Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1652
Peer-review started: June 5, 2022
First decision: June 22, 2022
Revised: July 4, 2022
Accepted: August 15, 2022
Article in press: August 15, 2022
Published online: August 27, 2022
Processing time: 81 Days and 12.5 Hours
Although the prognostic relevance of sarcopenia has been increasingly recognised in the context of liver disease, there is a paucity of data evaluating body composition in patients with chronic hepa
To evaluate the association between components of sarcopenia and demographic, clinical, lifestyle, nutritional, and biochemical variables in CHB patients.
Dual-energy X-ray absorptiometry (DXA) was used to assess muscle mass by quantifying appendicular lean mass (ALM) adjusted for body mass index (ALMBMI). Muscle function was evaluated by hand grip strength (HGS) and the timed up and go test. Metabolic-associated fatty liver disease (MAFLD) was defined according to the criteria proposed by an international expert panel. A body shape index and the International Physical Activity Questionnaire were used to assess central obesity and physical activity level, respectively.
This cross-sectional study included 105 CHB outpatients followed at the tertiary care ambulatory centre (mean age, 48.5 ± 12.0 years; 58.1% males; 76.2% without cirrhosis; 23.8% with compensated cirrhosis). The DXA-derived fat mass percentage was inversely correlated with the ALMBMI (r = - 0.87) and HGS (r = - 0.63). In the multivariable analysis, MAFLD, sedentarism and central obesity were positively and independently associated with low ALMBMI. MAFLD and central obesity were independently associated with low HGS.
MAFLD and central obesity were associated with low muscle mass and strength in patients with chronic hepatitis B, independent of the liver disease stage.
Core Tip: Recently, the clinical significance of sarcopenia in hepatic disease has been increasingly recognised. In patients with chronic hepatitis B, metabolic-associated fatty liver disease and central obesity were associated with low muscle mass and strength. Metabolic and skeletal muscle abnormality appraisal should be encouraged among individuals chronically infected with hepatitis B virus.
- Citation: Santos CML, Brito MD, Castro PASV, Vries TP, Viana NL, Coelho MPP, Malheiro OB, Bering T, Gonzalez MC, Teixeira R, Cambraia RD, Rocha GA, Silva LD. Metabolic-associated fatty liver disease is associated with low muscle mass and strength in patients with chronic hepatitis B. World J Hepatol 2022; 14(8): 1652-1666
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1652.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1652
Globally, approximately 462 million adults are underweight, whereas 1.9 billion are either overweight or obese[1,2]. In this scenario, according to the World Health Organization definition, the double burden of malnutrition is “characterized by the coexistence of undernutrition along with overweight, obesity or diet-related noncommunicable diseases, within individuals, households and populations, and across the life-course”[1,2]. Translating this definition into the hepatic disease context, several investigations have demonstrated that malnutrition and overweight can simultaneously be present in a patient[3-8]. Malnutrition contributes to the development of skeletal muscle abnormalities[3,4]. The loss of skeletal muscle mass, function and performance is considered primary when it is associated with ageing itself, i.e., primary sarcopenia; however, it can also be related to chronic diseases, i.e., secondary sarcopenia[9,10]. Furthermore, abnormalities in muscle mass and function may coexist with obesity, resulting in sarcopenic obesity, which is associated with liver-related complications and adverse outcomes[5,6]. The interaction between skeletal muscle abnormalities and metabolic factors such as obesity, insulin resistance and metabolic syndrome play a key role in the progression of liver fibrosis[5-8].
In real-world settings, researchers have identified an overlap between two or more factors associated with the progression of fibrosis in a substantial number of patients with cirrhosis[11-15]. Although in patients with chronic hepatitis B (CHB), long-term antiviral therapy is effective in discontinuing viral replication and reducing the development of cirrhosis and/or hepatocellular carcinoma (HCC), subgroups of patients are still prone to fibrosis progression, even achieving virological sustained response with potent nucleos(t)ide analogue therapy[16-18]. This evidence sheds light on putative risk factors for fibrosis advancement other than hepatitis B virus (HBV)-related factors. Among these factors, host and environmental factors should be highlighted, such as nutritional and metabolic characteristics.
With respect to nutritional status, in a previous study including individuals chronically infected with HBV or hepatitis C virus (HCV), sarcopenia was identified in 7.1%, 11.8%, and 21.9% of noncirrhotic, compensated cirrhotic (Child-Turcotte-Pugh A), and decompensated cirrhotic (Child-Turcotte-Pugh B/C) patients, respectively[19]. More recently, Han and colleagues examined the influence of sarcopenia on liver fibrosis among 506 patients with CHB[7]. Sarcopenia was significantly associated with liver disease severity, especially among HBV-positive subgroups with obesity, insulin resistance, metabolic syndrome and liver steatosis[7]. Although secondary sarcopenia is a well-known predictor of liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD), the interaction between sarcopenia and CHB is poorly understood. On the other hand, in line with the increasing prevalence of NAFLD, the coexistence of HBV infection and fatty liver disease has frequently been identified worldwide[8,20].
Recently, an international expert panel outlined metabolic-associated fatty liver disease (MAFLD) as hepatic steatosis in the presence of overweight, diabetes, and/or a combination of other metabolic disorders[21]. In contrast to the previous criteria for the diagnosis of NAFLD, the diagnosis of MAFLD is based on the degree of metabolic derangement and does not require the exclusion of other aetiologies of hepatic disease[21]. The role of superimposed MAFLD in CHB progression is still unclear. Despite the risks and consequences associated with low muscle mass in subjects chronically infected with HBV, there is a paucity of data evaluating body composition in this population. Thus, the aim of this study was to investigate the association between components of sarcopenia and demographic, clinical, lifestyle, nutritional, and biochemical variables in patients chronically infected with HBV.
This was a cross-sectional study comprising 105 consecutive outpatients who were aged > 18 years with confirmed CHB diagnosis attending the Viral Hepatitis Outpatient Clinic, University Hospital, Belo Horizonte, Brazil, between 2017 and 2020. Each patient met the inclusion criteria of the study for CHB as confirmed by the presence of specific HBV seromarkers and HBV-DNA.
The Viral Hepatitis Outpatient Clinic is an outpatient care ambulatory of a metropolitan tertiary teaching hospital that admits patients for the treatment of chronic viral hepatitis. All participants signed the informed consent form. The study was designed and conducted following the Declaration of Helsinki and was approved by the Ethics Committee of Federal University of Minas Gerais/UFMG (ETIC 0404.0.203.000 - 10; CAAE, 07761212.2.0000.5149).
All patients were screened for other hepatic diseases. The following patients were excluded from the study: those aged < 18 years; women who were pregnant or breastfeeding; those with hepatic encephalopathy, HBV/HCV or HBV/human immunodeficiency virus (HIV) coinfection; patients who had causes of liver disease other than HBV infection and advanced diseases such as chronic kidney disease, heart failure, chronic pulmonary disease, and neoplasia, including HCC. Patients were also excluded if they were using drugs known to be associated with fatty liver disease.
