients were re-evaluated 3 mo after initiation of S. boulardii or placebo treatment.
| The test arm, n = 24 | The control arm, n = 16 | P value | |
| Age, yr | 48.5 (42.5-59.0) | 53.5 (44.5-59.0) | 0.730 |
| Body mass index, kg/m2 | 25.4 (22.3-27.9) | 26.5 (24.3-28.7) | 0.553 |
| Male/female | 8/16 | 9/7 | 0.134 |
| Etiology of cirrhosis: Alcohol | 12 (50.0) | 8 (50.0) | > 0.050 |
| Metabolically associated fatty liver disease | 2 (8.3) | 0 | |
| HBV | 2 (8.3) | 0 | |
| HCV | 3 (12.5) | 2 (12.5) | |
| Mixed | 3 (12.5) | 3 (18.8) | |
| Cryptogenic | 2 (8.3) | 3 (18.8) | |
| Child–Pugh score | 9 (8-10) | 9 (8-10) | 0.730 |
| Child–Pugh class, B/C | 17/7 | 12/4 | 0.533 |
| End-diastolic volume of the left ventricle, mL | 109 (84-116) | 100 (90-143) | 0.689 |
| Ejection fraction of the left ventricle, % | 61.5 (59.4-62.8) | 59.8 (57.5-62.0) | 0.263 |
| Stroke volume, mL | 67 (52-72) | 58 (55-82) | 0.689 |
| Heart rate, bpm | 71 (69-75) | 70 (69-74) | 0.709 |
| Cardiac output, L/min | 4.8 (3.6-5.2) | 4.4 (4.0-5.8) | 0.473 |
| Mean blood pressure, mmHg | 84.7 (80.3-89.7) | 87.3 (79.8-93.7) | 0.499 |
| Systemic vascular resistance, dyn· s· cm−5 | 1442 (1243-1874) | 1470 (1255-1744) | 0.945 |
| Mean pulmonary artery pressure, mmHg | 23.4 (22.1-25.8) | 23.4 (20.9-26.4) | 0.749 |
| Esophageal varices (Grade 1), n (%) | 8 (33.3) | 6 (37.5) | 0.338 |
| Esophageal varices (Grade 2-3), n (%) | 16 (66.7) | 7 (43.8) | |
| Minimal hepatic encephalopathy, n (%) | 18 (75.0) | 10 (62.5) | 0.484 |
| Overt hepatic encephalopathy, n (%) | 2 (8.3) | 2 (12.5) | |
| Hepatic encephalopathy, n (%) | 20 (83.3) | 12 (75.0) | 0.399 |
| Ascites, n (%) | 22 (91.7) | 12 (75.0) | 0.160 |
| Minimal ascites, n (%) | 15 (62.5) | 6 (37.5) | 0.249 |
| Clinically significant ascites, n (%) | 7 (29.2) | 6 (37.5) | |
| Red blood cells as 1012 cell/L | 3.7 (3.3-4.3) | 3.6 (3.1-4.1) | 0.689 |
| White blood cells as 109 cell/L | 4.3 (3.2-5.6) | 3.8 (2.6-7.2) | 0.553 |
| Platelets as 109 cell/ L | 94 (69-107) | 94 (48-104) | 0.669 |
| Serum total protein, g/L | 73 (63-77) | 70 (61-77) | 0.649 |
| Serum albumin, g/L | 33 (31-36) | 33 (29-37) | 0.967 |
| Serum total bilirubin, μmol/L | 37 (27-64) | 55 (29-67) | 0.499 |
| International normalized ratio | 1.48 (1.39-1.68) | 1.58 (1.31-1.73) | 0.978 |
| Serum cholesterol, mmol/L | 4.1 (3.1-5.5) | 3.5 (2.6-4.6) | 0.230 |
| Serum triglyceride, mmol/L | 1.1 (0.7-1.4) | 1.0 (0.7-1.4) | 0.626 |
| Serum creatinine, mg/dL | 76 (65-88) | 78 (70-105) | 0.448 |
| Serum sodium, mmol/L | 140 (139-141) | 141 (140-142) | 0.235 |
| Serum potassium, mmol/L | 4.3 (4.0-4.8) | 4.4 (4.1-4.6) | 0.804 |
| Serum glucose, mmol/L | 4.7 (4.2-5.7) | 4.8 (4.6-5.4) | 0.464 |
| Serum iron, μmol/L | 13.9 (8.1-20.6) | 11.3 (7.3-22.0) | 0.761 |
| Alanine aminotransferase, U/L | 31 (20-46) | 26 (20-51) | 0.782 |
| Aspartate aminotransferase, U/L | 54 (34-72) | 52 (38-79) | 0.945 |
| Gamma glutamyl transferase, U/L | 82 (28-299) | 84 (49-118) | 0.934 |
| Alkaline phosphatase, U/L | 268 (221-395) | 214 (173-274) | 0.098 |
| Cholinesterase, U/L | 3650 (2861-3961) | 3803 (2778-4215) | 0.827 |
| C-reactive protein, mg/L | 9 (6-15) | 7 (2-20) | 0.347 |
| Beta blockers, n (%) | 17 (70.8) | 11 (68.8) | 0.580 |
