INTRODUCTION
Advances in organ preservation techniques, perioperative care, and immunosuppression have resulted in greatly improved long-term survival in patients undergoing liver transplantation (LT). A continued assessment of the liver allograft to ensure optimal graft function is becoming increasingly important[1]. Recurrent or de novo injury is one of the most common causes of chronic hepatitis and fibrosis following LT[2,3]. However, no obvious cause can be identified in many adult recipients who have their original disease under control. The same is the case in the majority of paediatric LT recipients who have been transplanted for non-recurrent liver disease[4]. Centres which perform serial post-LT surveillance allograft biopsies have noted histological abnormalities without any clinical or biochemical dysfunction. Whether such abnormalities progress to long-term graft loss remains unknown and requires careful study[5-7].
This narrative review summarises the factors predisposing to long-term liver allograft fibrosis (LAF), highlighting the putative role of idiopathic post-LT hepatitis (IPLTH) and chronic antibody mediated rejection (CAMR) in its pathogenesis.
LONG-TERM LIVER ALLOGRAFT FIBROSIS
Allograft fibrosis is defined as the excessive accumulation of extracellular matrix proteins (including collagen) within the transplanted liver. The central event is the activation of hepatic stellate cells and portal fibroblasts in response to chronic injury[8]. When unchecked, progressive LAF inevitably leads to graft failure and loss.
Moreover, the prevalence and severity of LAF are reported to increase over time. It has been shown that 10 years after LT, normal histology is likely to be present in only 30% of patients. Data from six European transplant centres show an increasing incidence of LAF over time (54% at 5 years, 79% at 10 years)[7]. Interestingly, this phenomenon has been observed to occur more commonly in the paediatric population. Late post-transplant liver biopsies performed in this cohort of patients reveal LAF in 69% to 97% of all cases. Scheenstra et al[9] reported that the prevalence of LAF increased from 34% to 48%, 65%, and 69% among children at 1, 3, 5, and 10 years after LT, respectively.
Furthermore, apart from the incidence, the etiology and mechanism of LAF appear to be distinct between the adult and paediatric LT recipients. In adults, the original indication for LT is clearly important. Recurrent hepatitis C virus (HCV) infection is a common diagnosis and an important cause of LAF[10]. Venturi et al[11] noted a correlation between portal fibrosis and prolonged ischemic time, deceased graft, and post-transplant lymphoproliferative disease. In their study, they also highlighted biliary complications as a related factor for sinusoidal fibrosis, while vascular complications, positive autoantibodies, and high gamma-globulin level were related to centrilobular fibrosis. Immunosuppression with steroid therapy was not associated with decreased fibrosis. In a study by Rhu et al[12], liver scarring was common in patients with no clinical signs of graft dysfunction. Repeated transaminitis, positive autoantibodies, elevated gamma-glutamyl transferase, and experience of post-transplant lymphoproliferative disease were suspicious signs for fibrosis. HLA-DRB1 × 03/04 allele in LT recipients has also been shown to be significantly associated with portal fibrosis without influencing inflammation[13]. Other viral diseases including hepatitis E have been reported to cause LAF[14-19]. Furthermore, immune related indications of LT (auto-immune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, etc.) have been known to recur in the allograft, causing remarkable histological abnormalities. De novo autoimmune hepatitis has also been implicated as a causative factor in adult LAF.
On the other hand, in the paediatric population, where the great majority of transplants are carried out for non-recurring disease, changes seen in late biopsies have no obvious attributable cause apart from a chronic immune-related damage (discussed below). Hence, while in adults a recurrence of primary disease is the most common cause of late graft dysfunction, in children, unexplained idiopathic hepatitis and liver fibrosis are the main causes. Furthermore, compared to the adult population, wherein the histological abnormalities manifest as abnormal graft function, up to 90% of children who are otherwise clinically and biochemically normal will have some abnormality on protocol biopsy.