Since fluid overload interferes with body composition assessment, the Child-Pugh-Turcotte score was assessed for each patient, and those with a Child-Pugh-Turcotte score > 7 points and/or decompensated cirrhosis were not included in the study[22,23]. The diagnosis of cirrhosis was based on standard clinical, biochemical, radiological, and histological parameters[15]. Each patient underwent a detailed physical examination, particularly for the presence of bilateral lower extremity oedema and ascites. Additionally, all included patients had serum albumin levels ≥ 3.5 g/dL and the absence of ascites confirmed by abdominal ultrasound.
Blood samples were obtained from each patient after 12 h of overnight fasting for HBV diagnosis and biochemical and haematological evaluation. Fasting blood glucose levels, glycated haemoglobin, total cholesterol and fractions, triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase, alkaline phosphatase, albumin, total bilirubin, prothrombin activity, complete blood count test and creatinine were evaluated by routine laboratory methods.
CHB infection was classified as recommended by the EASL Clinical Practice Guidelines[24]. HBeAg-positive or HBeAg-negative chronically infected patients who presented HBV DNA > 2000 IU/mL, ALT > 2 × upper limits of normal and/or at least moderate liver necroinflammation or fibrosis during clinical follow-up were categorised as having CHB and underwent antiviral treatment[24]. All of them had undetectable HBV-DNA viral loads. Patients who were treatment-naïve with intrinsically low HBV viral load met the HBV chronic infection classification[24].
HBV status and HBV-DNA viral load were evaluated by chemiluminescence immunoassay (Ortho-Clinical Diagnostics™ VITROS™, Cumberland County, NJ) and a commercial test (Abbott Real Time HBV Viral Load, Lake Bluff, IL), respectively, according to the manufacturers’ instructions.
Hypertension, diabetes mellitus, dyslipidaemia and metabolic syndrome were defined in accordance with international guidelines[25-28]. Hepatic steatosis was diagnosed as the presence of fatty liver determined by ultrasound and/or histological assessment. The diagnosis of liver steatosis on ultrasound was based on increased hepatic echogenicity, hepatic attenuation of the ultrasound beam and hepatorenal index[29-31]. In addition, the hepatic steatosis index (HSI), a quantitative method for the evaluation of fatty liver disease validated for patients with HBV, was calculated according to the following formula: 8 × (ALT/AST ratio) + BMI (+2, if female; +2, if diabetes mellitus)[32,33]. MAFLD was defined according to the International Expert Consensus Statement[21].
The METAVIR score was used to assess the severity of fibrosis and the degree of liver inflammation/activity[34]. The grading and staging of fatty liver were defined using criteria proposed by Brunt et al[35] for histological lesions.
A current/past history of alcohol use was investigated as part of the lifestyle evaluation. Risky alcohol consumption was defined as a consumption of more than 20 g and 30 g of alcohol daily for women and men, respectively, for more than five years[36].
Participant habitual physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) short version validated for the Brazilian population[37]. Physical activity was dichotomised into normal [moderate-to-high categorical scale of IPAQ ≥ 600 metabolic equivalent of task (MET)-min/wk] or low (< 600 MET-min/wk). A trained person administered the questionnaires.
A nutritionist carried out all nutritional evaluations (C.M.L.S.). Weight and height were measured with a mechanical platform-type Filizola® (Filizola, São Paulo, Brazil). Light indoor clothing could be worn, excluding sweaters, belts, and shoes. We used Quetelet's formula to calculate BMI as a ratio between weight in kilograms and height in metres squared (kg/m2), and for elderly subjects, we used the Lipschitz classification[38,39].
Waist circumference (WC) was measured in the horizontal plane midway between the lower rib edge and the upper iliac crest in the standing position with a nonstretchable tape (cm). Central obesity was diagnosed as waist circumference > 102 cm in males and > 88 cm in females[28].
“A body shape index” (ABSI), an indirect measure of central obesity, was calculated as WC/(BMI2/3 × height1/2) and expressed in m11/6.kg−2/3[40-42]. The original ABSI values were < 0.1 and were multiplied by 1,000 to derive numbers on the order of magnitude of WC[42]. The fourth sex-specific quartile was used as the cut-off point to categorise the patients into the following groups: “higher ABSI” (> 82.4 for men and > 83.2 for women) and “nonhigher ABSI”.
Malnutrition was evaluated by using subjective global assessment (SGA). Patients were classified as follows: Nourished (SGA A), suspected to be malnourished or moderately malnourished (SGA B), and severely malnourished (SGA C)[43].
Whole-body dual-energy X-ray absorptiometry (DXA) exams were performed according to the procedures recommended by the manufacturer on a Discovery W densitometer (Hologic, Inc., Bedford, MA), software version 3.3.0. All procedures were carried out by blinded assessors and interpreted by the same operator (O. B. M.). The analysis included whole-body DXA measurements as fat mass (FM) and appendicular lean mass (ALM) or appendicular lean soft tissue (ALST), which is the sum of the lean mass of the arms and legs (kg)[44]. ALM was adjusted for BMI (ALMBMI), and patients in the first sex-specific quintile (< 0.767 for men and < 0.501 for women) were considered to have low ALMBMI. The criteria were adapted from the Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project consensus (FNIH Consensus)[10].
High DXA-derived FM was considered greater than 27.0% for men and 38.0% for women[45].
Handgrip strength, used to evaluate muscle strength, was measured with the hand-held dynamometer JAMAR® (Asimow Engineering Co., Los Angeles, CA). Subjects were seated with their elbows flexed at 90° and supported at the time of the measurement[46]. During handgrip strength measurement, we asked the patient to grip the dynamometer with maximum strength and hold the grip for 3 s. We collected three measurements from each hand in an alternating manner, and the maximum strength was defined as the greatest of the six measurements[46]. Handgrip strength was considered low when it was < 30 kg for males and < 20 kg for females (1.0 SD below the mean of a reference Brazilian population)[47,48].
The timed up-and-go test (TUG) measures the time it takes an individual to stand up from an armchair, walk a distance of three metres, turn, walk back to the chair and sit down again[49]. Patients in the fourth age- and sex-specific quartile were considered to have low physical performance according to the TUG values [for both men and women (age in years), 20-29 years, 9 s; 30-39 years, 10 s; 40-39 years, 11 s; 50-59 years, 12 s. For men 60-80 years, 14 s and for women 60-80 years, 18 s] modified from Furlanetto et al[50].
To improve the accuracy of the results, biochemical evaluation, abdominal ultrasound, liver biopsy, DXA, interview as well as lifestyle evaluation, anthropometric assessment and nutritional status were obtained from each patient at the time of her or his inclusion in the study.
Data were analysed with IBM SPSS (IBM Corp., Armonk, NY), statistical software package version 26.0. Descriptive statistics were used to provide information regarding the demographic, clinical, metabolic, lifestyle, nutritional, and biochemical data. The Shapiro-Wilk test was used to evaluate whether the data were normally distributed. For the comparison of percentages, the asymptotic Pearson's χ2 test was used. The Mann-Whitney U test or Kruskal-Wallis test was used for comparing the medians, and Student's t test or ANOVA was used for comparing the means.
The strength of the associations between, FM and ALMBMI and FM and HGS was analysed by Spearman's correlation. The correlation coefficient was interpreted as follows: 0.00-0.30 negligible, 0.30-0.50 Low, 0.50-0.70 moderate, 070-0.90 high and 0.90-1.00 very high[51].
