Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1584
Peer-review started: March 30, 2022
First decision: June 8, 2022
Revised: June 22, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: August 27, 2022
Processing time: 148 Days and 21.9 Hours
Acute severe variceal bleeding (AVB) refractory to medical and endoscopic therapy is infrequent but associated with high mortality. Historical cohort studies from 1970-1980s no longer represent the current population as balloon tamponade is no longer first-line therapy for variceal bleeding; treatments including vasoactive therapies, intravenous antibiotics, endoscopic variceal band ligation are routinely used, and there is improved access to definitive treatments including transjugular intrahepatic portosystemic shunts. However, only a few studies from the current era exist to describe the practice of balloon tamponade, its outcomes, and predictors with a requirement for further updated information.
To describe current management of AVB requiring balloon tamponade and identify the outcomes and predictors of mortality, re-bleeding and complications.
A retrospective multi-centre cohort study of 80 adult patients across two large tertiary health networks from 2008 to 2019 in Australia who underwent balloon tamponade using a Sengstaken-Blakemore tube (SBT) were included for analysis. Patients were identified using coding for balloon tamponade. The primary outcome of this study was all-cause mortality at 6 wk after the index AVB. Secondary outcomes included re-bleeding during hospitalisation and complications of balloon tamponade. Predictors of these outcomes were determined using univariate and multivariate binomial regression.
The all-cause mortality rates during admission and at 6-, 26- and 52 wk were 48.8%, 51.2% and 53.8%, respectively. Primary haemostasis was achieved in 91.3% and re-bleeding during hospitalisation occurred in 34.2%. Independent predictors of 6 wk mortality on multivariate analysis included the Model for Endstage Liver disease (MELD) score (OR 1.21, 95%CI 1.06-1.41, P = 0.006), advanced hepatocellular carcinoma (OR 11.51, 95%CI 1.61-82.20, P = 0.015) and re-bleeding (OR 13.06, 95%CI 3.06-55.71, P < 0.001). There were no relevant predictors of re-bleeding but a large proportion in which this occurred did not survive 6 wk (76.0% vs 24%). Although mucosal trauma was the most common documented complication after SBT insertion (89.5%), serious complications from SBT insertion were uncommon (6.3%) and included 1 patient who died from oesophageal perforation.
In refractory AVB, balloon tamponade salvage therapy is associated with high rates of primary haemostasis with low rates of serious complications. Re-bleeding and mortality however, remain high.
Core Tip: Acute severe variceal bleeding requiring balloon tamponade remains associated with high mortality rates of approximately 50%. Sengstaken-Blakemore tube achieves excellent primary haemostasis rates in > 90% however re-bleeding is common at approximately 30% with subsequent death in approximately 75%. Predictors of all-cause mortality at 6 wk included a greater Model for Endstage Liver disease score, re-bleeding and advanced hepatocellular carcinoma. The most commonly reported complication from SBT was mucosal trauma, which was conservatively managed, with only a small proportion resulting in serious complications (6.3%). There was significant variability amongst technical aspects of balloon tamponade insertion which may result from the infrequent need to perform this procedure.
- Citation: Keung CY, Morgan A, Le ST, Robertson M, Urquhart P, Swan MP. Survival outcomes and predictors of mortality, re-bleeding and complications for acute severe variceal bleeding requiring balloon tamponade. World J Hepatol 2022; 14(8): 1584-1597
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1584.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1584
Acute severe variceal bleeding (AVB) refractory to endoscopic variceal band ligation (EVBL) and injection therapy occurs infrequently in 10%-20% of variceal haemorrhage but is associated with significant mortality rates of over 30%[1]. In this situation, the main salvage strategy has traditionally involved balloon tamponade with various devices including the Sengstaken-Blakemore tube (SBT)[2], the Minnesota tube and the Linton-Nachlas tube, which are similar devices that differ in terms of the number of balloons and ports[3]. While covered self-expandable metallic oesophageal stents have more recently become available, with potential advantages of improved safety and efficacy over balloon tamponade[1,4], oesophageal stents are still not routinely available in many treating centres. Both these rescue techniques serve a temporising role while awaiting further definitive options including transjugular intrahepatic portosystemic shunt (TIPS), balloon-occluded retrograde transvenous oblit
Previous retrospective cohort studies published in the 1970s and 1980s demonstrated that balloon tamponade successfully achieved primary haemostasis in 40-98% of cases, however it was associated with a high risk of both re-bleeding (35%-70%) and procedural complications[8-12]. Importantly, the management of AVB has evolved significantly since this time and thus these studies are not reflective of current practice. For example, balloon tamponade is no longer employed as a first-line management option and endoscopic sclerotherapy has long been superseded by EVBL. In addition, the therapeutic armamentarium for AVB has significantly expanded and now encompasses vasoactive treatment, empiric antibiotics, endoscopic therapies and radiologic procedures such as TIPS and BRTO. Finally, expert opinion-based consensus guidelines for variceal bleeding are also now available[5,6]. Currently there is a paucity of literature examining the clinical outcomes of patients treated with current standards of care, who require balloon tamponade for AVB[13,14]. Subsequently this study aims to: (1) Describe the current clinical practice surrounding management of endoscopically uncontrollable AVB requiring balloon tamponade; (2) Identify the outcomes; and (3) Predictors of mortality, re-bleeding and complications of balloon tamponade.
A multi-centre retrospective cohort study was undertaken across Monash Health and Eastern Health, two large metropolitan tertiary health care services in Victoria, Australia. All consecutive adult patients (> 18 years) who underwent balloon tamponade using a SBT for refractory AVB between 1st January 2008 until 31st December 2019 were included. Patients were identified by the International Classification of Diseases-10 procedure code for gastro-oesophageal balloon tamponade. Data extracted from medical records included baseline demographic information, liver disease severity indicators, clinical and biochemical data relating to variceal bleeding, practice surrounding insertion and monitoring of balloon tamponade devices and clinical outcomes including re-bleeding, survival up to 52 wk and complications of both variceal bleeding and balloon tamponade. All patients were risk stratified using the AIMS65, Rockall, pre-endoscopy Rockall (pre-Rockall), Child-Pugh and Model for Endstage Liver disease (MELD) scores on admission prior to index gastroscopy.
AVB was managed according to published United Kingdom and United States guidelines[5,6]. Patients with suspected variceal bleeding received intravenous (IV) antibiotics (ceftriaxone or piperacillin-tazobactam) and vasoactive therapy with either an octreotide infusion (50 microgram (mcg) bolus, followed by a 25-50 mcg/hour infusion) or IV terlipressin (0.85-1.7 mg 6 hourly). A restrictive blood transfusion policy is standard at the treating centres and patients typically receive packed red cells if their haemoglobin is < 70 g/L (or < 80 g/L in the presence of ischaemic heart disease) with a target haemoglobin level of 80-90 g/L. Endoscopy was performed in either a dedicated endoscopy suite or operating theatre with sedation administered by an anaesthetist in all cases. Bleeding oesophageal varices were treated with EVBL and bleeding gastric varices were treated with variceal obturation using histoacryl and lipiodol or thrombin. In cases of AVB not amenable to endoscopic therapy, both interventional radiology (including TIPS or BRTO) and upper gastrointestinal surgery services were available.