From a pathophysiological perspective, LAF is the result of sustained wound healing in response to repeated hepatocyte injury, leading to scar tissue formation and loss of hepatic architecture. Nonetheless, it is imperative to realise that the conventional concept of irreversible fibrosis has evolved and it is now considered to be a dynamic and reversible process. Hence, when the inciting injury stimulus is removed, LAF has shown to regress over time.
RISK FACTORS FOR LONG-TERM LIVER ALLOGRAFT FIBROSIS
Significant insights into the risk factors and natural history of LAF in clinically stable LT recipients have been obtained by correlating clinical and biochemical with histological findings on surveillance biopsy tissue obtained 5 and 10 years after paediatric LT[9]. LAF was strongly correlated with transplant-related factors such as prolonged cold ischemia time, young age at LT, high donor/recipient age ratio, and the use of a partial graft[9,10]. Venturi et al[11] noted a higher incidence of LAF in the presence of factors like prolonged ischemic time, deceased donor grafts, and post-transplant lymphoproliferative disease. The authors subdivided the risk factors based on the type of fibrosis. While biliary complications were more likely to result in sinusoidal fibrosis, vascular complications and high gamma-globulin levels were related to centrilobular fibrosis. Interestingly, episodes of rejection, chronic hepatitis, and the type of immunosuppression were not related to allograft scarring.
Other factors predicting a higher risk of LAF include the presence of autoantibodies with elevated immunoglobulin levels, repeated transaminitis, de novo hepatitis C infection, and hepatitis E (HEV) infection (genotype 3)[12,16]. The interplay of immunosuppressants and HEV is noteworthy. Post-LT HEV infection is usually acquired from the community. However, cases of HEV infection acquired from blood products or donor organs have also been reported[17,18]. Immunosuppression utilizing tacrolimus has been postulated as a risk factor for chronic liver disease, possibly by promoting viral replication[16]. Approximately 50%-80% of LT recipients infected with HEV develop chronic infection and 10-15% progress to diffuse scarring. Similarly, Torque Teno Virus (TTV), which is part of the normal human virome, may result in direct LAF without hepatitis[19]. HLA-DRB1 × 03/04 allele in LT recipients has also shown to be significantly associated with portal fibrosis without inflammation[12,13]. It is nonetheless sobering to realise that liver scarring is common in patients with no probable risk factors or clinical signs of graft dysfunction[12].
MECHANISMS OF LONG-TERM LIVER ALLOGRAFT FIBROSIS
It is noteworthy to consider that the paediatric immune system is quite distinctive from that of the adult population[20,21]. Depending upon the age at LT, their immune system is in various stages of development and maturation.
Multiple studies have proven that innate immunity plays a key role in the development of LAF[22]. At the cellular level, interferon (IFN)-λ stimulates LAF whereas both IFN-α/β and IFN-γ inhibit this event. Toll-like receptors (TLRs) participate in the development of fibrosis, while liver dendritic cells regulate inflammation and fibrosis in the liver microenvironment. Kupffer cells stimulate liver fibrosis whereas natural killer (NK) cells inhibit LAF by lysing activated hepatic stellate cells (HSCs) and inhibiting IFN-γ production. The imbalance between pro- and anti-fibrogenic agents is created by a common pathway which is incited by damaged hepatocytes. These cells in turn stimulate and activate HSCs by releasing damage-related reactive oxygen species and other fibrogenic substances. They also do it by recruitment of immune cells which promote cytokines and chemokines, causing further collagen deposition. This mutual stimulation between inflammation and profibrotic cells leads to a vicious circle of LAF. The two strongly associated precursor/ inciting events for ‘idiopathic’ LAF are IPLTH and CAMR which benefit from further elucidation, as below.