Multiple logistic regression models were used to appraise the factors independently associated with the components of sarcopenia, low ALMBMI, low HGS and low physical performance (dependent variables, categorised as 0, absent or 1, present). We selected the following independent variables: demographics (age and sex); anthropometrics (ABSI); stage of liver disease (with compensated cirrhosis and without cirrhosis); metabolic derangement (MAFLD); sedentary lifestyle (IPAQ < 600 MET-min/wk); and prescribed medications (polypharmacy). Associations were evaluated by univariate analysis, and all variables with P values < 0.20 were included in the full models of logistic regression. Odds ratios and 95% confidence intervals were used as estimates of the risk. The Hosmer-Lemeshow test was used to assess the adequacy of the models.
To avoid the effect of collinearity, muscle abnormalities, low ALMBMI and low HGS were not included in the same logistic regression models.
The level of significance was set at P values ≤ 0.05.
The baseline characteristics of the patients are summarised in Table 1. The mean age of the patients was 48.5 ± 12.0 years, and 58.1% were men. At clinical follow-up, 61 (58.1%) and 44 (41.9%) patients met the criteria of CHB and HBV chronic infection, respectively[24]. Those categorised as CHB underwent antiviral treatment for at least 12 mo and had undetectable viral loads (Table 1). Out of 105 patients, 94 (89.5%) were diagnosed as HBeAg-negative, and 25/105 (23.8%) had compensated cirrhosis, which was more frequent in men than in women.
| Variables | Total (n = 105) | Male (n = 61) | Female (n = 44) | P value |
| Demographic | ||||
| Age (yr)1 | 48.5 ± 12.0 | 48.9 ± 12.9 | 48.0 ± 10.7 | 0.69 |
| HBV infection | ||||
| HBeAg negative n (%) | 94 (89.5) | 52 (85.2) | 42 (95.5) | 0.12 |
| HBeAg positive n (%) | 11 (10.5) | 9 (14.8) | 2 (4.5) | |
| HBV-DNA log10 (IU)/mL2 | 3.23 (2.59; 4.33) | 3.66 (2.75; 5.12) | 2.97 (2.53; 3.70) | 0.05 |
| Phases of HBV infection3 | ||||
| HBeAg-positive or -negative HBV chronic infection n (%) | 44 (41.9) | 19 (31.2) | 25 (56.8) | 0.008 |
| HBeAg-positive or -negative chronic hepatitis B n (%) | 61 (58.1) | 42 (68.8) | 19 (43.2) | |
| Time of HBV diagnosis (years)2 | 13.0 (5.0; 19.0) | 19.5 (15.0; 24.0) | 8.0 (4.0; 15.0) | 0.17 |
| Antiviral therapy | ||||
| Entecavir n (%) | 35 (33.3) | 29 (47.6) | 6 (13.6) | 0.009 |
| Tenofovir disoproxil fumarate n (%) | 26 (24.8) | 13 (21.3) | 13 (29.6) | |
| Time of antiviral treatment (months)2 | 36.0 (12.0; 60.0) | 36.0 (12.0; 60.0) | 39.0 (12.0; 49.5) | 0.58 |
| Stage of liver disease | ||||
| Without cirrhosis n (%) | 80 (76.2) | 37 (60.7) | 43 (97.7) | < 0.001 |
| Compensated cirrhosis n (%) | 25 (23.8) | 24 (39.3) | 1 (2.3) | |
| Child-Pugh-Turcotte score (A5/A6) | 19/6 | 18/6 | 1/0 | |
| Biochemical parameters2 | ||||
| Serum albumin, g/dL | 4.4 (4.1; 4.6) | 4.5 (4.2; 4.7) | 4.2 (4.1; 4.5) | 0.02 |
| Clinical and metabolic abnormalities n (%) | ||||
| Blood hypertension | 34 (32.4) | 20 (32.8) | 14 (31.8) | 0.92 |
| Diabetes mellitus | 11 (10.5) | 9 (14.8) | 2 (4.5) | 0.12 |
| Dyslipidaemia | 19 (18.1) | 11 (18.0) | 8 (18.2) | 0.98 |
| Overweight/obesity4 | 60 (57.1) | 31 (50.8) | 29 (65.9) | 0.12 |
| Metabolic syndrome5 | 19 (18.1) | 10 (16.4) | 9 (20.5) | 0.59 |
| Hepatic steatosis | 40 (38.1) | 27 (44.3) | 13 (29.6) | 0.13 |
| Metabolic associated fatty liver disease6 | 29 (27.6) | 18 (29.5) | 11 (25.0) | 0.61 |
| Polypharmacy7 | 10 (9.5) | 6 (9.8) | 4 (9.1) | 0.9 |
| Lifestyle data n (%) | ||||
| Low IPAQ (<600 met-min/week) | 65 (61.9) | 38 (62.3) | 27 (61.4) | 0.92 |
| Current alcohol consumption8 | 7 (6.7) | 4 (6.6) | 3 (6.8) | 1 |
| Risk drinking consumption9 | 3 (2.9) | 2 (3.3) | 1 (2.3) | 1 |
With respect to the nutritional data, ALMBMI (0.882 ± 0.147 vs 0.589 ± 0.097; P < 0.001) and HGS (43.5 ± 11.0 vs 24.9 ± 4.8; P < 0.001) were significantly higher in men than in women. Women had a significantly higher mean BMI (27.4 ± 4.6 vs 25.5 ± 4.1 kg/m2; P = 0.02) and mean FM (40.9 ± 5.2 vs 26.7 ± 6.2; P < 0.001) than men. Most patients (98.1%) were well nourished according to the SGA [SGA = 103 and SGB = 2 (1.9%)]. No differences were observed in mean or median of waist circumference (90.3 ± 12.2 vs 89.9 ± 12.2 cm; P = 0.87), ABSI [80.0 (77.0; 82.4) vs 78.9 (75.7; 83.2) (m11/6. kg-2/3); P = 0.37] or the timed up and go test [10.0 (8.9; 11.7) vs 10.0 (8.1; 10.9) (sec); P = 0.42] between men and women, respectively.
Overweight/obesity (57.1%) was the most frequent clinical and metabolic abnormality, followed by hepatic steatosis (38.1%), blood hypertension (32.4%), dyslipidaemia (18.1%), metabolic syndrome (18.1%) and diabetes mellitus (10.5%). As an elevated prevalence of clinical and metabolic abnormalities was identified, the patients were categorised into non-MAFLD and MAFLD groups. MAFLD was diagnosed in 29 of 105 patients with CHB. Among these patients, 14 (48.2%) had overweight or obesity; 20.7% had overweight/obesity, hypertension and diabetes mellitus; 10.3% had overweight/obesity, hypertension and dyslipidaemia; 6.9% had overweight/obesity, hypertension, diabetes mellitus and dyslipidaemia; 6.9% had hypertension and diabetes mellitus; 3.5% had hypertension and dyslipidaemia; and 3.5% had dyslipidaemia.