| Spironolactone, n (%) | 22 (91.7) | 14 (87.5) | 0.529 |
| Loop diuretics, n (%) | 11 (45.8) | 7 (43.8) | 0.578 |
| Ademetionine, n (%) | 15 (62.5) | 9 (56.3) | 0.472 |
| Entecavir or tenofovir, n (%) | 2 (8.3) | 0 | 0.354 |
All patients underwent a standard medical check-up for evaluation of cirrhosis and for measurement of indicators of systemic hemodynamics before and 3 mo after initiation of S. boulardii or placebo treatment (the first and second visit, respectively). There were no additional visits or examinations between these two time points. The outcomes included changes in cardiac output, SVR, the extent of systemic inflammation (represented by serum C-reactive protein levels), severity of ascites and hepatic encephalopathy, serum levels of liver biomarkers, and Child-Pugh scale scores.
Echocardiography was performed at rest based on the guidelines of the American Society of Echocardiography[37-40]. The systolic and diastolic blood pressure and heart rate were measured using an automatic oscillometric sphygmomanometer (A and D, Japan) simultaneously with measurement of the stroke volume. Table 2 shows the hemodynamic parameters calculated in this study[37-42].
| Parameter | Calculation |
| End-diastolic and end-systolic volume of the left ventricle | Modified Simpson’s disk method |
| Ejection fraction of the left ventricle | [(End-diastolic volume) – (end-systolic volume)]/(end-diastolic volume) |
| Stroke volume | (Doppler velocity time integral) × (cross-sectional aorta area)[41] |
| Mean arterial pressure | [(systolic blood pressure)+2 × (diastolic blood pressure)]/3 |
| Cardiac output | (Stroke volume) × (heart rate) |
| Systemic vascular resistance | (Mean arterial pressure)/(cardiac output) |
| Systolic pulmonary artery pressure | (Right atrium pressure estimated from diameter of inferior vena cava and respiratory changes) + 4 × (the peak velocity of the tricuspid valve regurgitant jet)2[39,40] |
| Mean pulmonary artery pressure | 0.61 × (systolic pulmonary artery pressure) + 2 mmHg[42] |
The degree of ascites was determined based on the International Ascites Club scale as follows: 0 = No ascites; 1 = Minimal ascites (measurable only with instrumental methods); 2 = Clinically significant ascites (determined on physical examination); and 3 = Gross ascites[43].
The degree of hepatic encephalopathy was determined based on the following scale: 0 = No hepatic encephalopathy; 1 = Minimal hepatic encephalopathy; and 2 = Overt hepatic encephalopathy[44].
Statistical analysis was performed with STATISTICA 10 (StatSoft Inc., Tulsa, OK, United States) software. The data were presented as medians interquartile ranges]. Differences between continuous variables were assessed with the Mann-Whitney test because many variables were not distributed normally. Fisher’s exact test was used to assess the differences between categorical variables. P values ≤ 0.05 were considered as statistically significant. We performed per-protocol analysis.
The study included 40 patients [24 (test group) and 16 (control group)] (Figure 1). No significant differences were observed between the groups at the time of study inclusion (Table 1). All included patients completed the study. None of the patients were hospitalized between the visits.