IDIOPATHIC POST-LIVER TRANSPLANT HEPATITIS
Identified in 5%-85% of adults and 32%-97% of children with normal liver biochemistry, IPLTH is an inclusive term for unexplained portal and/or lobular inflammatory lesions in the allograft[9,10,23-30]. These pathological features have been variedly labelled as nonspecific portal and/or lobular inflammation, unexplained hepatitis, interface hepatitis, portal lymphocytic inflammation, portal/parenchymal mononuclear inflammation, and allograft inflammation, leading to an underestimation of its true incidence[30-33]. Moreover, variations in centre-specific protocols of surveillance liver biopsies make this conundrum even more byzantine[2]. Nevertheless, there are certain pathological features which are frequently observed in IPLTH. These include predominantly mononuclear (lymphocytes, histiocytes, and some plasma cells) portal inflammatory infiltrate associated with no significant bile duct damage or portal venulitis (Figure 1)[2,34]. Variable interface activity and/or centrilobular inflammation with spotty to confluent necrosis have also been reported. Further, periportal necroinflammatory activity is generally mild, and features of T-cell mediated rejection may occasionally be observed.
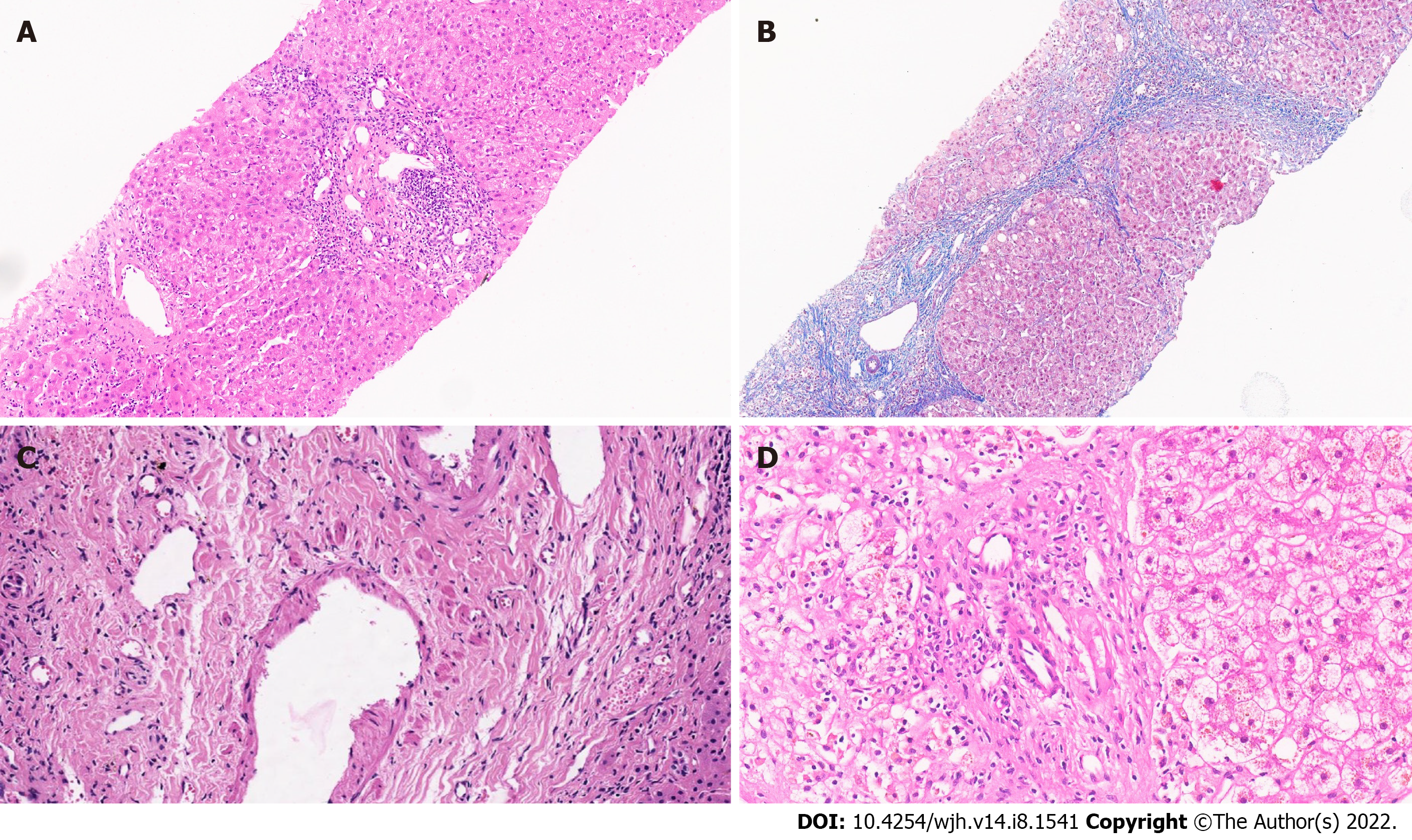
Figure 1 Liver allograft biopsy.