Concerning hepatic steatosis assessment, all patients underwent liver ultrasound evaluation, and liver biopsy was available in 41 patients (39%). In the MAFLD group, hepatic steatosis was diagnosed by ultrasound in 17/29 (58.6%) patients and by both histological analysis and ultrasound in 12 (41.4%) patients. The HIS [median (interquartile range, 25th - 75th percentile)] was significantly higher in the MAFLD group [42.5 (37.6-44.8)] than in the non-MAFLD group [34.8 (30.9-40.4); P < 0.001].
Out of 105 participants, 8 (7.6%) had low ALMBMI and HGS combined, and 5 (4.8%) had low ALMBMI, HGS and physical performance combined.
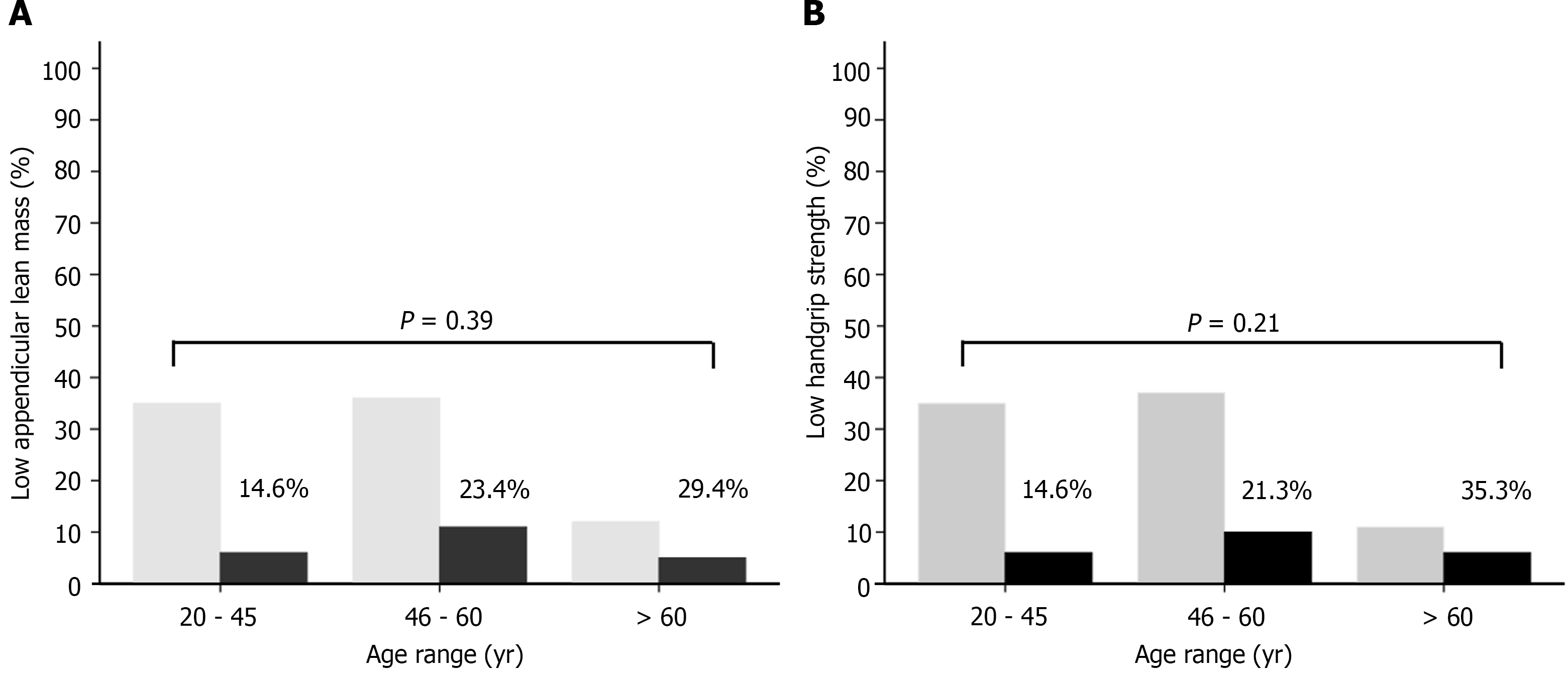
Patients with low ALMBMI were older, had a higher prevalence of general or central obesity, high DXA-derived FM, low HGS, compensated cirrhosis, clinical and metabolic disorders, sedentary lifestyle and risky alcohol consumption (Supplementary Table 1). General or central obesity, high FM, low ALMBMI, polypharmacy, and clinical and metabolic abnormalities were more frequent in patients with low HGS than in those without low muscle strength (Supplementary Table 2). There were no significant differences between low ALMBMI (Figure 1A) and low HGS (Figure 1B) within different age range groups.
Polypharmacy tended to be more frequent in CHB patients with low physical performance (19.4%) than in those without abnormal functional performance (5.4%, P = 0.06) (Supplementary Table 3). Angiotensin-converting inhibitor, angiotensin-receptor blockers, amlodipine, amitriptyline, atenolol, carvedilol, diltiazem, entecavir, furosemide, hydrochlorothiazide, indapamide, insulin, metformin, omeprazole, propranolol, spironolactone, statin and tenofovir disoproxil fumarate were the medications used by the patients. None of the individuals taking statins had myalgia, muscle weakness or increased creatine phosphokinase.
Neither ALMBMI nor muscle function was associated with antiviral therapy use. All patients with coexisting low ALMBMI, HGS and physical performance had MAFLD, central obesity, sedentary lifestyle and high FM (Supplementary Table 4).