After a 3-mo course of the probiotic in comparison to the control group, we observed evidence of amelioration of hyperdynamic circulation (a decrease in cardiac output and end-diastolic volume and an increase in SVR) and systemic inflammation (a decrease in serum C-reactive protein levels), improved liver function (an increase in serum albumin and cholinesterase levels and a decrease in the value of Child-Pugh score), regression of ascites and hepatic encephalopathy, increased serum sodium levels, as well as a reduction in the severity of cholestasis (a decrease in serum alkaline phosphatase and serum gamma-glutamyl transpeptidase activity), and hypersplenism (an increase in platelet count). However, in contrast to patients in the test group, those in the control group showed an increase in mean blood pressure. No significant changes were observed in the levels of other variables, including in the grade of esophageal varices and the international normalized ratio (Table 3).
| The test arm (n = 24) | The control arm (n = 16) | P value | |
| Body mass index, kg/m2 | -0.2 (-0.9-1.5) | 0.0 (0.0-0.5) | 0.281 |
| Child–Pugh score | -1.5 [-3.0-(-0.5)] | 0 (-0.5-1.5) | 0.001 |
| Child–Pugh class (from B or C to A), n (%) | 8 (33.3) | 1 (6.3) | 0.048 |
| End-diastolic volume of the left ventricle, mL | -13 [-17-(-10)] | 0 (-3-12) | < 0.001 |
| Ejection fraction of the left ventricle, % | 0.5 (-0.7-0.3) | 0.0 (0.0-0.3) | 0.590 |
| Stroke volume, mL | -7 [-12-(-4)] | 0 (-3-8) | < 0.001 |
| Heart rate, bpm | 2 (-4-8) | -2 (-7-7) | 0.234 |
| Cardiac output, L/min | -0.5 [-1.0-(-0.1)] | 0.3 (-0.3-0.9) | 0.026 |
| Mean blood pressure, mmHg | 1.8 (-1.7-7.5) | 3.8 (-3.3-15.3) | 0.847 |
| Systemic vascular resistance, dyn· s· cm−5 | 237 (39-358) | 30 (-200-227) | 0.026 |
| Mean pulmonary artery pressure, mmHg | 0.0 (-2.1-0.6) | 1.2 (0.0-1.8) | 0.043 |
| Esophageal varices, a decrease in grade, n (%) | 2 (4.3) | 0 | 0.354 |
| Esophageal varices, an increase in grade, n (%) | 0 | 0 | - |
| Regression hepatic encephalopathy, n (%) | 8 (33.3) | 1 (6.3) | 0,048 |
| Ascites, a decrease in degree, n (%) | 15 (62.4) | 4 (25.0) | 0.022 |
| Ascites, an increase in degree, n (%) | 1 (4.2) | 3 (18.8) | 0.167 |
| Red blood cells as 1012 cell/L | 0.2 (-0.1-0.5) | 0.2 (-0.1-0.4) | 0.730 |
| White blood cells as 109 cell/L | 0.1 (-1.2-0.6) | -0.1 (-2.1-0.7) | 0.782 |
| Platelets as 109 cell/ L | 11 (4-32) | -6 (-14-1) | < 0.001 |
| Serum total protein, g/L | -0.2 (-4.2-8.9) | -1.4 (-3.5-5.6) | 0.793 |
| Serum albumin, g/L | 3.0 (0.6-7.7) | -3.1 (-5.1-2.4) | 0.001 |
| Serum total bilirubin, μmol/L | -5.1 [-23.9-(-0.9)] | -3.7 (-10.0-17.2) | 0.181 |
| International normalized ratio | 0.0 (-0.1-0.1) | 0.0 (-1.0-0.1) | 0.544 |
| Serum cholesterol, mmol/L | 0.2 (-0.4-1.2) | 0.5 (-1.0-2.0) | 0.571 |
| Serum triglyceride, mmol/L | 0.1 (-0.2-0.2) | 0.1 (-0.6-0.4) | 0.836 |
| Serum creatinine, mg/dL | 2.4 (-9.1-8.1) | -3.5 (-11.2-4.2) | 0.264 |
| Serum sodium, mmol/L | 1.5 (0.5-3.0) | -1 (-3-2) | 0.048 |
| Serum potassium, mmol/L | 0.0 (-0.4-1.2) | 0.1 (-0.2-0.3) | 0.523 |
| Serum glucose, mmol/L | 0.4 (-0.1-0.7) | 0.0 (-0.9-0.8) | 0.423 |
| Alanine aminotransferase, U/L | -1 (-15-6) | 2 (-15-8) | 0.740 |
| Aspartate aminotransferase, U/L | -9 (-32-1) | -2 (-26-20) | 0.116 |
| Gamma glutamyl transferase, U/L | -8 (-171-11) | 6 (-15-23) | 0.039 |
| Alkaline phosphatase, U/L | -40 (-95-3) | 26 (-57-91) | 0.016 |
| Cholinesterase, U/L | 257 (28-734) | -155 [-239-(-68)] | 0.016 |
| C-reactive protein, mg/L | -3.0 [-4.5-(-0.5)] | 1.0 (-2.0-4.5) | 0.044 |
In the test arm, an improvement in liver function (a decrease in the value of Child-Pugh score: -2 [-3-(-1)] vs -0.5 [-1-0]; P = 0.042) and a decrease in the degree of ascites (-1[-1-(-1)] vs 0 [0-0]; P = 0.015) was observed only in those patients (n = 18) who had a decrease in cardiac output after the course of the probiotic.
No patient developed acute-on-chronic liver failure and bleeding esophageal varices during the study, and no patient died during the study period.
Only 1 patient in the test arm developed self-limited itching as an adverse effect.
This is the first randomized controlled study that investigated the effect of probiotics on hemodynamic disturbances in patients with cirrhosis. Our results concur with those reported by a previous uncontrolled study[29], which showed that these drugs reduce cardiac output and increase SVR. In our study, the reduced cardiac output was attributable to a decrease in the end-diastolic volume, which may indicate a reduction in the effective circulating blood volume.
The use of the probiotic also increased serum albumin and sodium levels and decreased in the degree of ascites. We assume that the probiotics inhibit bacterial translocation and thereby ameliorate hyperdynamic circulation, with a consequent reduction in the degree of ascites, which corrects hypoalbuminemia and hyponatremia. Unfortunately, we could not evaluate the indicators of intrahepatic hemodynamics (for example, the hepatic venous pressure gradient, among other variables).
Interestingly, this study highlights that probiotics reduced serum levels of biomarkers of cholestasis (alkaline phosphatase and gamma-glutamyl transpeptidase). This is the first study to report these findings; further studies are warranted to investigate the mechanisms underlying these changes. We observed probiotic-induced reduction in the severity of hepatic encephalopathy, which is consistent with the results reported by previous research[28]. In our study, probiotic ingestion did not affect the degree of esophageal varices and prothrombin levels (indicated by the international normalized ratio); our results were consistent with those reported by a previous study[28].
Probiotic use was associated with a significant improvement in liver function in our study; 33.3% of patients in the probiotic arm and only 6.3% of patients in the control arm showed Child-Pugh class A cirrhosis after a course of probiotic or placebo administration. We observed no significant probiotic-induced adverse effects during the study. Overall, our study showed that probiotic administration may be a useful therapeutic strategy for correction of gut-heart-liver axis disturbances.
This is the first randomized placebo-controlled trial that confirms the role of probiotics in amelioration of hemodynamic disorders in cirrhosis, together with improvement in levels of several liver biomarkers, which serves as a strength of our study.
A limitation of our study is the fact that biomarkers of bacterial translocation and intestinal permeability, biomarkers of systemic inflammation in addition to C-reactive protein, as well as indicators of intrahepatic hemodynamics were not evaluated. Further research is warranted to overcome this challenge.
Probiotic administration was associated with amelioration of hyperdynamic circulation and the associated complications of cirrhosis.
Bacterial translocation exacerbates the hyperdynamic circulation observed in cirrhosis and contributes to a more severe disease course.
Probiotics may reduce bacterial translocation and may therefore be useful to redress the circulatory imbalance.