A: Liver allograft biopsy with portal fibrosis, portal inflammation, and mild centrilobular inflammation; (hematoxylin and eosin stain, ×10), B: Liver allograft biopsy with bridging fibrosis and portal/septal inflammation (Masson trichrome stain, ×10); C: Dense portal collagenization with portal venupathy in an allograft biopsy(hematoxylin and eosin stain, ×40); D: Bile duct loss in a portal tract. (hematoxylin and eosin stain, ×30).
Although the term “idiopathic” implies an unexplained cause, there is increasing evidence to suggest that many cases of so-called IPLTH probably represent an immune phenomenon. The majority of patients have auto/allo-antibodies and other uncharacterized serum factors which react with donor hepatocytes and/or bile ducts. Over a fourth of patients with moderate to severe portal inflammation show positive antinuclear antibodies and/or anti-smooth muscle antibodies (titres 1:40-1:640)[5]. Their history is also significant for episodes of T-cell mediated rejection and histopathology shows features of acute or chronic rejection. Furthermore, patients on a long-term maintenance dose of corticosteroids have shown lesser degrees of inflammation and fibrosis, further suggesting that the whole process is immune mediated and may represent a hepatitic form of chronic rejection[10,34-39].
Recent genomic studies shed further light on this association. By using modular analysis, Londoño et al[5] explored the correlation between groups of co-expressed genes and semi-quantitative histological scores across liver samples. Of the 23 modules of genes identified, two were selected for further analyses on the basis of their significant correlation with portal inflammation and fibrosis. A significant correlation was noted between the modules and a 13-gene set specific for T-cell mediated rejection. The two modules were enriched in gene sets previously identified as being associated with allograft rejection across a variety of experimental and clinical settings. Significantly, the majority of their patients were on a very low dose of immunosuppressants, indicating insufficient immunosuppression as a cause of the chronic hepatitis.
Prevention and an early diagnosis of IPLTH are crucial as it is implicated in causing LAF and cirrhosis[33-36]. A study based on 158 asymptomatic paediatric LT recipients followed for over 10 years showed that a significant number of those who received cyclosporine-A as primary immunosuppression with withdrawal of corticosteroids at 3 mo post-LT developed unexplained chronic hepatitis[10]. The incidence and intensity of this inflammation increased with time; 22%, 43%, and 64% at 1, 5, and 10 years, respectively, developed chronic hepatitis. Of those with chronic hepatitis, 52%, 81%, 91% at 1, 5, and 10 years, respectively, progressed to graft fibrosis. Additionally, 15% progressed to cirrhosis at 10 years[10]. In another study based on 1287 LT recipients who were followed for over a decade, almost 40% of patients with allograft cirrhosis had no identifiable etiology apart from IPLTH[36].
Other long-term follow-up series have also shown similar data with regards to incidence and progression of inflammation[5,30]. Liver biopsies in clinically well LT recipients at a median interval of 13 years from LT showed histological abnormalities in 76% of liver biopsies (35% interface hepatitis, 12% moderate to severe hepatic steatosis, 9% cirrhosis, and 8% chronic rejection). Varma et al[13] showed that when the inciting factors were removed, serial allograft biopsies showed a reduction in inflammation and fibrosis, thus suggesting that IPLTH is not a terminal and progressive phenomenon.