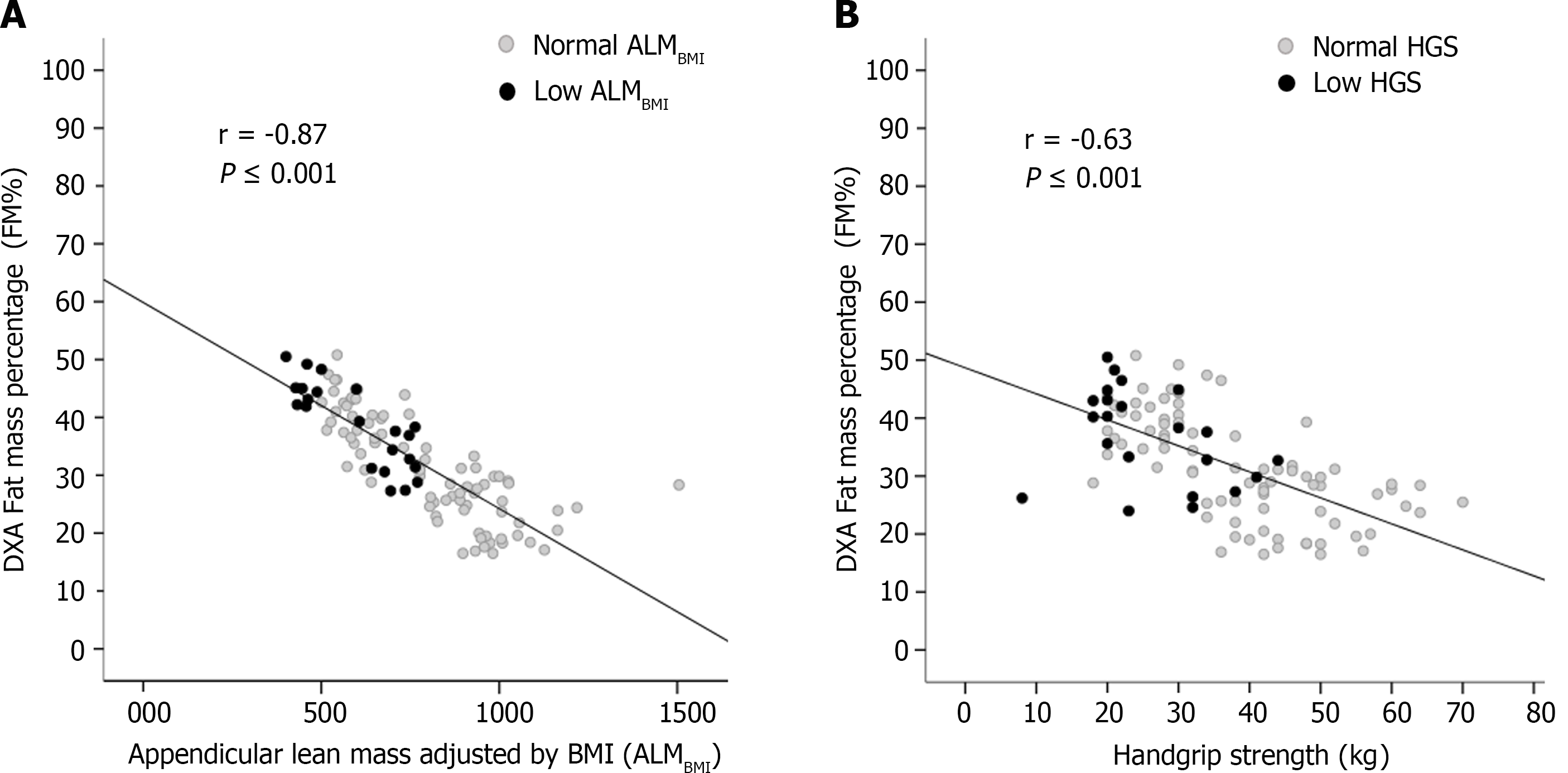
DXA-derived FM was inversely correlated with ALMBMI (r = -0.87; P < 0.001) (Figure 2A) and HGS (r = -0.63; P < 0.001) (Figure 2B).
Neither ALMBMI nor muscle function was associated with abnormal aminotransferase levels.
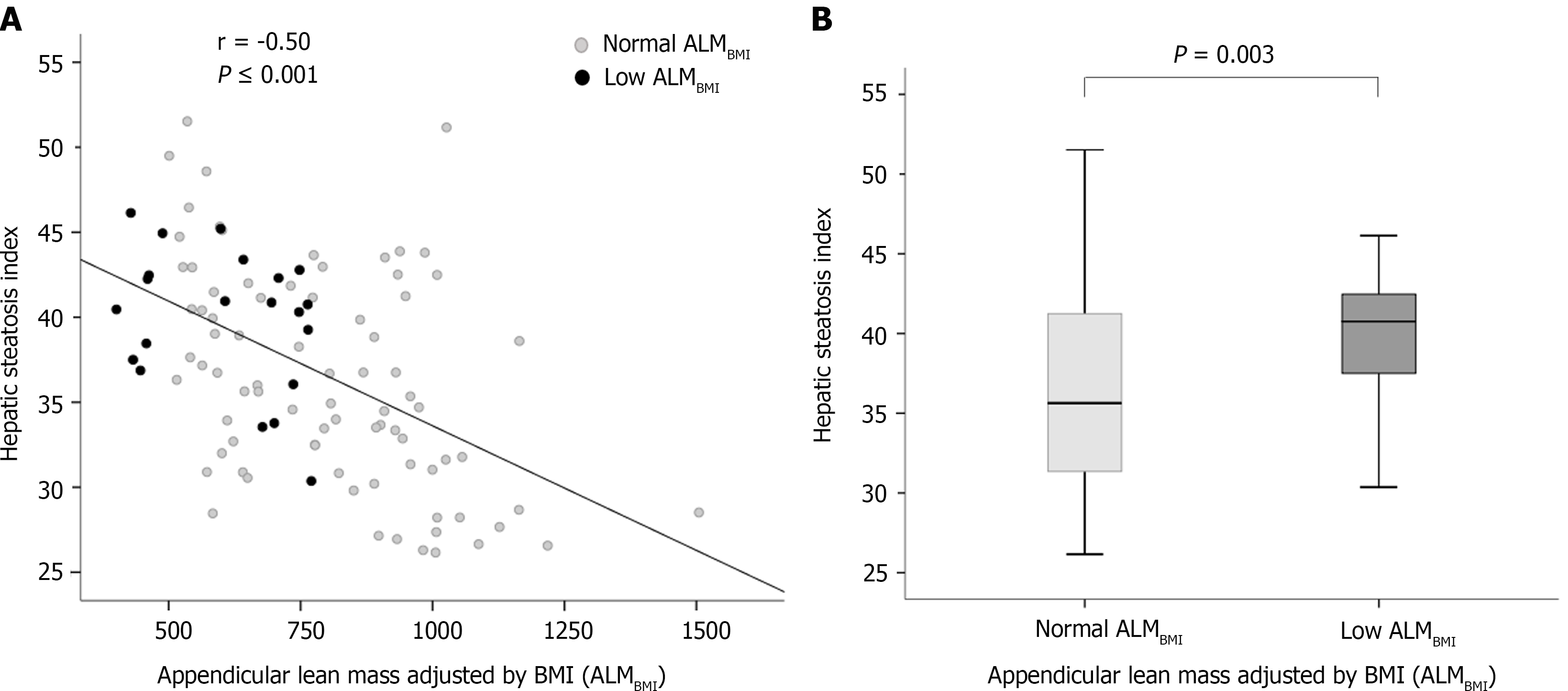
In the univariate analysis, age, high ABSI, compensated cirrhosis, MAFLD and low IPAQ (< 600 MET-min/wk) were included (Table 2). High ABSI, MAFLD and sedentary lifestyle remained positively and independently associated with low ALMBMI in the multivariable analysis (Table 2). In patients with hepatic steatosis, HSI was inversely correlated with ALMBMI (r = -0.50; P < 0.001) (Figure 3A), and HSI was higher in patients with low ALMBMI than in those without (Figure 3B).