To investigate the effect of probiotics on hemodynamic parameters, systemic inflammation, and complications of cirrhosis in this randomized placebo-controlled trial.
This single-blind randomized placebo-controlled study included patients with Child-Pugh class B and C cirrhosis that received probiotics (Saccharomyces boulardii) or a placebo for 3 mo. Liver function and the systemic hemodynamic status were evaluated pre- and post-intervention. Echocardiography and simultaneous blood pressure and heart rate monitoring were performed to evaluate systemic hemodynamic indicators. Cardiac output and systemic vascular resistance were calculated.
Following a 3-mo course of probiotics in comparison to the control group, we observed amelioration of hyperdynamic circulation and systemic inflammation with improved liver function, reduction in the severity of ascites, hepatic encephalopathy, and cholestasis, and an increase in platelet counts.
Probiotic administration was associated with amelioration of hyperdynamic circulation and the associated complications of cirrhosis.
To study the changes in the levels of the biomarkers of bacterial translocation, intestinal permeability, and in indicators of intrahepatic hemodynamics after the use of the probiotic in decompensated cirrhosis.
The authors are grateful to the staff of the Department of Hepatology: Alexei Lapshin, Shauki Ondos, Petr Tkachenko, Igor Tikhonov, and others.
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 1057] [Article Influence: 211.4] [Reference Citation Analysis (2)] |
| 2. | Ponziani FR, Zocco MA, Cerrito L, Gasbarrini A, Pompili M. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol. 2018;12:641-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Zhang L, Wu YN, Chen T, Ren CH, Li X, Liu GX. Relationship between intestinal microbial dysbiosis and primary liver cancer. Hepatobiliary Pancreat Dis Int. 2019;18:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Jin M, Kalainy S, Baskota N, Chiang D, Deehan EC, McDougall C, Tandon P, Martínez I, Cervera C, Walter J, Abraldes JG. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019;39:1437-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Zeng Y, Chen S, Fu Y, Wu W, Chen T, Chen J, Yang B, Ou Q. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat. 2020;27:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 6. | Kajihara M, Koido S, Kanai T, Ito Z, Matsumoto Y, Takakura K, Saruta M, Kato K, Odamaki T, Xiao JZ, Sato N, Ohkusa T. Characterisation of blood microbiota in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2019;31:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Chen Z, Xie Y, Zhou F, Zhang B, Wu J, Yang L, Xu S, Stedtfeld R, Chen Q, Liu J, Zhang X, Xu H, Ren J. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front Microbiol. 2020;11:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Zheng R, Wang G, Pang Z, Ran N, Gu Y, Guan X, Yuan Y, Zuo X, Pan H, Zheng J, Wang F. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020;9:4232-4250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Lapidot Y, Amir A, Nosenko R, Uzan-Yulzari A, Veitsman E, Cohen-Ezra O, Davidov Y, Weiss P, Bradichevski T, Segev S, Koren O, Safran M, Ben-Ari Z. Alterations in the Gut Microbiome in the Progression of Cirrhosis to Hepatocellular Carcinoma. mSystems. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 878] [Article Influence: 73.2] [Reference Citation Analysis (1)] |
| 11. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 12. | Liu Y, Jin Y, Li J, Zhao L, Li Z, Xu J, Zhao F, Feng J, Chen H, Fang C, Shilpakar R, Wei Y. Small Bowel Transit and Altered Gut Microbiota in Patients With Liver Cirrhosis. Front Physiol. 2018;9:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M, Yoshiji H, Tanaka Y. Gut Dysbiosis Associated With Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 14. | Maslennikov R, Ivashkin V, Efremova I, Alieva A, Kashuh E, Tsvetaeva E, Poluektova E, Shirokova E, Ivashkin K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J Hepatol. 2021;13:557-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 823] [Article Influence: 54.9] [Reference Citation Analysis (3)] |
| 16. | Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 17. | Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int. 2018;12:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, Gasbarrini A, Miele L, Grieco A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol. 2019;25:4814-4834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (4)] |
| 19. | Giannelli V, Di Gregorio V, Iebba V, Giusto M, Schippa S, Merli M, Thalheimer U. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol. 2014;20:16795-16810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 20. | Gómez-Hurtado I, Such J, Sanz Y, Francés R. Gut microbiota-related complications in cirrhosis. World J Gastroenterol. 2014;20:15624-15631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Di Pascoli M, Sacerdoti D, Pontisso P, Angeli P, Bolognesi M. Molecular Mechanisms Leading to Splanchnic Vasodilation in Liver Cirrhosis. J Vasc Res. 2017;54:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Møller S, Bendtsen F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018;38:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20:2555-2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (4)] |
| 24. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Gut-liver axis in cirrhosis: Are hemodynamic changes a missing link? World J Clin Cases. 2021;9:9320-9332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (3)] |
| 25. | Maslennikov R, Pavlov C, Ivashkin V. Is small intestinal bacterial overgrowth a cause of hyperdynamic circulation in cirrhosis? Turk J Gastroenterol. 2019;30:964-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Simbrunner B, Mandorfer M, Trauner M, Reiberger T. Gut-liver axis signaling in portal hypertension. World J Gastroenterol. 2019;25:5897-5917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Trush EA, Poluektova EA, Beniashvilli AG, Shifrin OS, Poluektov YM, Ivashkin VT. The Evolution of Human Probiotics: Challenges and Prospects. Probiotics Antimicrob Proteins. 2020;12:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Probiotics in hepatology: An update. World J Hepatol. 2021;13:1154-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 29. | Rincón D, Vaquero J, Hernando A, Galindo E, Ripoll C, Puerto M, Salcedo M, Francés R, Matilla A, Catalina MV, Clemente G, Such J, Bañares R. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 2014;34:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Cui B, Lin L, Wang B, Liu W, Sun C. Therapeutic potential of Saccharomyces boulardii in liver diseases: from passive bystander to protective performer? Pharmacol Res. 2022;175:106022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Kaźmierczak-Siedlecka K, Ruszkowski J, Fic M, Folwarski M, Makarewicz W. Saccharomyces boulardii CNCM I-745: A Non-bacterial Microorganism Used as Probiotic Agent in Supporting Treatment of Selected Diseases. Curr Microbiol. 2020;77:1987-1996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 32. | McFarland LV. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open. 2014;4:e005047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Buts JP, Bernasconi P, Vaerman JP, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 140] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Terciolo C, Dobric A, Ouaissi M, Siret C, Breuzard G, Silvy F, Marchiori B, Germain S, Bonier R, Hama A, Owens R, Lombardo D, Rigot V, André F. Saccharomyces boulardii CNCM I-745 Restores intestinal Barrier Integrity by Regulation of E-cadherin Recycling. J Crohns Colitis. 2017;11:999-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Schneider SM, Girard-Pipau F, Filippi J, Hebuterne X, Moyse D, Hinojosa GC, Pompei A, Rampal P. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J Gastroenterol. 2005;11:6165-6169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Generoso SV, Viana ML, Santos RG, Arantes RM, Martins FS, Nicoli JR, Machado JA, Correia MI, Cardoso VN. Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed Saccharomyces boulardii. Eur J Nutr. 2011;50:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6446] [Cited by in RCA: 9968] [Article Influence: 906.2] [Reference Citation Analysis (0)] |
| 38. | Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, Gottdiener J, Haluska B, Ofili E, Segers P, Senior R, Tapp RJ, Zamorano JL. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28:727-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 39. | Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685-713; quiz 786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4670] [Cited by in RCA: 5493] [Article Influence: 343.3] [Reference Citation Analysis (0)] |
| 40. | Bossone E, D’Andrea A, D’Alto M, Citro R, Argiento P, Ferrara F, Cittadini A, Rubenfire M, Naeije R. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013;26:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 352] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 41. | Sangkum L, Liu GL, Yu L, Yan H, Kaye AD, Liu H. Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth. 2016;30:461-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Chemla D, Castelain V, Humbert M, Hébert JL, Simonneau G, Lecarpentier Y, Hervé P. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 365] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 43. | Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 634] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 44. | Rudler M, Weiss N, Bouzbib C, Thabut D. Diagnosis and Management of Hepatic Encephalopathy. Clin Liver Dis. 2021;25:393-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lal A, United States; Wan XH, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Cai YX