CHRONIC ANTIBODY MEDIATED REJECTION
Conventionally, unlike cardiac or renal transplant recipients, LT recipients were believed to have an innate resistance to antibody mediated rejection (AMR) caused by donor specific antibodies (DSA)[40,41]. More recently, several series have reported an inferior survival in patients who were DSA positive, leading to a renewed interest in its effect on liver allograft structure and function and long-term outcome[42,43].
DSAs are antibodies formed by the recipient that bind to type I and type II human leukocyte antigens (HLAs) in the donor organ, potentially resulting in allograft injury[44]. Recipients exposed to a variety of non-self HLAs may have preformed DSAs prior to LT, whereas de novo DSAs form after LT in response to the new donor organ’s HLAs[45]. Anti-HLA class I antibodies tend to appear in the early post-LT period, while anti-HLA class II antibodies (particularly anti-HLA-DQ antibodies) occur in the long-term. Non-self class II HLA molecules expressed by endothelial cells within the liver allograft are DSA targets. These get significantly upregulated by proinflammatory signals, resulting in antibody binding, crosslinking, and triggering of effector mechanisms like inflammation and fibrosis[46].
Nonetheless, the liver allograft has numerous inherent mechanisms which make it relatively resistant to AMR[6]. These include Kupffer cell-based scavenging and clearance of activated complements and immune complexes, relatively lower expression of class II DSA targets as compared to the kidney or the heart, and a large sinusoidal vascular bed which dilutes antibody-binding across a larger endothelial cell surface. Other liver based protective factors include a dual blood supply protecting the organ from ischemic damage and a high regenerative capacity which enables the liver to heal and recover from injury[45,46]. In view of these liver specific dynamics, the presence of preformed DSAs and a positive crossmatch test are not considered contraindications to LT. Moreover, many LT units do not routinely perform a DSA test or cross-match prior to LT.
Descriptions of antibody subclasses and functional tests developed to separate complement binding and complement non-binding DSAs have provided better insights into the risks of DSAs in LT recipients. This understanding of the antibody mediated response in organ-transplant recipients is greatly facilitated by the introduction of solid-phase immunoassay technology for the detection and characterization of HLA antibodies[41]. The solid-phase immunoassay or Luminex assay which uses three types of antibody panels is more sensitive than complement-dependent lymphocytotoxicity assay and flow cytometry[44]. However, as described above, the clinical relevance of anti-HLA antibodies and DSAs detected on Luminex assay have not yet been conclusively elucidated. Moreover, there also remains the inability to sift through the panel and filter out the harmful antibodies from the more innocuous ones.
The lack of specific clinical, biochemical, or pathologic features makes CAMR a challenging diagnosis. The exact incidence of CAMR remains unknown but has been believed to occur in approximately 8%-15% of recipients who retain or form de novo DSAs against HLA class II molecules (especially DQ)[46]. Evidence in this regard has mostly come from long-term adult and paediatric LT recipients who had protocol liver biopsies or achieved operational tolerance. Histological findings strongly associated with persistent DSA include low-grade portal, periportal, and perivenular lymphoplasmacytic inflammation with low-grade interface and perivenular necro-inflammatory activity[47-50]. Dense portal collagenisation and obliterative portal venopathy have also been reported[48-50]. The above features, with or without positive complement component 4d (C4d) staining, is strongly associated with CAMR[48].
Several series have also indicated a direct correlation between DSAs against HLA class II molecules and LAF. A study from Japan showed that 88% of paediatric LT recipients with stable graft function and fibrosis or cirrhosis at 5 years post-LT exhibited DSA positivity[38]. Other series from Europe and Japan strongly associate the presence of de novo HLA antibodies to class II antigens (DSAs and non-DSAs) with CAMR, inflammation, and LAF[50-54]. Potential patho-mechanisms linking DSAs to LAF include destruction of microvasculature, non-microvascular antibody-dependent cell mediated cytotoxicity, activation of endothelial and stellate cells and portal myofibroblasts, and complement mediated chemotaxis[47,51,52].