| Variables | Univariate analysis | Multivariable analysis | ||||||
| Low ALMBMI | ||||||||
| Present n = 22 | Absent n = 83 | OR | 95%CI | P value | OR | 95%CI | P value | |
| Male/Female n (%) | 13 (59.1)/9 (40.9) | 48 (57.8)/35 (42.2) | 1.05 | 0.41-2.73 | 0.92 | - | - | - |
| Age > 50 yr | 13 (59.1) | 33 (39.8) | 2.19 | 0.84-5.70 | 0.1 | 1.03 | 0.98-1.08 | 0.2 |
| High ABSI (m11/6.kg−2/3) | 10 (45.5) | 16 (19.3) | 3.49 | 1.29-9.50 | 0.01 | 3.53 | 1.18-10.60 | 0.03 |
| Compensated cirrhosis | 9 (40.9) | 16 (19.3) | 2.9 | 1.06-7.96 | 0.03 | 1.64 | 0.51-5.27 | 0.41 |
| MAFLD | 11 (50.0) | 18 (21.7) | 3.61 | 1.35-9.68 | 0.008 | 3.81 | 1.30-11.19 | 0.02 |
| Low IPAQ (< 600 met-min/wk) | 19 (86.4) | 46 (55.4) | 5.09 | 1.40-18.55 | 0.01 | 3.13 | 1.17-8.32 | 0.02 |
| Polypharmacy1 | 4 (18.2) | 6 (7.2) | 2.85 | 0.73-11.17 | 0.21 | - | - | - |
| Variables | Low HGS | |||||||
| Present n = 22 | Absent n = 83 | OR | 95%CI | P value | OR | 95%CI | P value | |
| Male/Female n (%) | 12 (54.5)/10 (45.5) | 49 (59.0)/34 (41.0) | 0.83 | 0.32-2.14 | 0.7 | - | - | - |
| Age > 50 years | 11 (50.0) | 35 (42.2) | 1.37 | 0.58-3.51 | 0.51 | - | - | - |
| High ABSI (m11/6.kg−2/3) | 10 (45.5) | 16 (19.3) | 3.49 | 1.29-9.50 | 0.01 | 3.54 | 1.26-9.89 | 0.02 |
| Compensated cirrhosis | 8 (36.4) | 17 (20.5) | 2.22 | 0.80-6.15 | 0.12 | 1.45 | 0.46-4.55 | 0.53 |
| MAFLD | 10 (45.5) | 19 (22.9) | 2.81 | 1.05-7.50 | 0.04 | 2.85 | 1.02-7.91 | 0.04 |
| Low IPAQ (< 600 met-min/wk) | 13 (59.1) | 52 (62.7) | 0.86 | 0.33-2.25 | 0.76 | - | - | - |
| Polypharmacy1 | 5 (22.7) | 5 (6.0) | 4.59 | 1.19-17.63 | 0.02 | 3.13 | 0.74-13.22 | 0.12 |
| Variables | Low physical performance | |||||||
| Present n = 31 | Absent n = 74 | OR | 95%CI | P value | OR | 95%CI | P value | |
| Male/Female n (%) | 17 (54.8)/14 (45.2) | 44 (59.5)/30 (40.5) | 0.83 | 0.36-1.93 | 0.66 | - | - | - |
| Age > 50 years | 21 (67.7) | 61 (82.4) | 0.45 | 0.17-1.17 | 0.1 | 0.69 | 0.46-1.05 | 0.08 |
| High ABSI (m11/6.kg−2/3) | 7 (22.6) | 19 (25.7) | 0.84 | 0.32-2.27 | 0.74 | - | - | - |
| Compensated cirrhosis | 6 (19.4) | 19 (25.7) | 0.69 | 0.25-1.96 | 0.49 | - | - | - |
| MAFLD | 9 (29.0) | 20 (27.0) | 1.1 | 0.44-2.80 | 0.83 | - | - | - |
| Low IPAQ (< 600 met-min/wk) | 21 (67.7) | 44 (59.5) | 1.43 | 0.59-3.47 | 0.43 | - | - | - |
| Polypharmacy1 | 6 (19.4) | 4 (5.4) | 4.2 | 1.09-16.13 | 0.06 | 5.69 | 1.38-23.44 | 0.02 |
High ABSI, compensated cirrhosis, MAFLD and polypharmacy were included in the univariate analysis (Table 2). High ABSI and MAFLD remained positively and independently associated with low HGS in the univariate analysis (Table 2).
Age and polypharmacy were included in the univariate analysis (Table 2). Polypharmacy remained positively and independently associated with low physical performance in the multivariable analysis.
Muscle abnormalities have been identified in 13.0% to 40.0% of patients with liver cirrhosis, and recent reports have recognised their clinical significance[4-8]. However, there are limited data evaluating the loss of muscle quantity and quality in patients with CHB[7].
To the best of our knowledge, this is the first study to demonstrate that MAFLD and central obesity are associated with muscle abnormalities in the setting of CHB. Patients chronically infected with HBV with MAFLD had a 3.8-fold increased risk of muscle wasting compared to those without MAFLD. We also found that patients with central obesity had a threefold increased risk of muscle abnormalities in comparison with patients without central obesity.
In the current study, all CHB patients had quiescent virological activity. Although the long-term risk factors for cirrhosis and HCC, such as elevated levels of ALT and high HBV viral load, were not verified in our patients, 27.6% of them fulfilled the MAFLD criteria[14,21,52]. Thus, the presence of overweight, obesity or diet-related noncommunicable diseases should not be disregarded.
Concerning patients with cirrhosis, recent studies have shown that the presence of both obesity and muscle abnormalities was associated with higher rates of mortality than either condition alone[5,6]. Myosteatosis, the infiltration of fat in skeletal muscle, has been associated with worse survival in cirrhotic patients compared to those with normal body composition[53]. In individuals with NAFLD, the presence of low muscle volume and high muscle fat has been associated with poor functional performance and metabolic comorbidities[53].
Conversely, investigations exploring muscle composition abnormalities in patients with CHB are scarce. Our findings are similar to those of a previous study reporting that the frequency of obesity was higher in CHB patients with muscle abnormalities than in those without this condition[7]. When the authors categorised the participants according to metabolic factors, a strong association between muscle abnormalities and advanced fibrosis was identified in patients with obesity, insulin resistance, metabolic syndrome and hepatic steatosis[7]. These data suggest that mechanisms associated with muscle abnormalities identified in patients with NAFLD/NASH could be found in patients chronically infected with HBV[8,53].
Regarding fatty liver disease, we must bear in mind the complexity of mechanisms implicated in skeletal muscle damage. Lee and colleagues identified that up to 12.0% of patients diagnosed with NAFLD had sarcopenia independent of obesity and insulin resistance, and approximately 30.0% of sarcopenic individuals without metabolic syndrome and obesity had NAFLD[54,55]. These results point to a bidirectional muscle-liver axis as a possible pathophysiological contributor to either nonhepatic- or hepatic-related complications. The mechanisms involved in muscle-liver crosstalk include insulin resistance, increased inflammation, myokines secreted by skeletal muscles, myostatin, adiponectin, vitamin D deficiency and physical inactivity[8]. Therefore, based on these facts, it remains of utmost importance to detect additional risk predictors, other than those related to HBV, for adverse liver and nonliver outcomes.
Given the relevance of metabolic derangement in the liver disease course, an expert panel proposed a new definition for metabolic dysfunction in the presence of liver disease, renaming NAFLD as MAFLD, which, unlike NAFLD, does not require the exclusion of other hepatic diseases[21]. Large longitudinal cohort investigations demonstrated that superimposed MAFLD, NAFLD and nonalcoholic steatohepatitis (NASH) in adults with CHB were associated with advanced fibrosis, necroinflammatory activity, liver-related complications and all-cause mortality[11-14].
Nevertheless, the impact of coexisting hepatic steatosis on HBV-related disease progression remains complex and controversial[20]. A recent investigation reported that although coexisting fatty liver was observed in approximately 34.0% of CHB patients receiving HBV antiviral therapy, hepatic steatosis was associated with a low risk of HCC[56].
In our study, low physical performance was associated with polypharmacy. Recently, Venter and colleagues[57] observed a significantly greater weight gain in patients with HIV treated with dolutegravir plus two prodrugs of tenofovir (tenofovir disoproxil fumarate and tenofovir alafenamide fumarate), especially in combination with TAF, than in participants who were treated with the standard-care regimen. Translating this evidence into the CHB context, it is important to mention that prodrugs of tenofovir have been extensively used worldwide as first-line options and long-term therapy for patients with chronic HBV infection[24]. The effects of nucleos(t)ide analogues on body composition in HBV-infected individuals have scarcely been investigated. However, previous studies have demonstrated fat body increases and mitochondrial alterations with long-term antiviral treatment[58,59].
Limitations of our study were the inclusion of patients attending a referral centre, which may have made them not be representative of all patients with CHB, and the cross-sectional nature of our investigation that precluded the possibility of recognising any cause-effect relationship between adverse skeletal muscle status and the coexisting fatty liver in patients with CHB. In addition, a detail of DXA-derived measurements is their restraint in discerning any level of intramuscular fat infiltration. Furthermore, the inclusion of a control group of patients with MAFLD but without CHB should be assessed in a sequential investigation.
Although skeletal muscle abnormalities have been highly important in the course of chronic liver disease, there is no universal consensus to define and diagnose this condition in this population. Especially in patients with CHB, there is a need to endorse the definitions and cut-off values for assessing muscle mass (quantity/quality) and function. Regarding issues related to coexisting liver steatosis and myosteatosis[8,56], body composition assessment could shed light on the interplay among muscle, adipose tissue and liver in patients with CHB[7,8,53].
In conclusion, MAFLD and central obesity were associated with muscle abnormalities in patients with CHB, independent of the stage of liver disease. These findings point to crosstalk between metabolic factors and skeletal muscle abnormalities in CHB. Both clinicians and researchers should emphasise the importance of holistic and integrated management of patients infected with HBV. The coexistence of CHB, muscle abnormalities, obesity, and metabolic dysregulation may be involved in the patho
Recently, the clinical significance of sarcopenia in hepatic disease has been increasingly recognised. However, in chronic hepatitis B patients, the factors linked to skeletal muscle abnormalities have scarcely been investigated. Among them, host and environmental factors, such as nutritional and metabolic characteristics, should be evaluated.
Sarcopenia was identified in 7.1%, 11.8%, and 21.9% of noncirrhotic, compensated cirrhotic (Child-Turcotte-Pugh A), and decompensated cirrhotic (Child-Turcotte-Pugh B/C) patients, respectively. More recently, Han and colleagues observed that sarcopenia was significantly associated with liver disease severity, especially among hepatitis B virus (HBV)-positive subgroups with obesity, insulin resistance, metabolic syndrome and liver steatosis.
To investigate the association between components of sarcopenia and demographic, clinical, lifestyle, nutritional, and biochemical variables in HBV-chronically infected patients.
Dual-energy X-ray absorptiometry (DXA) was used to assess muscle mass by quantifying appendicular lean mass (ALM) adjusted for body mass index (ALMBMI). Muscle function was evaluated by hand grip strength (HGS) and the timed up and go test. Metabolic-associated fatty liver disease (MAFLD) was defined according to the criteria proposed by an international expert panel. A Body Shape Index and the International Physical Activity Questionnaire were used to assess central obesity and physical activity level, respectively.
This cross-sectional study included 105 chronic hepatitis B (CHB) outpatients followed at the tertiary care ambulatory centre (mean age, 48.5 ± 12.0 years; 58.1% males; 76.2% without cirrhosis; 23.8% with compensated cirrhosis). The DXA-derived fat mass percentage was inversely correlated with the ALMBMI (r = - 0.87) and HGS (r = - 0.63). In the multivariable analysis, MAFLD, sedentarism and central obesity were positively and independently associated with low ALMBMI. Central obesity was independently associated with low HGS. MAFLD and central obesity were independently associated with low HGS.
Among patients with CHB, metabolic-associated fatty liver disease (MAFLD) and central obesity were associated with low muscle mass and strength. Metabolic and skeletal muscle abnormality appraisal should be encouraged among HBV-chronically infected individuals.
Further large-scale case-control studies are needed to evaluate the role of MAFLD in HBV-chronically infected patients, including individuals with MAFLD but without CHB.
The authors appreciate all participants for their contribution to this study and the Outpatient Clinic of Viral Hepatitis staff of the Instituto Alfa de Gastroenterologia, Faculdade de Medicina, Universidade Federal de Minas Gerais.
| 1. | World Health organization. The double burden of malnutrition. Policy brief. Geneva: World Health Organization, 2017. [cited 20 April 2022]. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-17.3. |
| 2. | NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4095] [Cited by in RCA: 3639] [Article Influence: 363.9] [Reference Citation Analysis (0)] |
| 3. | Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S147-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (1)] |
| 4. | Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 464] [Article Influence: 46.4] [Reference Citation Analysis (1)] |
| 5. | Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (2)] |
| 7. | Han E, Lee YH, Kim BK, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH, Kim SU. Sarcopenia is associated with the risk of significant liver fibrosis in metabolically unhealthy subjects with chronic hepatitis B. Aliment Pharmacol Ther. 2018;48:300-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Chakravarthy MV, Siddiqui MS, Forsgren MF, Sanyal AJ. Harnessing Muscle-Liver Crosstalk to Treat Nonalcoholic Steatohepatitis. Front Endocrinol (Lausanne). 2020;11:592373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 8875] [Article Influence: 1267.9] [Reference Citation Analysis (1)] |
| 10. | Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1846] [Cited by in RCA: 1877] [Article Influence: 156.4] [Reference Citation Analysis (0)] |
| 11. | van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, Janssen HLA, de Knegt RJ, Sonneveld MJ. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3:100350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Khalili M, Kleiner DE, King WC, Sterling RK, Ghany MG, Chung RT, Bhan AK, Rosenthal P, Lisker-Melman M, Ramachandran R, Lok AS; and the Hepatitis B Research Network (HBRN). Hepatic Steatosis and Steatohepatitis in a Large North American Cohort of Adults With Chronic Hepatitis B. Am J Gastroenterol. 2021;116:1686-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, Janssen HLA, Patel K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 14. | Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 15. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 1057] [Article Influence: 211.4] [Reference Citation Analysis (2)] |
| 16. | Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, Nam SW, Bae SH, Choi JY, Yoon SK, Han NI, Jang JW. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2020;69:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 788] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 18. | Lee YB, Moon H, Lee JH, Cho EJ, Yu SJ, Kim YJ, Zoulim F, Lee J, Yoon JH. Association of Metabolic Risk Factors With Risks of Cancer and All-Cause Mortality in Patients With Chronic Hepatitis B. Hepatology. 2021;73:2266-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Hiraoka A, Michitaka K, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, Yamago H, Suga Y, Tomida H, Miyamoto Y, Azemoto N, Mori K, Miyata H, Tsubouchi E, Ninomiya T, Hirooka M, Abe M, Matsuura B, Hiasa Y. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2016;28:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Lin S, Jiang D, Li M, Chen Y, Li J, Fan J. Chronic hepatitis B and non-alcoholic fatty liver disease: Conspirators or competitors? Liver Int. 2020;40:496-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 3158] [Article Influence: 526.3] [Reference Citation Analysis (2)] |
| 22. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 23. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G, Politi F, Luca A, Virdone R, Licata A, Pagliaro L. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 393] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 24. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement D, Coca A, De Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen S, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder R, Shlyakhto E, Tsioufis K, Aboyans V, Desormais I. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27:314-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 26. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2340] [Article Influence: 292.5] [Reference Citation Analysis (0)] |
| 27. | Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Monique Verschuren WM, Vlachopoulos C, Wood DA, Luis Zamorano J; Additional Contributor, Cooney MT. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Rev Esp Cardiol (Engl Ed). 2017;70:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10941] [Article Influence: 643.6] [Reference Citation Analysis (0)] |
| 29. | Meek DR, Mills PR, Gray HW, Duncan JG, Russell RI, McKillop JH. A comparison of computed tomography, ultrasound and scintigraphy in the diagnosis of alcoholic liver disease. Br J Radiol. 1984;57:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 562] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 31. | Sberna AL, Bouillet B, Rouland A, Brindisi MC, Nguyen A, Mouillot T, Duvillard L, Denimal D, Loffroy R, Vergès B, Hillon P, Petit JM. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice recommendations for the management of non-alcoholic fatty liver disease: evaluation of their application in people with Type 2 diabetes. Diabet Med. 2018;35:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 32. | Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1185] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 33. | Chang JW, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Kim SU. Hepatic Steatosis Index in the Detection of Fatty Liver in Patients with Chronic Hepatitis B Receiving Antiviral Therapy. Gut Liver. 2021;15:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3128] [Article Influence: 104.3] [Reference Citation Analysis (1)] |
| 35. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2925] [Article Influence: 108.3] [Reference Citation Analysis (3)] |
| 36. | U S. Department of Agriculture, U. S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th Edition. [Cited 20 April 2022]. Available from: https://www.dietaryguidelines.gov. |
| 37. | Hallal PC, Cordeira K, Knuth AG, Mielke GI, Victora CG. Ten-year trends in total physical activity practice in Brazilian adults: 2002-2012. J Phys Act Health. 2014;11:1525-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | World Health Organization. Physical status, the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization technical report series 2015. [cited 20 April 2022]. Available from: http://www.who.int/childgrowth/publications/physical_status/en/. |
| 39. | Lipschitz DA. Screening for nutritional status in the elderly. Prim Care. 1994;21:55-67. [PubMed] |
| 40. | Biolo G, Di Girolamo FG, Breglia A, Chiuc M, Baglio V, Vinci P, Toigo G, Lucchin L, Jurdana M, Pražnikar ZJ, Petelin A, Mazzucco S, Situlin R. Inverse relationship between "a body shape index" (ABSI) and fat-free mass in women and men: Insights into mechanisms of sarcopenic obesity. Clin Nutr. 2015;34:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7:e39504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 462] [Cited by in RCA: 871] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 42. | Christakoudi S, Tsilidis KK, Muller DC, Freisling H, Weiderpass E, Overvad K, Söderberg S, Häggström C, Pischon T, Dahm CC, Zhang J, Tjønneland A, Halkjær J, MacDonald C, Boutron-Ruault MC, Mancini FR, Kühn T, Kaaks R, Schulze MB, Trichopoulou A, Karakatsani A, Peppa E, Masala G, Pala V, Panico S, Tumino R, Sacerdote C, Quirós JR, Agudo A, Sánchez MJ, Cirera L, Barricarte-Gurrea A, Amiano P, Memarian E, Sonestedt E, Bueno-de-Mesquita B, May AM, Khaw KT, Wareham NJ, Tong TYN, Huybrechts I, Noh H, Aglago EK, Ellingjord-Dale M, Ward HA, Aune D, Riboli E. A Body Shape Index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci Rep. 2020;10:14541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 43. | Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1939] [Cited by in RCA: 2008] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 44. | Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 560] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 45. | Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2670] [Cited by in RCA: 2804] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 46. | Luna-Heredia E, Martín-Peña G, Ruiz-Galiana J. Handgrip dynamometry in healthy adults. Clin Nutr. 2005;24:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Budziareck MB, Pureza Duarte RR, Barbosa-Silva MC. Reference values and determinants for handgrip strength in healthy subjects. Clin Nutr. 2008;27:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Amaral CA, Amaral TLM, Monteiro GTR, Vasconcellos MTL, Portela MC. Hand grip strength: Reference values for adults and elderly people of Rio Branco, Acre, Brazil. PLoS One. 2019;14:e0211452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 49. | Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8741] [Cited by in RCA: 9742] [Article Influence: 278.3] [Reference Citation Analysis (0)] |
| 50. | Furlanetto KC, Correia NS, Mesquita R, Morita AA, do Amaral DP, Mont'Alverne DGB, Pereira DM, Pitta F, Dal Corso S. Reference Values for 7 Different Protocols of Simple Functional Tests: A Multicenter Study. Arch Phys Med Rehabil. 2022;103:20-28.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69-71. [PubMed] |
| 52. | Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, You SL, Wang LY, Chen CJ; R. E.V.E.A.L.-HBV Study Group. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 53. | Linge J, Ekstedt M, Dahlqvist Leinhard O. Adverse muscle composition is linked to poor functional performance and metabolic comorbidities in NAFLD. JHEP Rep. 2021;3:100197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 54. | Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology. 2016;63:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 55. | Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, Kang ES, Han KH, Lee HC, Cha BS. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol. 2015;63:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 56. | Fan R, Niu J, Ma H, Xie Q, Cheng J, Rao H, Dou X, Xie J, Zhao W, Peng J, Gao Z, Gao H, Chen X, Chen J, Li Q, Tang H, Zhang Z, Ren H, Cheng M, Liang X, Zhu C, Wei L, Jia J, Sun J, Hou J; Chronic Hepatitis B Study Consortium. Association of central obesity with hepatocellular carcinoma in patients with chronic hepatitis B receiving antiviral therapy. Aliment Pharmacol Ther. 2021;54:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, Serenata C, Akpomiemie G, Qavi A, Chandiwana N, Norris S, Chersich M, Clayden P, Abrams E, Arulappan N, Vos A, McCann K, Simmons B, Hill A. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019;381:803-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 58. | Yao J, Zhou L, Hua X, Kong M, Chen Y, Duan Z. Effects of nucleos(t)ide analogs on body composition in HBV-infected men: An age- and BMI-matched, cross-sectional study. Nutrition. 2016;32:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Madeddu G, Soddu A, Mannu F, Muredda AA, Garrucciu G, Bandiera F, Zaru S, Mura MS, Babudieri S. Body fat changes and mitochondrial alterations during HBV treatment: a warning for long term administration. J Infect. 2012;65:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sripongpun P, Thailand; Yin GQ, China S-Editor: Wang LL L-Editor: A P-Editor: Cai YX