The primary outcome measure of this study was all-cause mortality after AVB requiring balloon tamponade which was assessed at 6 wk and followed up at 26 and 52 wk. Secondary outcomes assessed included re-bleeding after insertion of SBT and complications of balloon tamponade during the hospital admission. Primary haemostasis was defined as the clinical cessation of variceal bleeding after balloon tamponade during the index hospitalisation and re-bleeding defined as further bleeding after primary haemostasis was achieved upon removal or balloon deflation of the SBT. Patients without cirrhosis (non-cirrhotic portal hypertension) who required balloon tamponade for AVB were excluded from the predictors of mortality analyses but included in remaining analyses surrounding balloon tamponade practice.
The Monash Health Human Research Ethics Committee assessed this study as low risk (RES-21-0000-218Q-70254) and did not require participant informed consent.
Descriptive statistics was used to analyse continuous variables expressed as median and interquartile range (IQR) for continuous non-parametric variables, and absolute frequencies between groups for categorical variables. Analysis was performed on factors potentially contributing to death, re-bleeding and balloon tamponade complications using Mann-Whitney U test for continuous variables and Fisher’s Exact Test for dichotamous variables. Univariate binomial regression was used to identify potential clinically relevant variables predictive of death, re-bleeding and complications and those that reached statistical significance (P < 0.10) were then included in a multivariate binomial regression analysis. Missing data was excluded from multivariate analysis. Statistical analysis was performed using licensed SPSS software (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). Figures for survival analysis were prepared using licensed GraphPad Prism software (GraphPad Software for Windows, Version 9.0.0, Sand Diego, California).
Overall, there were 81 adult patients who required balloon tamponade with SBT for endoscopically uncontrollable AVB. Insufficient information was available for 1 patient who was subsequently excluded from the analysis (n = 80). Cirrhosis was diagnosed in 75 (93.8%) patients but 5 (6.3%) had non-cirrhotic portal hypertension and were not included in the predictors of mortality analyses.
Most of the patients were male (61, 76.3%) with a median age of 56 years (range 34 to 80 years). Most patients with cirrhosis had advanced cirrhosis with a median Child-Pugh score 9 (IQR 8-11) and median MELD score 17 (IQR 13-21). The most common aetiology of cirrhosis was alcohol-related liver disease (54, 72.0%) of which 34 (63.0%) were actively still consuming alcohol, followed by chronic hepatitis B or C (30, 40.0%) and then non-alcoholic steatohepatitis (7, 9.3%). Eleven (14.7%) patients with hepatocellular carcinoma (HCC) had Stage C (Advanced) or Stage D (Terminal) staging as per the Barcelona Clinic Liver Cancer classification[15].
The presence of varices had been documented in 51 (63.8%) patients prior to the index bleed. Of these patients, 47.1% of these had prophylactic EVBL prior to the index AVB at a median duration of 3 wk prior (IQR 2-12 wk). Non-selective beta blocker use was documented in 23 (28.8%) patients at the time of variceal haemorrhage.
The baseline characteristics of the patients are presented in Table 1 which compares characteristics of those who survived and those who died at 6 wk after the index variceal bleed. Compared to patients who survived, patients who died were noted to have significantly higher Child-Pugh and MELD scores (P = 0.004 and P < 0.001, respectively), international normalised ratio (P < 0.001), albumin (P = 0.034), bilirubin (P = 0.003), sodium (P = 0.025), creatinine (P = 0.014) and lactate levels (P = 0.007) at the time of presentation to hospital. In addition, the diagnosis of HCC was significantly more prevalent in patients who died within 6 wk (P = 0.019).
| Variable (n = 75) | Survived (n = 40) | Death (n = 35) | P value |
| Male sex | 30 (75.0) | 28 (80.0) | 0.783 |
| Age in years | 58 (48, 65) | 54 (49, 65) | 0.629 |
| Cirrhosis aetiology: Alcoholic liver disease, Chronic viral hepatitis, NASH | 26 (65.0), 16 (40.0), 6 (15.0) | 28 (80.0), 14 (40.0), 1 (2.9) | 0.199, 1.00, 0.113 |
| Child-Pugh score | 8 (7, 10) | 11 (8, 12) | 0.004 |
| MELD score | 14 (11, 18) | 19 (16, 24) | < 0.001 |
| AIMS65 score | 2 (1, 2.5) | 3 (2, 3) | 0.004 |
| Glasgow-Blatchford score | 12 (9, 14) | 12 (9, 15) | 0.908 |
| Complete Rockall score | 8 (7, 8) | 8 (7, 8) | 0.159 |
| HCC | 2 (5.0) | 9 (25.7) | 0.019 |
| Portal vein thrombosis | 6 (15.0) | 4 (11.4) | 0.742 |
| Antiplatelets or anticoagulants | 7 (17.5) | 2 (5.7) | 0.162 |
| Glasgow Coma Scale | 15 (14, 15) | 15 (14, 15) | 0.503 |
| Systolic blood pressure | 101 (91, 123) | 96 (76, 124) | 0.328 |
| Heart rate | 95 (82, 114) | 104 (91, 118) | 0.304 |
| Significant hypoxia (FiO2 > 35%) | 10 (25.0) | 13 (37.1) | 0.319 |
| Haemoglobin g/L | 86 (74, 103) | 78 (63, 111) | 0.538 |
| Platelets × 109/L | 96 (72, 123) | 110 (75, 150) | 0.204 |
| Albumin g/L | 26 (22, 28) | 22 (19, 28) | 0.034 |
| INR | 1.5 (1.3, 1.7) | 1.9 (1.5, 2.3) | < 0.001 |
| Bilirubin μmol/L | 29 (14, 54) | 51 (29, 119) | 0.003 |
| Serum sodium mmol/L | 139 (135, 141) | 135 (129, 140) | 0.025 |
| Serum creatinine μmol/L | 74 (62, 92) | 95 (73, 124) | 0.014 |
| Serum pH | 7.36 (7.26, 7.44) | 7.30 (7.10, 7.41) | 0.072 |
| Lactate mmol/L | 3.10 (1.65, 5.35) | 5.20 (2.65, 9.80) | 0.007 |
Including all patients who required balloon tamponade (n = 80), at presentation of the index variceal bleed, 48.8% (39) of patients were tachycardic with a heart rate over 100 beats/min and 28.7% (23) were hypotensive with a systolic blood pressure < 90 mmHg. A reduced Glasgow Coma Scale score was recorded in 24 (30%) patients and 30% (24) required oxygen supplementation at concentrations of at least FiO2 35% for hypoxia. Almost all patients received vasoactive agent therapy with either terlipressin or octreotide (79, 98.8%) and IV antibiotics (77, 96.3%) in the emergency department. Most patients received vitamin K (65, 81.3%) and 16.5% (13 patients) received human prothrombin complex concentrate (Prothrombinex®) in an attempt to correct coagulopathy.
Table 2 summarises the medical and endoscopic management of the index variceal bleed and the clinical practice surrounding insertion of SBT for salvage therapy. The median time to initial endoscopy after AVB was 6.8 h (IQR 4.2-19.0 h). The source of bleeding was noted to be oesophageal varices in 80.0% (64 patients) with 20.0% (16 patients) due to gastric varices. Initial endoscopic therapy was performed in 45 patients (56.4%). Insertion of balloon tamponade devices were performed by specialist endoscopists in all cases, most commonly during the initial gastroscopy. The indications for balloon tamponade with SBT were incomplete haemostasis (39, 48.8%), poor endoscopic views (26, 32.5%) or both (15, 18.8%). The SBT insertion approach was documented to be oral in 49 (61.3%) and nasal in 22 (31.0%), while no documentation was available in 9 (11.3%). Confirmation of SBT position by either direct endoscopic vision or chest X-ray was documented in 80.5% of procedures. The gastric balloon was inflated in all cases with a median volume of 285 mL air (range 50-500 mL), while the oesophageal balloon was inflated in 22 (27.5%) cases with a median volume of 100 mL air (range 20-500 mL) (Table 2). Documentation of devices used to maintain traction on the inflated SBT was very inconsistent. Repeat gastroscopy was performed in 61 (76.3%) patients and generally occurred in the following 24 to 48 h after the index gastroscopy with repeat endoscopic therapy performed in 33.8% (27 patients). Patients that did not undergo repeat gastroscopy had rapidly deteriorated and died.
| Variable | Value (n = 80) |
| Vasoactive agent | 79 (98.8) |
| Terlipressin | 30 (37.5) |
| Octreotide | 49 (61.3 |
| Empiric antibiotics | 77 (96.3) |
| Time to endoscopy from bleed, h | 6.8 (4.2, 19.0) |
| Site of variceal bleed | |
| Oesophageal varices | 64 (80.0) |
| Gastric varices | 16 (20.0) |
| Initial endoscopic therapy | |
| Variceal band ligation | 43 (53.8) |
| Cyanoacrylate injection | 2 (2.5) |
| Indication for SBT insertion | |
| Incomplete haemostasis | 54 (67.5) |
| Poor endoscopic views | 41 (51.2) |
| Approach for SBT insertion (n = 71) | |
| Nasal | 22 (31.0) |
| Oral | 49 (69.0) |
| Confirmation of SBT position | |
| X-ray | 44 (55.0) |
| Direct endoscopic vision | 18 (22.5) |
| No documentation | 15 (18.8) |
| Gastric balloon inflation (n = 76) | |
| Volume, mL | 285 (250, 300) |
| Time inflated, h | 26.8 (16.9, 44.7) |
| Oesophageal balloon inflation (n = 22) | |
| Volume, mL | 100 (39, 100) |
| Time inflated, h | 20.5 (10.2, 36.8) |
| Therapy post-SBT deflation (n = 62) | |
| Repeat endoscopic therapy | 27 (43.5) |
| Further balloon tamponade | 16 (20.0) |
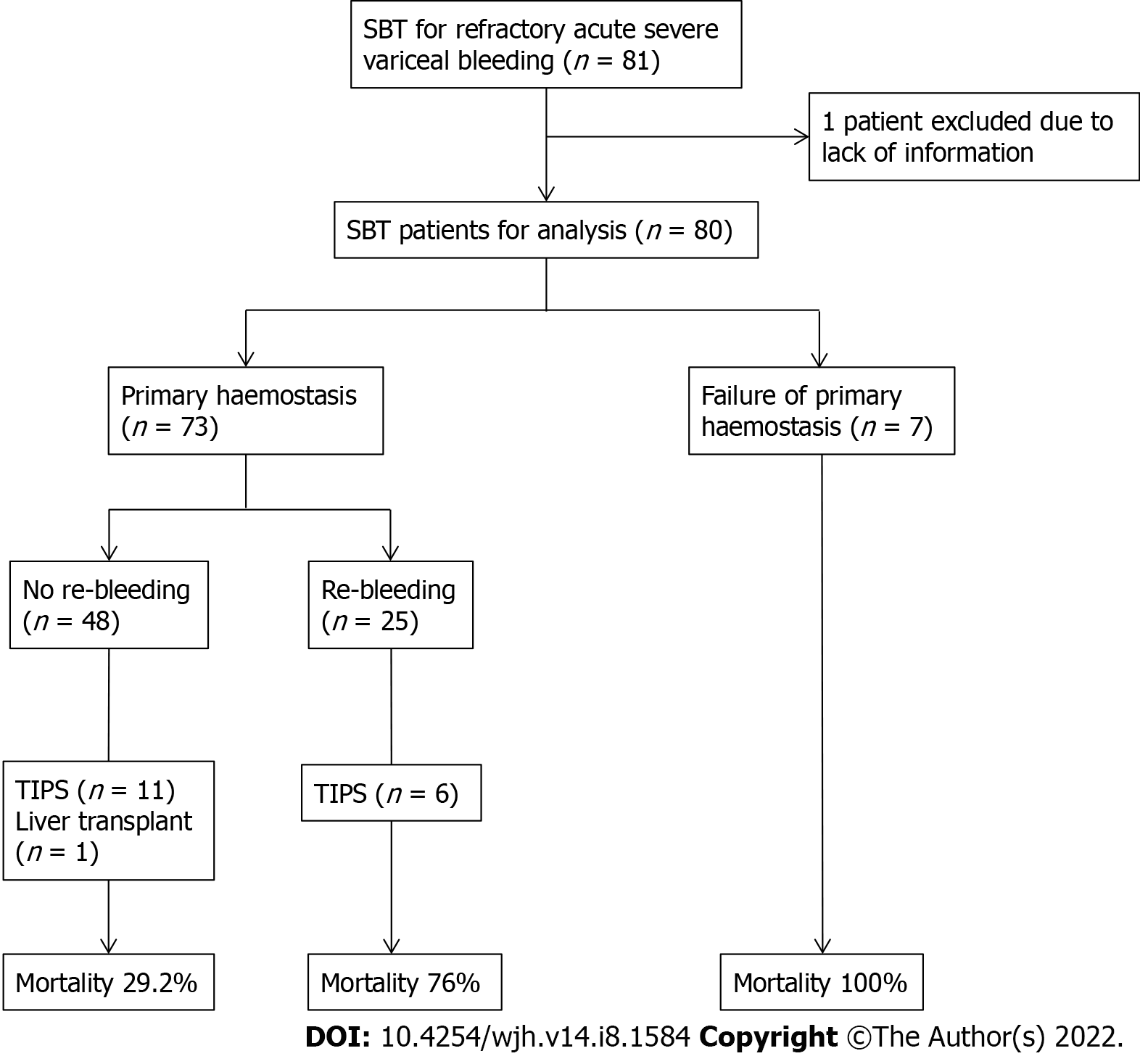
The outcomes of mortality, re-bleeding and complications from balloon tamponade are summarised in Figure 1 and Table 3. Inpatient mortality was 48.8% (39 deaths), and the mortality rates at 6-, 26- and 52 wk were 48.8% (39 deaths), 51.2% (41 deaths) and 53.8% (43 deaths), respectively. The causes of death during the index inpatient hospitalisation included refractory bleeding with failure to achieve haemostasis (20, 51.3%), sepsis with multiorgan failure (14, 35.9%), aspiration pneumonia (3, 7.7%) and 1 patient died from an oesophageal perforation due to SBT (2.6%). This patient had his initial gastroscopy and SBT inserted in a regional hospital prior to transfer, where a chest X-ray revealed the gastric balloon was either inflated or migrated into the oesophagus and caused perforation and mediastinitis.
| Variable | Value (n = 80) |
| Length of stay, d | |
| Total hospital LOS | 11.0 (7.0, 19.5) |
| ICU LOS | 4.90 (2.3, 9.1) |
| Duration of mechanical ventilation, h | 89.5 (39.3, 153.8) |
| Blood product transfusion | |
| Packed red cells, units | 6 (4, 10) |
| Platelets, units | 1 (0, 3) |
| Fresh frozen plasma, units | 2 (1, 6) |
| Achieved primary haemostasis | 73 (91.3) |
| Re-bleeding | 25 (34.2) |
| Complications of SBT | 19 (34.5) |
| Mucosal ulceration and tears (without perforation) | 17 (89.5) |
| Aspiration pneumonia | 4 (21.0) |
| Perforation | 1 (5.3) |
| TIPS | |
| Underwent TIPS | 17 (21.3) |
| Time to TIPS from SBT, d | 2.95 (1.4, 4.1) |
| Survival | |
| Survived hospital admission | 41 (51.2) |
| Alive at 6 wk | 41 (51.2) |
| Alive at 26 wk | 39 (48.8) |
| Alive at 52 wk | 37 (46.3) |
The insertion of SBT successfully achieved primary haemostasis in 73 (91.3%) patients, with no survivors amongst those where this was not achieved. Re-bleeding occurred in 34.2% (25) after achieving primary haemostasis, of which further balloon tamponade was performed in 16 of these patients. Of the 25 patients who had experienced re-bleeding, the inpatient mortality rate was 76.0%. TIPS was performed in 17 (21.3%) patients at a median of 2.95 d from balloon tamponade insertion, of which 5 patients died. One patient underwent liver transplantation and survived.
Complications associated with SBT insertion were documented in 19 (23.8%) patients. The most common complication (17, 89.5%) was superficial mucosal trauma without perforation which was managed conservatively. Only a few serious complications occurred in 5 patients (6.3%) and included aspiration pneumonia recorded in 4 patients (of which 2 died during the index hospitalisation) and 1 patient died from oesophageal perforation as mentioned above.
As most patients who survived their hospital admission continued to survive to 52 wk after the index variceal bleed, the mortality rates and thus the predictors on univariate and multivariate analyses are very similar for all study time points. Subsequently results for predictors will be presented for the primary endpoint of 6 wk mortality after index variceal bleed for cirrhotic patients only (n = 75).
Upon univariate analyses, variables that significantly predicted 6 wk mortality included: Markers of liver disease severity (Child-Pugh score, MELD score, international normalised ratio, bilirubin, serum creatinine and sodium), pH and serum lactate, the presence of HCC, the AIMS65 score and re-bleeding. Of the validated upper gastrointestinal bleeding risk scoring algorithms used to predict outcomes, only the AIMS65 score[16] reached significance at univariate analysis (OR 1.96, 95%CI 1.15- 3.35, P = 0.014) while the Glasgow-Blatchford score (GBS) (OR 0.98, 95%CI 0.86-1.15, P = 0.767), pre-endoscopy and complete Rockall scores (both OR 1.44, 95%CI 0.86-2.43, P = 0.168) were not significant[17-19]. Results of the univariate analyses are detailed in Table 4.
| Variable (n = 75) | Univariate analysis | Multivariate analysis (n = 68) | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age | 0.99 (0.95-1.04) | 0.669 | - | - |
| Male sex | 1.33 (0.45-3.98) | 0.606 | - | - |
| Haemodynamic instability | 2.01 (0.75-5.41) | 0.167 | - | - |
| INR | 8.19 (2.10-31.89) | 0.002 | - | - |
| Albumin | 0.924 (0.85-1.01) | 0.063 | - | - |
| Bilirubin | 1.02 (1.01-1.030) | 0.009 | - | - |
| Creatinine | 1.02 (1.01-1.035) | 0.024 | - | - |
| Sodium | 0.93 (0.86-0.99) | 0.045 | - | - |
| pH | 0.02 (0.01-0.68) | 0.030 | - | - |
| Lactate | 1.16 (1.02-1.31) | 0.019 | - | - |
| AIMS65 score | 1.96 (1.15-3.35) | 0.014 | - | - |
| GBS | 0.98 (0.86-1.15) | 0.767 | ||
| Rockall score | 1.44 (0.86-2.43) | 0.168 | ||
| Child-Pugh score | 1.40 (1.01-1.80) | 0.007 | - | - |
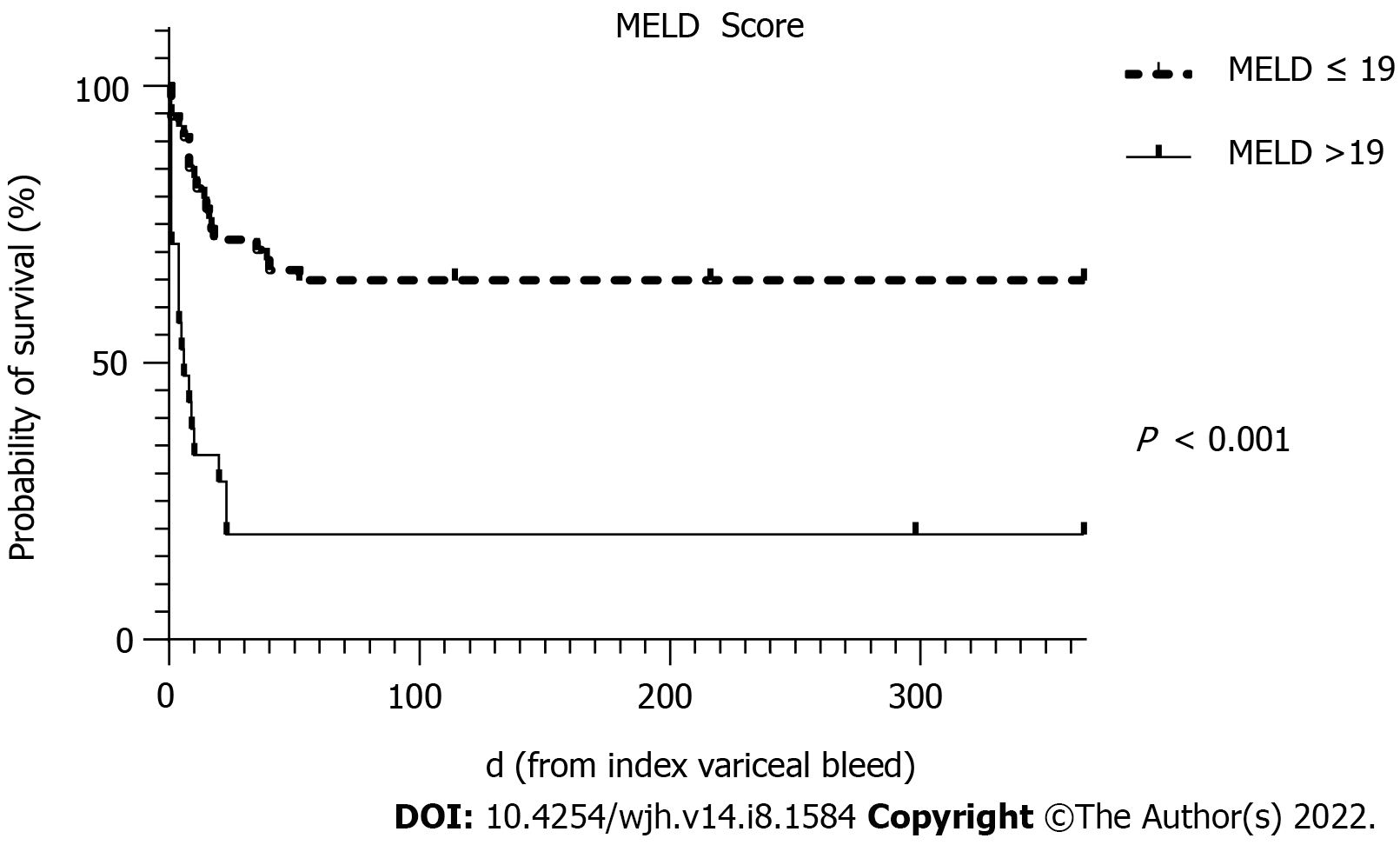
| MELD score | 1.22 (1.09-1.37) | < 0.001 | 1.21 (1.06-1.41) | 0.006 |
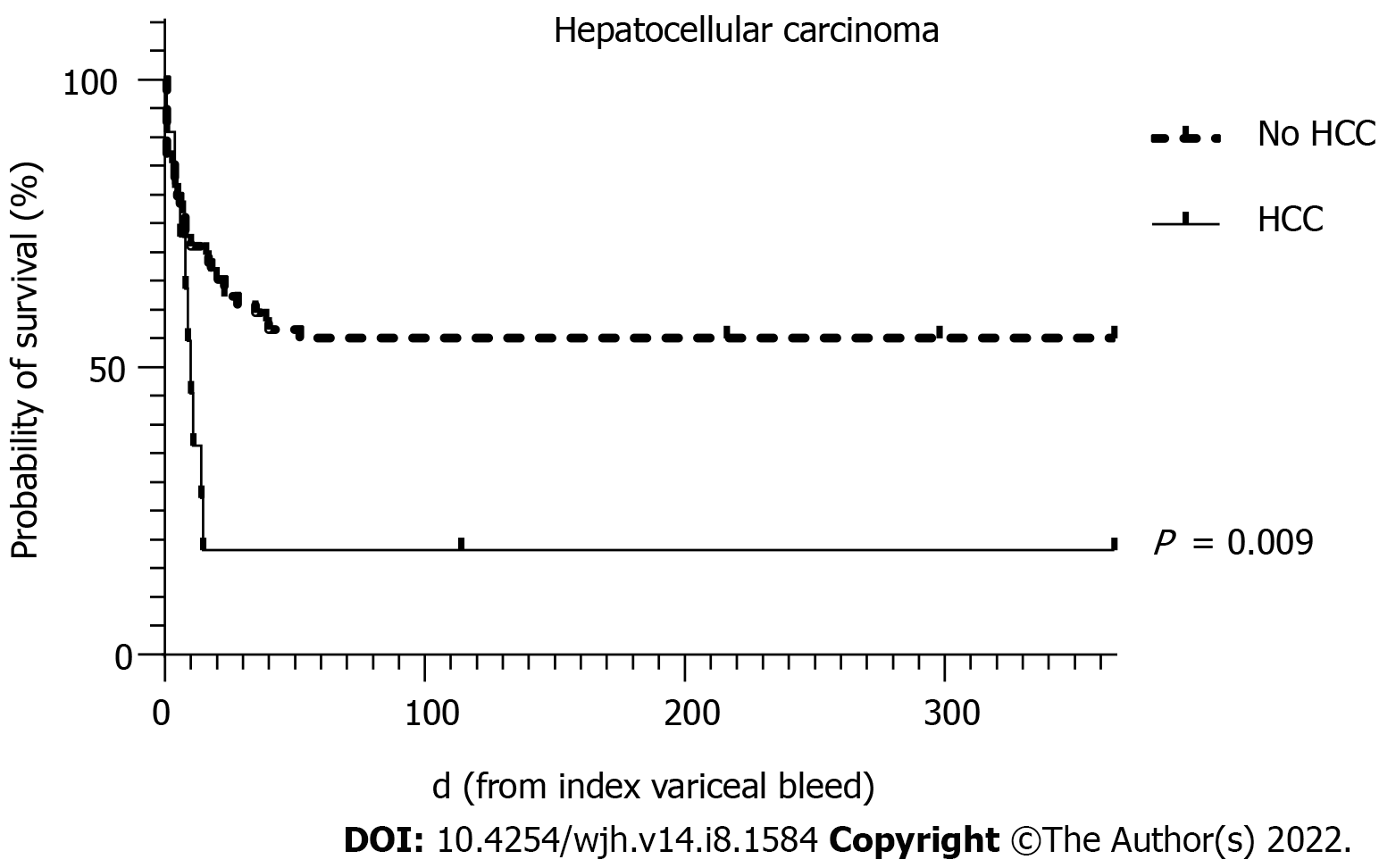
| HCC | 6.58 (1.31-32.95) | 0.022 | 11.51 (1.61-82.20) | 0.015 |
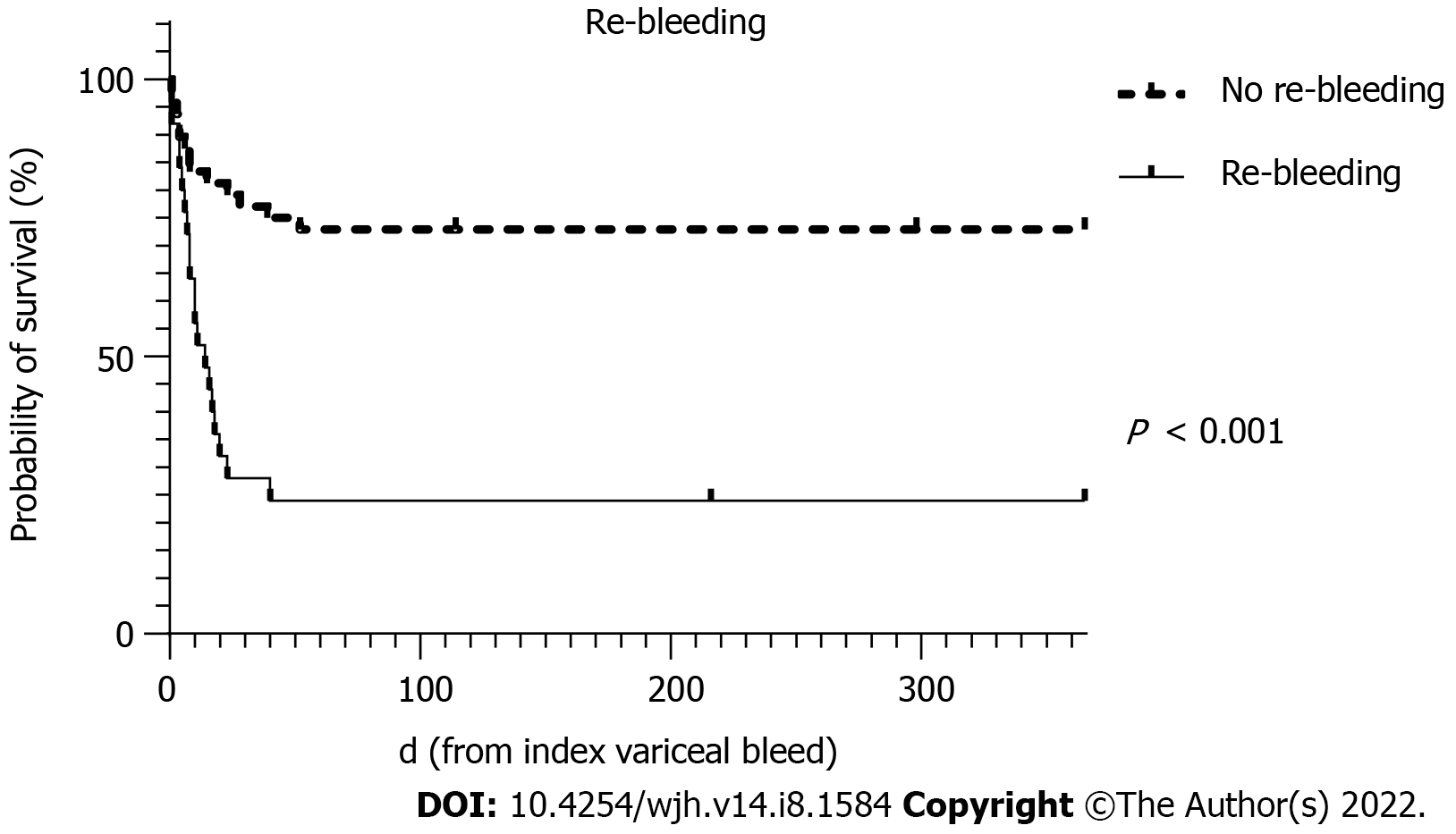
| Re-bleeding | 8.76 (2.77-27.73) | < 0.001 | 13.06 (3.06- 55.71) | < 0.001 |
| SBT complications | 1.40 (0.44-4.51) | 0.573 | - | - |
| TIPS performed | 0.30 (0.09-1.04) | 0.058 | - | - |
To avoid collinearity, the only liver disease severity indicator used in the multivariate analysis was the MELD score. MELD scores of > 19 have been shown to predict 6 wk mortality of > 20% for AVB[20]. Predictors of 6 wk mortality on multivariate analysis in this cohort showed that the MELD score, the presence of HCC and re-bleeding were statistically significant independent predictors.
The survival curves over 52 wk for MELD score >19, HCC and re-bleeding are shown in Figures 2-4, respectively.
On univariate analysis, there were no relevant predictors for re-bleeding after salvage therapy using SBT for AVB. When comparing 6 wk outcomes in those that re-bled after primary haemostasis to those that did not, re-bleeding was resulted in significantly greater mortality (76.0% vs 27.1%, P < 0.001), a longer duration of mechanical ventilation (P = 0.026) and higher transfusion requirements for packed red cells (P = 0.001) and fresh frozen plasma (P = 0.001) as shown in Table 5.
| Variable (n = 73) | No Re-bleeding (n = 48) | Re-bleeding (n = 25) | P value |
| Duration of mechanical ventilation (h) | 86.0 (46.0, 134.5) | 138.0 (79.0, 210.0) | 0.026 |
| No. of packed RBC transfused | 5 (3, 7) | 10 (5, 16) | 0.001 |
| No. of platelets transfused | 1 (0, 2) | 1 (0, 4) | 0.430 |
| No. of FFP transfused | 2 (0, 4) | 6 (2, 14) | 0.001 |
| 6 wk mortality | 13 (27.1) | 19 (76.0) | < 0.001 |
Non-serious mucosal trauma which was conservatively managed was not thought to be a significant complication in the life-threatening context of refractory AVB requiring balloon tamponade. Given that the incidence of serious complications from SBT insertion were uncommon and occurred in only 5 patients, no further analyses was performed to identify predictors.
AVB represents a life-threatening emergency in patients with liver cirrhosis and portal hypertension. However, with current treatment paradigms, 6 wk mortality has improved to 10%-15%[21]. Variceal bleeding refractory to first-line therapy requiring salvage therapy with balloon tamponade reflects a serious life-threatening condition in advanced liver disease that is associated with significant mortality. We demonstrate a 6 wk mortality rate of 48.8% in this cohort of patients despite current standards of care. Balloon tamponade with a SBT was found to be a very effective rescue therapy in refractory AVB, achieving primary haemostasis in 91.3% of patients with a low serious complication rate of 6.3%. On multivariate analysis, increasing MELD score, the presence of HCC and re-bleeding were all associated with a significantly increased odds of mortality.
This study represents one of the largest series to examine the efficacy of SBT in patients presenting with AVB treated with current standards of care; an era where nearly all patients routinely receive vasoactive therapy and IV antibiotics, timely access to emergency endoscopic therapies and access to early TIPS. Balloon tamponade now represents a rescue therapy utilised in the 10-20% of patients with AVB in whom haemostasis cannot be achieved with vasoactive therapy and endoscopic techniques such as EVBL. Our 6 wk mortality rate of 48.8% is comparable to other modern cohorts at 41%-60%[4,13,14]. In comparison with older cohorts from 1970-1980s with pooled 30-day to 6 wk mortality rates of 32.5%[1], the modern studies counterintuitively demonstrate a higher mortality rate. However, the historical cohorts often used balloon tamponade as a first-line treatment option and thus the cohorts are not readily comparable. Interestingly, in 2017 Nadler et al[13] reported similar survival rates to our study even though the rate of TIPS performed was much higher than in our cohort at 55.9% overall (19 of 34 patients). In our cohort, only 21.3% underwent TIPS at a median of 70.8 h (IQR 34.3-97.4 h) although variability in both expertise and availability of this radiological procedure throughout the years in our health services may have existed and the proportion of patients in whom TIPS may have been contraindicated remains unclear. Consideration of early TIPS insertion is currently recommended in all Child Pugh C patients and Child Pugh B patients with active bleeding who present with AVB[22]. TIPS placement is generally performed within 72 h (but ideally within 24 h) due to a high risk of treatment failure[7]. The early re-bleeding rate of 34.2% and high associated mortality found in our cohort highlights the propensity for serious complications in patients with AVB refractory to first-line treatments. Thus, if TIPS is considered in this cohort of patients, it should ideally be performed as soon as possible after primary haemostasis is achieved while the patient remains haemodynamically stable.
This study supports previous evidence that balloon tamponade with a SBT remains very effective at achieving primary haemostasis in 91.3%. Of the 7 patients who did not achieve primary haemostasis, all had clinical evidence of ongoing bleeding despite SBT placement and rapidly deteriorated with haemodynamic instability and death within h despite maximal vasopressor and inotropic support. Apart from 1 patient where the gastric balloon was inflated to 100 mL, all others had inflation of the gastric balloon to adequate volumes (250-400 mL) with the oesophageal balloon also documented to be inflated in 2 patients. Our rates of primary haemostasis are comparable with historical larger cohorts published in the 1970 and 1980s at 90.7% and 88.5%[11,23]. However, compared to the other current studies, our rates of primary haemostasis are higher than those reported by Choi et al[14] and Escorsell et al[4] at 75.8% and 47%, respectively. Our re-bleeding rates lie between that of the 1970-1980s cohort (43%)[11] and Choi et al (22%)[14], and similarly we did not identify any significant relevant predictors for re-bleeding. We have showed that re-bleeding was also associated with greater mortality (76.1% vs 27.1%, P = 0.001) and required greater use of resources including blood products and mechanical ventilation. However, the serious complication rates of 6.3% we observed from SBT insertion was significantly lower than studies from the 1970s-1980s (approximately 32%)[1,11].
In our study, the main predictors of 6 wk mortality on univariate and multivariate analysis were similar to those previously reported for AVB in cirrhosis and largely reflect liver disease severity eg. Child-Pugh and MELD scores (and its components) or severe biochemical systemic disturbance eg. pH and lactate[14,24]. In terms of validated tools for prognostication of upper gastrointestinal bleeding, we identified that the AIMS65 score significantly predicted 6 wk mortality but not the GBS or Rockall scores. A previous study has also demonstrated superiority of the AIMS65 score over the GBS and pre-endoscopy Rockall scores[25]. In addition, we also found advanced HCC and re-bleeding independently predicted 6 wk mortality. With regards to advanced HCC, 9 of 11 patients with SBT for acute severe variceal bleeding died during the admission suggesting that the utility of this SBT in this patient subgroup needs to be considered in context of the futility of the situation, particularly as it is inevitably resource-heavy, requiring invasive monitoring and intensive care admission.
We also identified significant variability amongst several aspects of clinical practice around SBT insertion at our centres, particularly around the inflation volumes of air into the gastric and oesophageal balloons. General guidelines[3] have recommended approximately 250-400 mL insertion of air into the gastric balloon based on clinical assessment, however 20.0% used < 250 mL with several of these noting migration of the SBT on confirmation chest X-ray due to under-filling. The oesophageal balloon is generally inflated to 25-40 mmHg or approximately 150 mL however 45.5% of oesophageal balloons were inflated to < 70 mL which is likely inadequate. Varying degrees of experience are expected with SBT insertion as most centres may only encounter this situation a few times every year, and formalised training is likely beneficial to optimise survival rates by appropriate tamponade technique and to prevent complications of oesophageal perforation, which may occur with balloon migration into the oesophagus from under-filling the gastric balloon. A previous survey of United States gastroenterologists and hepatologists from the American Association for the Study of Liver Diseases found that most respondents had not received training for balloon tamponade over the last 2 years and no trainees at that time were comfortable with balloon tamponade[26].
This cohort study has certain limitations, particularly its retrospective nature and that identification of the study population relies on accurate coding. However, due to the infrequent need for this procedure, prospective data collection remains challenging. While there was variation in SBT balloon volume inflation, which may result in suboptimal use of this technique, this is the only study that attempts to provide the technical information surrounding this procedure in a real world cohort. Also, none of the patients at any of our health centres had oesophageal stents inserted for haemostasis in the study time period, which have more recently been shown to be superior to balloon tamponade[4]. Nonetheless, to our knowledge this is the largest cohort study available in the current era with most patients treated according to clinical practice guidelines. Other modern cohort studies of acute severe variceal bleeding requiring balloon tamponade remain scarce and the SBT insertion was often not performed by trained specialist gastroenterologists.
In conclusion, in the modern era of standardised medical and endoscopic therapies to treat AVB, salvage techniques such as balloon tamponade remain relevant for the time being. Overall, this condition remains associated with a high mortality of approximately 50% and although rates of primary haemostasis remain excellent, rates of re-bleeding occur in around one third of cases with high rates of subsequent death. These outcomes have not significantly changed when compared with the 1970-1980s even with improved therapies. However, rates of serious complications are low. Patients who survived the admission were likely to survive until at least 52 wk. Independent predictors for mortality include a higher MELD score, re-bleeding and advanced HCC which may assist in further stratification of at-risk individuals for either early definitive therapy with TIPS or early palliation.
Salvage treatment using balloon tamponade techniques such as Sengstaken-Blakemore tubes (SBT) represents the most severe end of the spectrum of acute variceal bleeding (AVB), where failure to achieve primary haemostasis inevitably results in death. However, few studies report on the clinical practice and outcomes of this procedure in the current era, and only include small study populations where balloon tamponade is often performed by non-specialists in the emergency department setting. This retrospective multi-centre cohort study is the largest study including 80 patients over a decade who have undergone SBT for salvage therapy performed by gastroenterologists during endoscopy in tertiary hospitals. This study provides detailed technical aspects of the SBT insertion procedure and provides insight into the success rate, clinical outcomes of patients who undergo SBT insertion for refractory AVB and predictors of mortality, re-bleeding and complications from SBT.
The main topics of this study include detailed descriptions regarding the real-world practice of SBT performed by gastroenterologists in tertiary hospitals, and the clinical outcomes and predictors of short- and long-term mortality after SBT for AVB, the success rate of balloon tamponade in achieving primary haemostasis and the rate of re-bleeding and complications arising from SBT insertion. Information regarding these topics are not currently available for the current era which significantly differs from historical cohorts from the 1970-1980s due to a very different patient population where balloon tamponade was often first-line therapy. Currently, there are clear expert opinion-based consensus guidelines using a range of medical and endoscopic therapies and definitive treatment with radiologic procedures or liver transplantation for AVB. Furthermore, performing salvage technique with SBT is highly resource-intense and thus appropriate risk stratification to optimise outcomes for patients is required.
To assess the primary outcome which was all-cause mortality of AVB requiring SBT in the short-term (6 wk) as well as long-term (52 wk) and the secondary outcomes of re-bleeding and complications after SBT insertion. The predictors of these outcomes were also analysed. These objectives were all achieved apart from the predictors of complications from SBT as serious complications were infrequent.
Due to the infrequent need to perform SBT for AVB, an appropriate method to undertake this study resulted in a multi-centre retrospective cohort study including 80 adult patients with SBT for refractory AVB from 2008 to 2019. The study population was identified using International Classification of Diseases-10 codes and clinical data was collected from medical records. Descriptive statistics, univariate and multivariate binomial regression and survival analyses were used to analyse the data collected.
SBT salvage for refractory AVB is a life-threatening condition with high mortality rates of 48.8% at 6 wk and 53.8% at 52 wk. The SBT procedure was highly successful in achieving primary haemostasis in 91.3% of patients but re-bleeding was common at 34.2% and associated with very high mortality of 76.0%. The predictors of mortality after SBT insertion included increased severity of liver disease, severe metabolic disturbance, presence of hepatocellular carcinoma (HCC) and re-bleeding. Serious complications from SBT insertion were uncommon at 6.3% and the main complications were superficial mucosal trauma without perforation which was managed conservatively. Despite this procedure being performed by specialist gastroenterologists in this study, there was still significant variation amongst technical aspects of the SBT procedure particularly amongst gastric and oesophageal balloon inflation volumes.
In the current era, SBT as a salvage therapy for refractory AVB continues to be associated with high short and long-term mortality rates. The utilisation of this temporising procedure remains relevant and is associated with high rates of primary haemostasis over 90%. As the mortality rate exceeds 75% after re-bleeding, this highlights the importance of prompt treatment with definitive therapies such as transjugular intrahepatic portosystemic shunts to optimise clinical outcomes. Furthermore, as SBT is associated with intense use of resources with even greater mortality in the presence of advanced HCC, this study suggests early palliation may be more appropriate in this futile setting.
Future directions of this research should focus on strategies to optimise the clinical outcomes for this cohort of severe refractory AVB including prevention, the use of covered self-expandable oesophageal stents and prompt transition to definitive treatments before re-bleeding occurs. Further studies into risk stratification for optimal outcomes is required as well to assist clinicians in decision making regarding whether or not salvage therapy should be performed at all.
| 1. | Rodrigues SG, Cárdenas A, Escorsell À, Bosch J. Balloon Tamponade and Esophageal Stenting for Esophageal Variceal Bleeding in Cirrhosis: A Systematic Review and Meta-analysis. Semin Liver Dis. 2019;39:178-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Sengstaken RW, Blakemore AH. Balloon tamponage for the control of hemorrhage from esophageal varices. Ann Surg. 1950;131:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Bridwell RE, Long B, Ramzy M, Gottlieb M. Balloon Tamponade for the Management of Gastrointestinal Bleeding. J Emerg Med. 2022;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Gundling F, Tiller M, Schepp W. Comment on "esophageal balloon tamponade vs esophageal stent in controlling acute refractory variceal bleeding: A multicenter RCT". Hepatology. 2017;65:2120-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, Christie JM; Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 426] [Article Influence: 38.7] [Reference Citation Analysis (2)] |
| 6. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1499] [Article Influence: 166.6] [Reference Citation Analysis (3)] |
| 7. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2355] [Article Influence: 214.1] [Reference Citation Analysis (4)] |
| 8. | Cook D, Laine L. Indications, technique, and complications of balloon tamponade for variceal gastrointestinal bleeding. J Intensive Care Med. 1992;7:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Chojkier M, Conn HO. Esophageal tamponade in the treatment of bleeding varices. A decadel progress report. Dig Dis Sci. 1980;25:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 81] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Haddock G, Garden OJ, McKee RF, Anderson JR, Carter DC. Esophageal tamponade in the management of acute variceal hemorrhage. Dig Dis Sci. 1989;34:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 11. | Panés J, Terés J, Bosch J, Rodés J. Efficacy of balloon tamponade in treatment of bleeding gastric and esophageal varices. Results in 151 consecutive episodes. Dig Dis Sci. 1988;33:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 134] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Avgerinos A, Armonis A. Balloon tamponade technique and efficacy in variceal haemorrhage. Scand J Gastroenterol Suppl. 1994;207:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Nadler J, Stankovic N, Uber A, Holmberg MJ, Sanchez LD, Wolfe RE, Chase M, Donnino MW, Cocchi MN. Outcomes in variceal hemorrhage following the use of a balloon tamponade device. Am J Emerg Med. 2017;35:1500-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Choi JY, Jo YW, Lee SS, Kim WS, Oh HW, Kim CY, Yun EY, Kim JJ, Lee JM, Kim HJ, Kim TH, Jung WT, Lee OJ, Kim RB. Outcomes of patients treated with Sengstaken-Blakemore tube for uncontrolled variceal hemorrhage. Korean J Intern Med. 2018;33:696-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2915] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 16. | Saltzman JR, Tabak YP, Hyett BH, Sun X, Travis AC, Johannes RS. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 17. | Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 709] [Article Influence: 27.3] [Reference Citation Analysis (2)] |
| 18. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 918] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 19. | Vreeburg EM, Terwee CB, Snel P, Rauws EA, Bartelsman JF, Meulen JH, Tytgat GN. Validation of the Rockall risk scoring system in upper gastrointestinal bleeding. Gut. 1999;44:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S, Berzigotti A, Ma M, Genescà J, Bosch J, García-Pagán JC, Abraldes JG. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412-19.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 288] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 21. | Amitrano L, Guardascione MA, Manguso F, Bennato R, Bove A, DeNucci C, Lombardi G, Martino R, Menchise A, Orsini L, Picascia S, Riccio E. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol. 2012;107:1872-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 864] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 23. | Terés J, Planas R, Panes J, Salmeron JM, Mas A, Bosch J, Llorente C, Viver J, Feu F, Rodés J. Vasopressin/nitroglycerin infusion vs esophageal tamponade in the treatment of acute variceal bleeding: a randomized controlled trial. Hepatology. 1990;11:964-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Bambha K, Kim WR, Pedersen R, Bida JP, Kremers WK, Kamath PS. Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut. 2008;57:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Robertson M, Majumdar A, Boyapati R, Chung W, Worland T, Terbah R, Wei J, Lontos S, Angus P, Vaughan R. Risk stratification in acute upper GI bleeding: comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems. Gastrointest Endosc. 2016;83:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Bajaj JS, Ananthakrishnan A, Saeian K. Survey of attitudes of AASLD members toward balloon tamponade. Hepatology. 2005;41:1435-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Gastroenterological Society of Australia, 100139.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen MCY, Taiwan; Pop TL, Romania S-Editor: Wang LL L-Editor: A P-Editor: Wang LL