Given the persistent ambiguity which hinders an unequivocal diagnosis of CAMR, a scoring system to better elucidate the features of CAMR has been proposed for “putative CAMR”[48]. The score is based on interface activity, lobular inflammation, portal tract collagenization, portal venopathy, presence of positive circulating DSAs, sinusoidal fibrosis, and HCV status. Nevertheless, it must be borne in mind that CAMR is a diagnosis of exclusion and other potentially confounding causes like recurrent disease and viral pathology need exclusion[55,56].
METRICS FOR LIVER ALLOGRAFT FIBROSIS
Irrespective of the etiology, there remains the need to qualify and quantify LAF. A scoring system for LAF allows for a reliable diagnosis, timely intervention, assessment of treatment efficacy, prognostication, and peer comparison. Currently available metrics for LAF have been adapted from those used for chronic hepatitis and, therefore, lack predictive power. A semiquantitative fibrosis scoring system specifically adapted to assess LAF has been proposed, which objectively defines portal, sinusoidal, and centrilobar fibrosis, providing a good representation of the whole hepatic acinus[57].Immunohistochemical assessment of alpha smooth muscle actin on graft biopsies have also been proposed as a modality, wherein a positive area percentage of over 1.05 predicted with a 90% specificity an increased risk of fibrosis on subsequent biospsies[8,58].
OPERATIONAL TOLERANCE AND LIVER ALLOGRAFT FIBROSIS: THE EQUIPOISE
An immunosuppression-free life remains the ultimate goal of transplantation. Allograft tolerance can be realised by immunological dampening and inhibition of the rejection response. True tolerance occurs when there is no demonstrable immunological response against the liver graft and is a rare event in transplantation[46]. Nonetheless, graft acceptance with minimal immunosuppression referred to as ‘prope tolerance’ can often be achieved in long-term post-LT survivors. Operational tolerance (OT) defined as the absence of rejection, and graft survival with normal function and histology in an immunosuppression-free, fully immunocompetent host on the other hand can be potentially achieved in up to 20% of well selected LT recipients[59]. It is important to realize that the current characterisation of CAMR resulted from such attempts at withdrawing immunosuppression[39]. The 2016 Banff update discussed pathological findings predictive of successful immunosuppression withdrawal and provided a guarded view on immunosuppression withdrawl[46].
Most evidence on immunosuppression withdrawal is anecdotal, retrospective, or lack a control-cohort. There also remains the undisputable fact that most protocol graft biopsies reveal sub-clinical histological damage. Furthermore, as detailed above, the risks of inadequate immunosuppression far outweigh the small potential for success. It is also crucial to note that OT is not a permanent stable state, but a dynamic one. Serial protocol liver biopsies are one way of evaluating OT, allowing for resumption of immunosuppression if there is injury to the graft. There always remains the need for immunological surveillance to ensure continued good graft histology and function. The lack of available, well-defined immune monitoring to detect immunoregulation or unresponsive states leads to an inability to objectively predict those who can successfully achieve OT. The key nonetheless, is the development of immune biomarkers which can reliably foretell the possibility of achieving OT, and at the same time predict the likelihood of its failure.
CONCLUSION
The incidence of allograft hepatitis and fibrosis continues to increase in long-term LT recipients and liver histology remains the only definite way to confirm these findings. There is emerging evidence that some of the graft fibrosis could be driven by inflammation, antibody mediated rejection, or even genetic predisposition. Protocol biopsies can identify cases of early allograft fibrosis, which then can potentially be reversed with optimised immunosuppression. Achieving OT remains the ultimate immunological goal of LT. However, in light of long-term sub-clinical immunological injury to liver grafts, this enthusiasm needs to be tempered. A prudent approach would be to base this decision on reliably predictive immune biomarkers.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Xu X, China; Zhou Y, China S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL