Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1421
Peer-review started: March 23, 2022
First decision: April 28, 2022
Revised: May 13, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: July 27, 2022
Processing time: 126 Days and 0 Hours
Platelet transfusion in acute variceal bleeding (AVB) is recommended by few guidelines and is common in routine clinical practice, even though the effect of thrombocytopenia and platelet transfusion on the outcomes of AVB is unclear.
To determine how platelet counts, platelets transfusions, and fresh frozen plasma transfusions affect the outcomes of AVB in cirrhosis patients in terms of bleeding control, rebleeding, and mortality.
Prospectively maintained database was used to analyze the outcomes of cirrhosis patients who presented with AVB. The outcomes were assessed as the risk of rebleeding at days 5 and 42, and risk of death at day 42, considering the platelet counts and platelet transfusion. Propensity score matching (PSM) was used to compare the outcomes in those who received platelet transfusion. Statistical comparisons were done using Kaplan-Meier curves with log-rank tests and Cox-proportional hazard model for rebleeding and for 42-d mortality.
The study included 913 patients, with 83.5% men, median age 45 years, and Model for End-stage Liver Disease score 14.7. Platelet count < 20 × 109/L, 20-50 × 109/L, and > 50 × 109/L were found in 23 (2.5%), 168 (18.4%), and 722 (79.1%) patients, respectively. Rebleeding rates were similar between the three platelet groups on days 5 and 42 (13%, 6.5%, and 4.7%, respectively, on days 5, P = 0.150; and 21.7%, 17.3%, and 14.4%, respectively, on days 42, P = 0.433). At day 42, the mortality rates for the three platelet groups were also similar (13.0%, 23.2%, and 17.2%, respectively, P = 0.153). On PSM analysis patients receiving platelets transfusions (n = 89) had significantly higher rebleeding rates on day 5 (14.6% vs 4.5%; P = 0.039) and day 42 (32.6% vs 15.7%; P = 0.014), compared to those who didn't. The mortality rates were also higher among patients receiving platelets (25.8% vs 23.6%; P = 0.862), although the difference was not significant. On multivariate analysis, platelet transfusion and not platelet count, was independently associated with 42-d rebleeding. Hepatic encephalopathy was independently associated with 42-d mortality.
Thrombocytopenia had no effect on rebleeding rates or mortality in cirrhosis patients with AVB; however, platelet transfusion increased rebleeding on days 5 and 42, with a higher but non-significant effect on mortality.
Core Tip: This is a retrospective study to assess the impact of thrombocytopenia at presentation and that of platelet transfusion in the management of acute variceal bleeding in patients with chronic liver disease. Ten percent of patients received platelet transfusions and were found to have significantly higher rebleed rates on day 5 and 42 after the index bleeding episode but did not result in significantly higher mortality rates in these patients. On multivariate analysis, platelet transfusion was an independent risk factor for 42-d rebleeding, while hepatic encephalopathy was a significant risk factor for 42-d mortality.
- Citation: Biswas S, Vaishnav M, Pathak P, Gunjan D, Mahapatra SJ, Kedia S, Rout G, Thakur B, Nayak B, Kumar R, Shalimar. Effect of thrombocytopenia and platelet transfusion on outcomes of acute variceal bleeding in patients with chronic liver disease. World J Hepatol 2022; 14(7): 1421-1437
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1421.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1421
Patients with cirrhosis are conventionally considered to be at a greater risk of bleeding than healthy controls due to “cirrhotic coagulopathy”, characterized by thrombocytopenia and deranged proth
Up to 15% of patients with cirrhosis experience an episode of variceal bleeding each year[4]. Thrombocytopenia is common in patients with cirrhosis. Severe thrombocytopenia (defined as platelets < 50 × 109/L) may be associated with an increased risk of procedural bleeding[5,6]. Several studies have demonstrated a lack of predictive value of platelet count for procedure-related bleeding in cirrhotics[7,8]. The impact of thrombocytopenia on the severity of acute variceal bleeding (AVB) is unclear. Prior studies have demonstrated that platelet counts greater than 56 × 109/L are required to control variceal bleeding, resulting in several clinical guidelines to advocate platelet transfusion for the control of bleeding[9,10]. However, neither of these studies were prospective controlled clinical trials, and the fact that patients undergoing liver transplantation (which is arguably one of the most invasive procedures a cirrhotic can undergo) show higher rates of hepatic arterial or venous thrombosis with increased use of platelet or fresh frozen plasma (FFP), casts doubt over the guiding principles advocating platelet transfusion[7,8]. Despite several major guidelines advocating against the use of platelets, the decision is largely empirical and based on local practices in a real-world clinical setting. Transfusion practices regarding the use of FFP are clearer, with a recent retrospective cohort study demonstrating the potential harm of FFP transfusion in patients with AVB[11]. Prophylactic blood product transfusion is common in clinical practice, as reported in various studies[12,13]. The current study aimed to determine how platelet counts, platelets transfusions, and FFP transfusions affect the outcomes of AVB in cirrhosis patients in terms of bleeding control, rebleeding, and mortality.
The study comprised cirrhosis patients with AVB who presented to the All India Institute of Medical Sciences, New Delhi, India, a tertiary care center. A prospectively managed database was used to include patients diagnosed with bleeding from esophageal or fundal varices on esophagogastroduodenoscopy (EGD) between October 2017 and October 2021. AVB was defined on EGD by visible spurt, white nipple, or signs of recent hemorrhage. Patients with variceal bleeding not associated with liver cirrhosis, such as non-cirrhotic portal fibrosis, extrahepatic portal venous obstruction, splenic vein thrombosis with chronic pancreatitis etc., were excluded, as were patients with non-variceal hemat
Ethical clearance was obtained from the institutional ethics committee (IECPG). Some of the patients were also part of a TEG-based transfusion trial (CTRI/2017/02/007864)[14] and secondary prophylaxis of gastric varices (CTRI/2021/02/031396).
Baseline treatment included resuscitation and airway management. Following resuscitation, patients were transfused packed red blood cells (based on existing guidelines) targeting a hemoglobin level of 7 gm/dL in cirrhotics without cardiac dysfunction and 10 gm/dL in patients with cardiac comorbidities. Inotropes were initiated in patients with shock to maintain a mean arterial pressure of 65-70 mmHg. Mechanical ventilation indications included respiratory failure or airway protection prior to EGD. All patients received prophylactic antibiotics and vasoactive therapy with somatostatin/terlipressin prior to EGD, which was performed within 12 h of presentation to the hospital. The vasoactive agents were continued until day 3 of admission. The patients were initiated on non-selective beta-blockers, such as carvedilol or propranolol, with doses titrated according to heart rate/or blood pressure. The decision for transfusion of blood products (FFP, platelets) was taken by the treating team in the emergency department or as part of the randomized controlled trial[14]. The decision for repeat endoscopy, balloon-occluded retrograde transvenous obliteration (BRTO) or rescue transjugular intrahepatic portosystemic shunt (TIPS) was taken by the treating team based on the patient's clinical condition.
Baseline demographic, hematologic, and biochemical parameters were collected. Child-Turcotte-Pugh (CTP) and Model for End-stage Liver Disease (MELD) scores were calculated on admission. The details of type and units of blood products transfused (FFP/platelet and PRBCs) were noted from the patient's chart. Requirements of rescue therapies: TIPS, Sengstaken-Blakemore tube (SB tube), self-expanding Ella Danis stent (SX-Ella Danis) or BRTO were noted.
Rebleeding or failure of therapy was defined as per the Baveno V consensus as follows[15]: (1) Death within 120 h; (2) Fresh hematemesis or nasogastric aspiration of 100 mL of fresh blood 2 h after starting a specific drug treatment or therapeutic endoscopy; (3) Development of hypovolemic shock; and (4) A 3g drop in hemoglobin (equivalent to a 9% drop in hematocrit) within any 24 h if no transfusion is administered
The primary outcome of the study was the rebleeding at days 5 and 42, and death at day 42 after an episode of AVB in the 3 platelet groups. We also analyzed the differences in the rebleeding and death rates between those who received platelet transfusions and those who did not. Propensity score matching was done to compare the outcomes in those who received and did not receive platelet transfusion. The secondary outcomes were rebleeding at days 5 and 42, and death at day 42, after an episode of AVB in patients receiving FFP alone or in combination with platelet transfusion. In addition, we assessed the risk factors for rebleeding and death on day 42.
The normality of the data was assessed using the Shapiro-Wilk test. Skewed continuous variables were expressed as median [interquartile range (IQR)], and non-skewed as mean (sd). The qualitative data were expressed as numbers (%). Kruskal–Wallis test was used to compare more than two groups with non-parametric data. Comparison of categorical variables was made using the Fisher’s exact test or Pearson’s chi-squared test. For statistical evaluation, patients were further classified into three groups based on platelet counts of < 20 × 109/L, 20 × 109-50 × 109/L, and > 50 × 109/L. Survival analysis and rebleeding at 5 and 42 d stratified as per the platelet counts and transfusion of blood products were performed using Kaplan-Meier and compared with the log-rank test. Mortality and rebleeding were used as endpoints, and patients were censored at last patient contact. Univariate and multivariate Cox-proportional model regression analysis was done to assess the predictors of rebleeding and mortality at 42 d. Effect sizes for the identified predictors were reported as hazard ratio with 95% confidence interval. A P value of 0.05 was considered statistically significant. The data were analyzed using IBM SPSS Statistics software (version 20.0, Chicago, IL, United States) and Medcalc software (version 15.11.4, MedCalc Software, Ostend, Belgium)
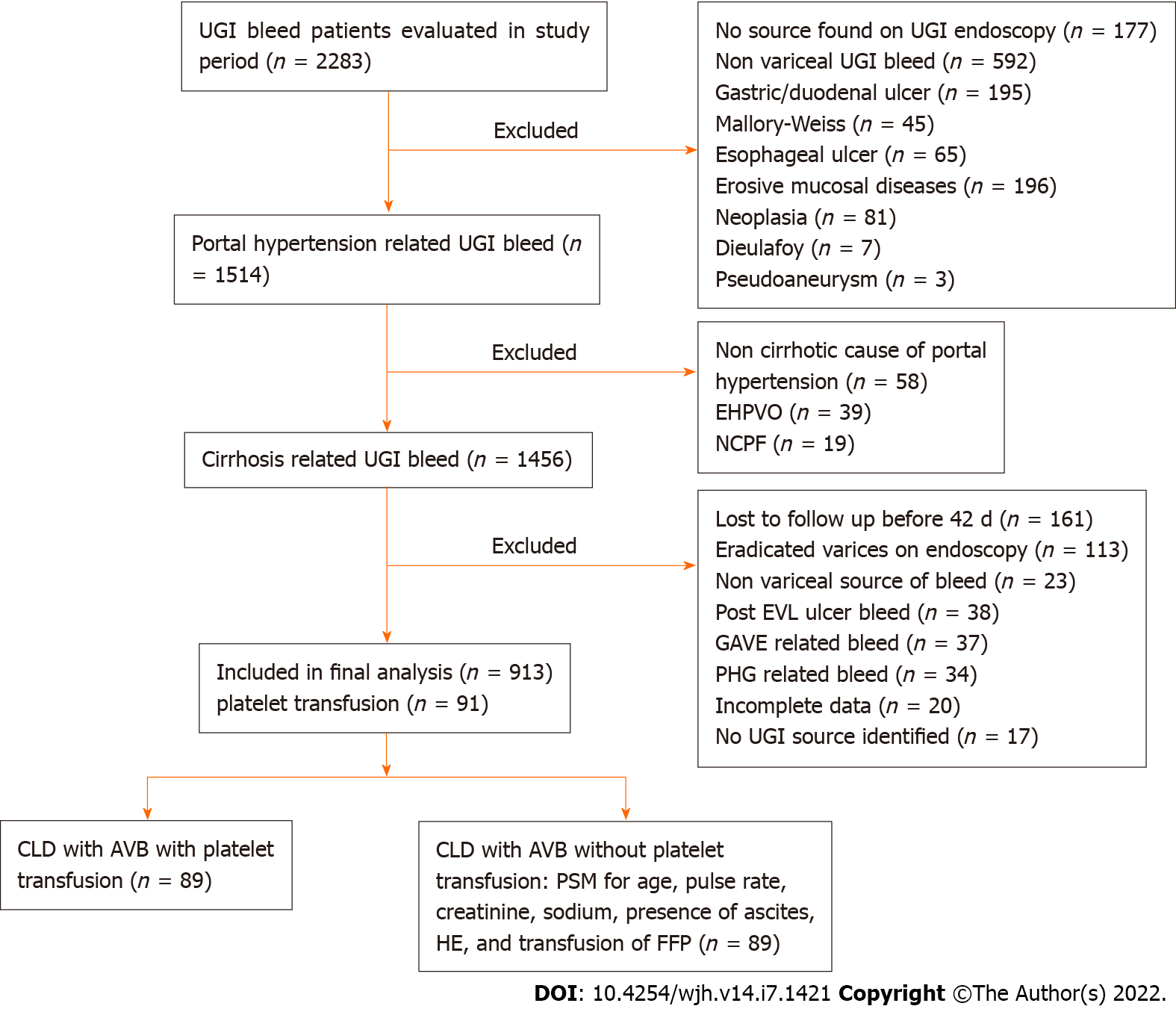
A total of 913 cirrhosis patients with AVB comprising 762 males (83.5%) and 151 females (16.5%) were enrolled (Figure 1). The median age of the patients’ cohort was 45 years (35-54), and their median MELD and CTP score were 14.7 (11.1-20.3) and 7 (6-9), respectively. At the time of presentation, the median hemoglobin level was 7.6 gm/dL (6.1-9.4 gm/dL), and platelet counts were 96 × 109/L (55 × 109-135 × 109/L). The number of patients in each of the three groups based on platelet counts < 20 × 109L, 20 × 109-50 × 109/L and > 50 × 109/L were 23 (2.5%), 168 (18.4%), and 722 (79.1%), respectively. The most common feature of decompensation was ascites in 456 patients (49.9%), followed by hepatic encephalopathy (HE) in 93 patients (10.2%). The most common etiology of cirrhosis was chronic alcohol use in 393 cases (43%). Endotherapy was offered to 711 patients (77.9%), and rebleeding was observed in 48 patients (5.3%) at 5 d and 138 patients (15.1%) at 42 d. Radiological interventions for management of rebleed were done in 17 (1.9%) patients and included TIPS in 8, BRTO in 3, SB tube in 2 and SX-Ella Danis stent placement in 4 patients (Table 1). The overall 42-d mortality rate was found to be 18.2% (n = 166).
| Characteristics | Total (n = 913) | Platelet count < 20 × 109/L (n = 23) | Platelet count 20-50 × 109/L (n = 168) | Platelet count > 50 × 109/L (n = 722) | P value |
| Age (years) | 45 (35-54) | 42.0 (33-46) | 43 (34-53) | 45 (36-54) | 0.068 |
| Sex (Males:Female) | 762 (83.5): 151 (16.5) | 20 (87.0): 3 (13.0) | 136 (81.0): 32 (19.0) | 606 (83.9): 116 (16.1) | 0.581 |
| Heart rate (per minute) | 96 (86-110) | 94 (86-100) | 94 (85-110) | 96 (86-110) | 0.397 |
| MAP (mm of Hg) | 82 (74-89) | 81 (74-84) | 81 (75-88) | 82 (73-89) | 0.771 |
| Hemoglobin (g/dl) | 7.6 (6.1-9.4) | 8.3 (5.7-9.6) | 7.4 (6.0-8.7) | 7.8 (6.1-9.5) | 0.168 |
| TLC (×109/L) | 6.5 (3.8-9.2) | 6.9 (3.7-9.6) | 5.1 (3.1-7.9) | 6.8 (4.2-9.7) | < 0.001b |
| Platelet count (×109/L) | 96 (55-135) | 12.0 (10.0-15.0) | 40.0 (34.0-46.0) | 118.0 (80.0-150.0) | < 0.001b,c |
| INR | 1.5 (1.3-1.9) | 1.7 (1.3-2.0) | 1.6 (1.3-1.9) | 1.5 (1.3-1.9) | 0.337 |
| Serum urea (mg/dL) | 37 (24-64) | 45 (22-101) | 36 (23-55) | 37 (25-66) | 0.298 |
| Creatinine (mg/dL) | 0.8 (0.6-1.2) | 1.1 (0.7-2.0) | 0.8 (0.6-1.1) | 0.8 (0.6-1.3) | 0.010a |
| Sodium (meq/L) | 139 (135-142) | 137 (131-141) | 140 (136-143) | 139 (135-142) | 0.065 |
| Bilirubin (mg/dL) | 1.6 (0.9-3.1) | 2.1 (1.0-7.7) | 1.7 (0.9-3.4) | 1.6 (0.9-2.9) | 0.317 |
| AST (IU/L) | 51 (34-86) | 49.0 (35.0-103.0) | 56.0 (38.0-82.0) | 50.0 (33.0-87.0) | 0.410 |
| ALT (IU/L) | 35 (23-55) | 36.0 (23.0-120.0) | 37.0 (24.0-56.0) | 34.0 (22.0-54.0) | 0.500 |
| Albumin (g/dL) | 3.2 (2.7-3.8) | 2.8 (2.1-3.7) | 3.1 (2.7-3.8) | 3.2 (2.7-3.8) | 0.146 |
| CTP | 7 (6-9) | 8.0 (7.0-10.0) | 7.0 (6.0-10.0) | 7.0 (6.0-9.0) | 0.044c |
| MELD scores | 14.7 (11.1-20.3) | 17.2 (10.0-28.3) | 14.4 (11.3-19.6) | 14.8 (11.1-20.3) | 0.551 |
| Ascites | 456 (49.9) | 12 (52.2) | 97 (57.7) | 347 (48.1) | 0.076 |
| HCC | 35 (3.8) | 0 | 8 (4.8) | 27 (3.7) | 0.515 |
| HE | 93 (10.2) | 2 (8.7%) | 22 (13.1) | 69 (9.6) | 0.382 |
| Endotherapy | 0.815 | ||||
| No therapy | 202 (22.1) | 7 (30.4) | 34 (20.2) | 161 (22.3) | |
| Glue | 105 (11.5) | 1 (4.3) | 19 (11.2) | 85 (11.8) | |
| Ethoxysclerol | 43 (4.7) | 2 (8.7) | 6 (3.6) | 35 (4.8) | |
| EVL | 537 (58.8) | 12 (52.2) | 102 (60.7) | 423 (58.6) | |
| APC | 2 (0.2) | 0 | 1 (0.6) | 1 (0.1) | |
| Glue and EVL | 24 (2.6) | 1 (4.3) | 6 (3.6) | 17 (2.4) | |
| Child Class | 0.047 | ||||
| A | 374 (41.0) | 5 (21.7) | 65 (38.7) | 304 (42.1) | |
| B | 361 (39.5) | 10 (43.5) | 61 (36.3) | 290 (40.2) | |
| C | 178 (19.5) | 8 (34.8) | 42 (25.0) | 128 (17.7) | |
| Etiology | 0.772 | ||||
| Alcohol | 393 (43.0) | 9 (39.1) | 76 (45.2) | 308 (42.7) | |
| Others | 520 (57.0) | 14 (60.9) | 92 (54.8) | 414 (57.3) | |
| RBC | 0.548 | ||||
| 0 | 542 (59.4) | 12 (52.2) | 91 (54.2) | 439 (60.8) | |
| 1 | 143(15.7) | 4 (17.4) | 29 (17.3) | 110 (15.2) | |
| ≥2 | 228 (25.0) | 7 (30.4) | 48 (28.6) | 173 (24.0) | |
| FFP transfusion | 108 (11.8) | 3 (13.0) | 23 (13.7) | 82 (11.4) | 0.689 |
| Number of FFP transfusion | 3 (3-4) | 3 (3-3) | 3 (3-4) | 3 (3-4) | 0.728 |
| Platelets transfusion | 91 (10.0) | 10 (43.5) | 53 (31.5) | 28 (3.9) | < 0.001a |
| Number of platelet transfusion | 3 (3-3) | 3 (2.7-3.2) | 3 (3-3) | 3 (3-3.7) | 0.728 |
| Rescue therapy (Radiological intervention) | 17 (1.9) | 2 (8.7) | 7 (4.2) | 8 (1.1) | 0.001a |
| Grade of varices low:high | 128 (14.0): 785 (86.0) | 4 (17.4): 19 (82.6) | 24 (14.3): 144 (85.7) | 100 (13.9): 622 (86.1) | 0.885 |
| Cause of bleed variceal | 0.898 | ||||
| Esophageal | 789 (86.4) | 21 (91.3) | 148 (88.1) | 620 (85.9) | |
| Fundal | 55 (6.0) | 1 (4.3) | 9 (5.4) | 45 (6.2) | |
| Esophageal and Fundal | 69 (7.6) | 1 (4.3) | 11 (6.5) | 57 (7.9) |
Demographic and vital parameters were well matched across the three groups. All groups had similar values of hemoglobin and INR. Patients with platelet counts < 20 × 109/L had significantly higher creatinine values at baseline as compared to the group with platelet count between 20-50 × 109/L (1.1 mg/dL vs 0.8 mg/dL, P < 0.001), however, there were no significant differences with the other two groups in terms of etiology of cirrhosis, liver related parameters, hepatocellular carcinoma at presentation, or features of decompensation (Ascites, HE). There were no differences in baseline MELD scores; however, the median CTP score was lower in the group with platelet counts > 50 × 109/L than those with platelet count < 20 × 109/L (7 vs 8, P = 0.044) (Table 1).
Among patients with platelet counts less than 20 x 109/L, 20-50 × 109/L and greater than 50 × 109/L, 10 (43.5%), 53 (31.5%) and 28 (3.9%) patients received platelet transfusion, respectively (P < 0.001). There were no significant differences in the source of bleeding, which was most commonly from high-grade esophageal varices, the requirement of PRBC or FFP transfusion, endotherapy offered, rebleeding rates at 5 and 42 d, or mortality at 42 d among the three groups when analyzed for baseline platelet counts (Table 2, Figure 2A and B).
| Characteristics | Total (n = 913) | Platelet count < 20 × 109/L (n = 23) | Platelet count 20-50 × 109/L (n = 168) | Platelet count > 50 × 109/L (n = 722) | P value |
| Rebleed at 5 d | 48 (5.3) | 3 (13.0) | 11 (6.5) | 34 (4.7) | 0.150 |
| Rebleed at 42 d | 138 (15.1) | 5 (21.7) | 29 (17.3) | 104 (14.4) | 0.433 |
| Death at 42 d | 166 (18.2) | 3 (13.0) | 39 (23.2) | 124 (17.2) | 0.153 |
On comparison of patients who underwent endotherapy vs no endotherapy, there was no difference in the rebleed at 5 d [36/711 (5.1%) vs 12/202 (5.9%), P = 0.595] and 42 d [102/711 (14.3%) vs 36/202 (17.8%), P = 0.223].
Ninety-one (10%) patients received platelet transfusions as a part of management, while 822 patients did not. There was a significant difference in age between the groups receiving platelets compared to those who did not (median age 42 vs 45 years, P = 0.012). As expected, platelet counts were significantly lower in the group receiving platelets than the non-receiving group with the median value 40 × 109/L vs 100 × 109/L, (P < 0.001). These patients also had lower median heart rate (90/min vs 96/min, P = 0.016), total leucocyte counts (5.6 × 109/L vs 6.6 × 109/L, P = 0.012) and serum creatinine (0.7 mg/dL vs 0.8 mg/dL, P = 0.003) than their counterparts (Table 3). There were no significant differences noted in the etiology of cirrhosis, alcohol use, liver-related parameters, CTP scores and MELD score, although patients who received platelets were more likely to present with ascites (64.8% vs 48.3%, P = 0.003) and HE (16.5% vs 9.5%, P = 0.044) than those who did not.
| Before PSM analysis | After PSM analysis | |||||
| Characteristics | Platelets transfusion (n = 91) | No platelets transfusion (n = 822) | P value | Platelet transfusion (n = 89) | No platelet transfusion (n = 89) | P value |
| Age (yr) | 42 (34-50) | 45 (35-54) | 0.012 | 42 (34-50) | 40 (30-50) | 0.716 |
| Sex (Male:Female) | 77 (84.6): 14 (15.4) | 685 (83.3): 137 (16.7) | 0.882 | 75 (84.3): 14 (15.7) | 80 (89.9): 9 (10.1) | 0.372 |
| Heart rate (per minute) | 90 (84-100) | 96 (86-110) | 0.016 | 90 (85-100) | 89 (82-100) | 0.546 |
| MAP (mm of Hg) | 81 (75-87) | 82 (74-90) | 0.341 | 81 (75-87) | 81 (73-88) | 0.968 |
| Hemoglobin (g/dL) | 7.7 (6.1-9.4) | 7.6 (6.1-9.4) | 0.890 | 7.7 (6.1-9.4) | 7.5 (6.3-9.0) | 0.720 |
| TLC (× 109/L) | 5.6 (3.1-8.3) | 6.6 (4.0-9.4) | 0.012 | 5.6 (3.1-8.3) | 7.0 (4.4-12.0) | 0.002 |
| Platelet count (× 109/L) | 40.0 (32.0-58.0) | 100.0 (63.0-139.0) | < 0.001 | 40.0 (32.0-58.0) | 81 (57-126) | < 0.001 |
| INR | 1.6 (1.3-2.0) | 1.5 (1.3-1.9) | 0.266 | 1.6 (1.3-2.0) | 1.7 (1.4-2.2) | 0.402 |
| Serum urea (mg/dL) | 41 (28-60) | 36 (24-64) | 0.864 | 41 (28-61) | 34 (24-69) | 0.369 |
| Creatinine (mg/dL) | 0.7 (0.5-1.0) | 0.8 (0.6-1.3) | 0.003 | 0.7 (0.5-1.0) | 0.8 (0.6-1.4) | 0.040 |
| Sodium (meq/L) | 140.2 (137.0-143.0) | 139.0 (135.0-142.0) | 0.023 | 140 (137-143) | 140 (135-143) | 0.529 |
| Bilirubin (mg/dL) | 1.7 (0.9-3.8) | 1.6 (0.9-3.0) | 0.771 | 1.7 (0.9-3.8) | 2.4 (1.3-4.8) | 0.071 |
| AST (IU/L) | 49 (34-79) | 51 (34-88) | 0.570 | 49 (34-79) | 67 (38-119) | 0.019 |
| ALT (IU/L) | 32 (22-58) | 35 (23-54) | 0.905 | 32 (22-58) | 41 (30-67) | 0.029 |
| Albumin (g/dL) | 3.2 (2.7-3.8) | 3.2 (2.7-3.8) | 0.897 | 3.2 (2.7-3.8) | 3.1 (2.6-3.6) | 0.355 |
| CTP | 7 (6-10) | 7 (6-9) | 0.119 | 8 (6-10) | 8 (6-10) | 0.186 |
| MELD | 14.6 (10.9-20.2) | 14.7 (11.1-20.3) | 0.878 | 14.6 (10.9-20.2) | 16.1 (12.5-24.1) | 0.079 |
| Ascites | 59 (64.8) | 397 (48.3) | 0.003 | 59 (66.3) | 58 (65.2) | 1.000 |
| HCC | 6 (6.6) | 29 (3.5) | 0.150 | 6 (6.7) | 4 (4.5) | 0.747 |
| HE | 15 (16.5) | 78 (9.5) | 0.044 | 15 (16.9) | 22 (24.7) | 0.268 |
| Endotherapy (yes) | 71 (78.0) | 640 (77.9) | 1.000 | 72 (80.9) | 71 (79.8) | 1.000 |
| Child class | 0.210 | 0.313 | ||||
| A | 33 (36.3) | 341 (41.5) | 31 (34.8) | 22 (24.7) | ||
| B | 34 (37.4) | 327 (39.8) | 34 (38.2) | 37 (41.6) | ||
| C | 24 (26.4) | 154 (18.7) | 24 (27.0) | 30 (33.7) | ||
| Etiology | 0.824 | 0.176 | ||||
| Alcohol | 38 (41.8) | 355 (43.2) | 37 (41.6) | 47 (52.8) | ||
| Other | 53 (58.2) | 467 (56.8) | 52 (58.4) | 42 (47.2) | ||
| RBC | 0.483 | 0.294 | ||||
| 0 | 49 (53.8) | 493 (60.0) | 48 (53.9) | 56 (62.9) | ||
| 1 | 15 (16.5) | 128 (15.6) | 14 (15.7) | 15 (16.9) | ||
| ≥ 2 | 27 (29.7) | 201 (24.5) | 27 (30.3) | 18 (20.2) | ||
| FFP transfusion | 22 (24.2) | 86 (10.5) | < 0.001 | 22 (24.7) | 22 (24.7) | 1.000 |
| Grade of varices low:high | 71 (78.0) | 714 (86.9) | 0.026 | 69 (77.5) | 84 (94.4) | 0.002 |
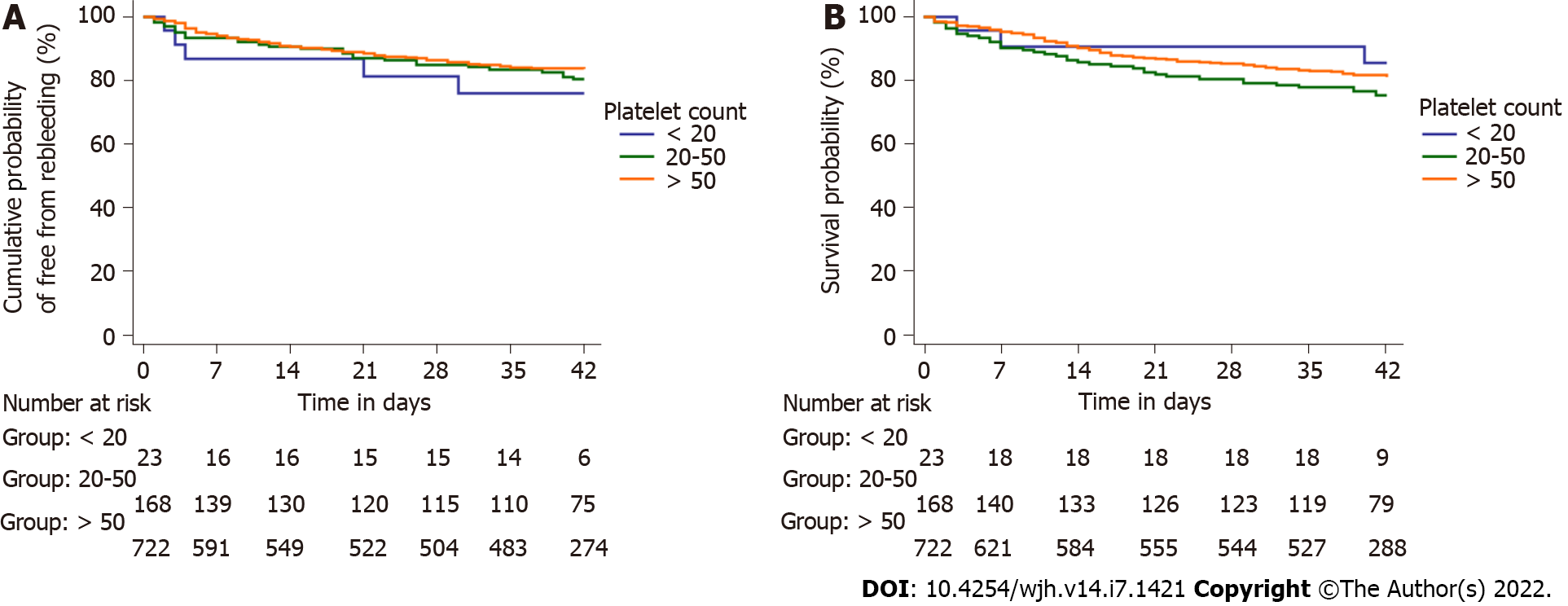
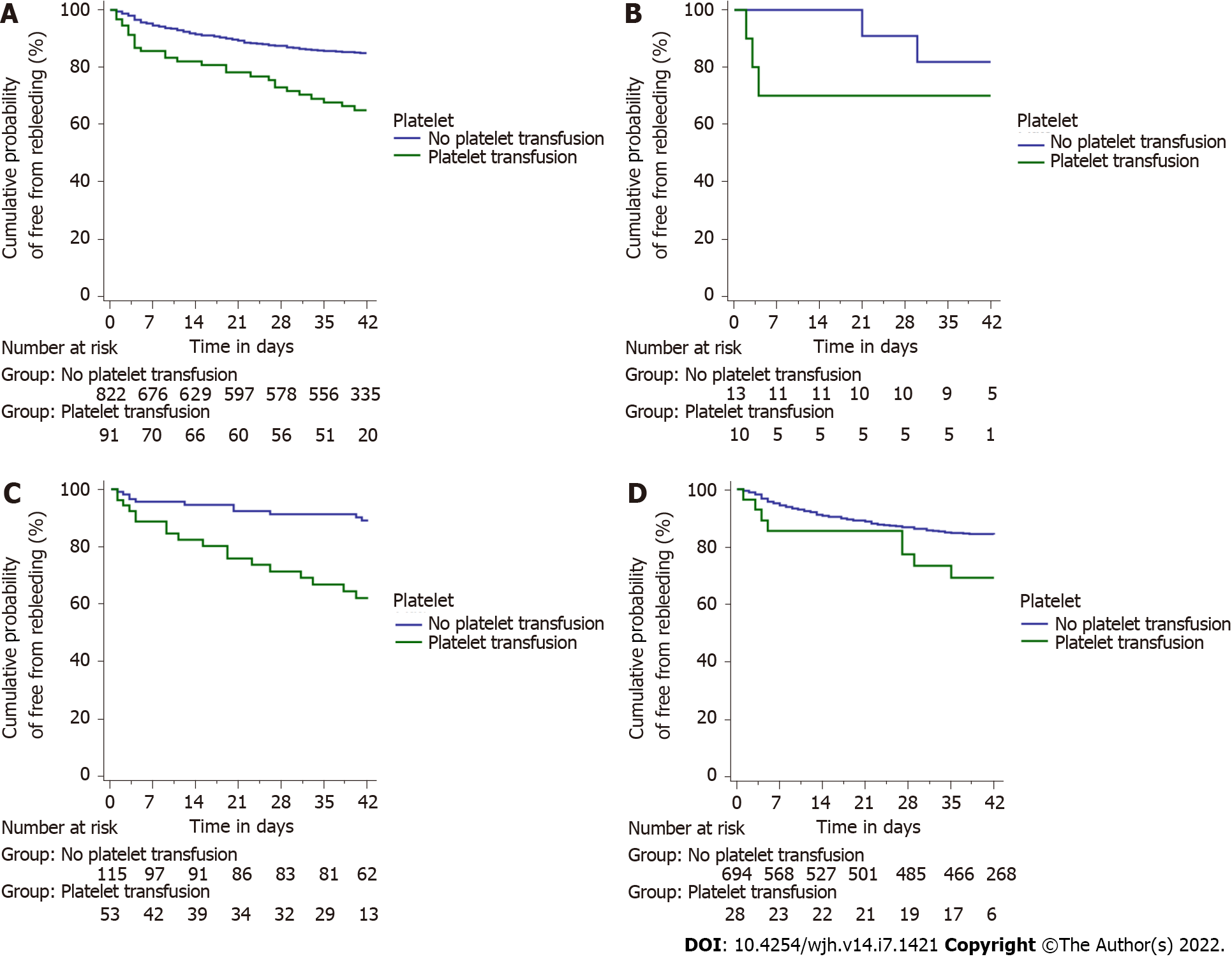
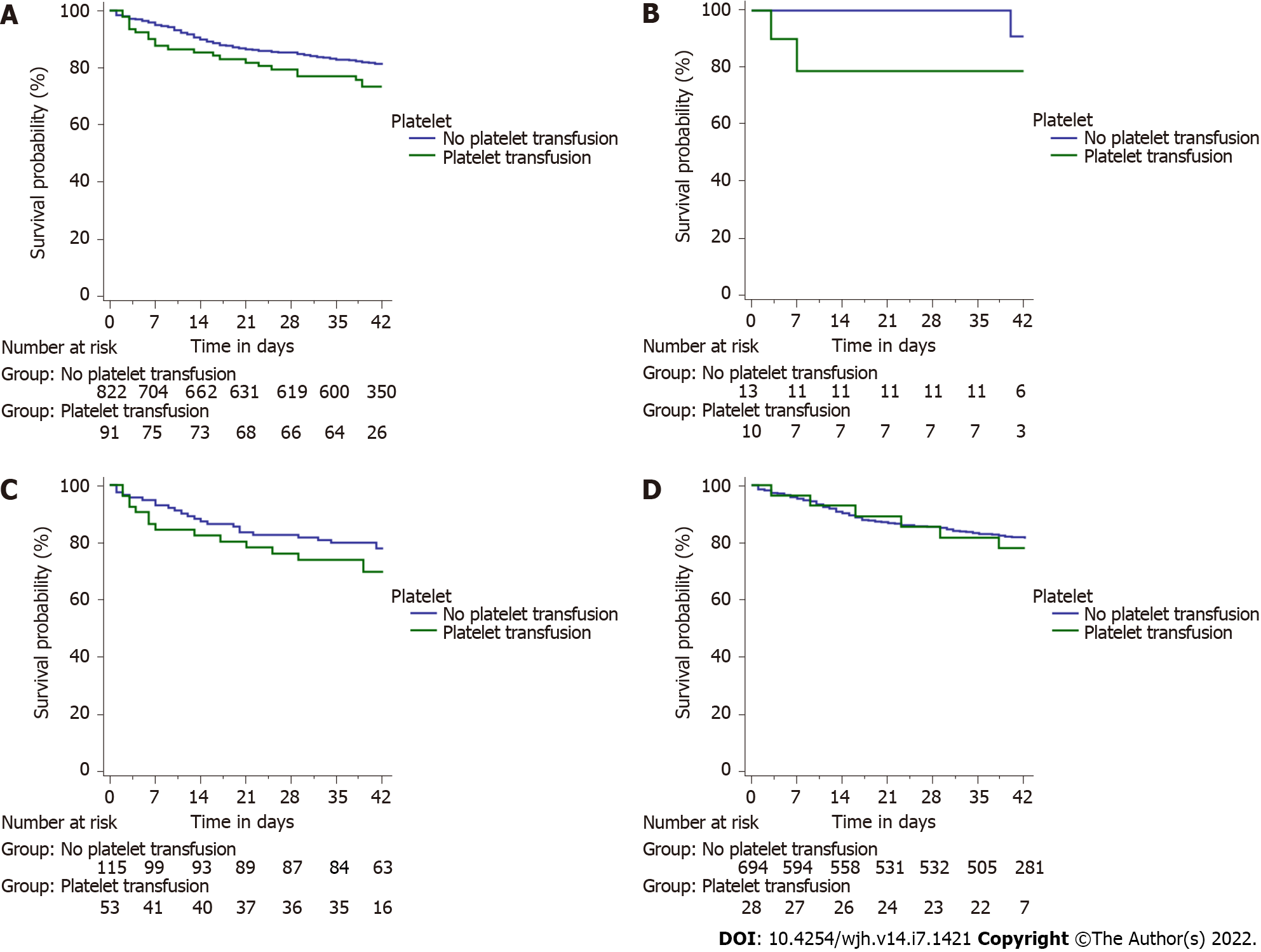
The most common bleeding source in either group was high-grade esophageal varices (84.6% and 86.6%, respectively). There was no difference in endotherapy rates offered to patients in either group. Patients receiving platelets had significantly higher rebleeding rates at day 5, 13/91 (14.3%) as compared to those who did not 35/822 (4.3%) (P < 0.001). The rate of rebleeding among those receiving platelets was even higher 29/91 (31.9%) at day 42 as compared to those who did not 109/822 (13.3%) (P < 0.001) (Figure 3A). Patients who received transfusions had a significantly greater rate of rebleeding in the groups with platelet counts between 20 × 109/L and 50 × 109/L (log-rank P < 0.001) and > 50 × 109/L (log-rank P = 0.038), but not in the group with platelet count < 20 × 109/L (log-rank P = 0.303) (Figure 3B-D). Patients receiving platelets had higher mortality rates overall 23/91 (25.3%) as compared to those who did not 143/822 (17.4%), although the difference was not significant (P = 0.074) (Figure 4A). There were no significant differences in mortality rates when assessed for group-wise outcomes (Figure 4B-D).
To compare the outcomes in those who received and those who did not receive platelet transfusion, we matched the 2 groups for variables such as age, heart rate, creatinine, sodium, presence of ascites, HE, and transfusion of FFP. The comparison of the 2 groups is shown in Table 3.
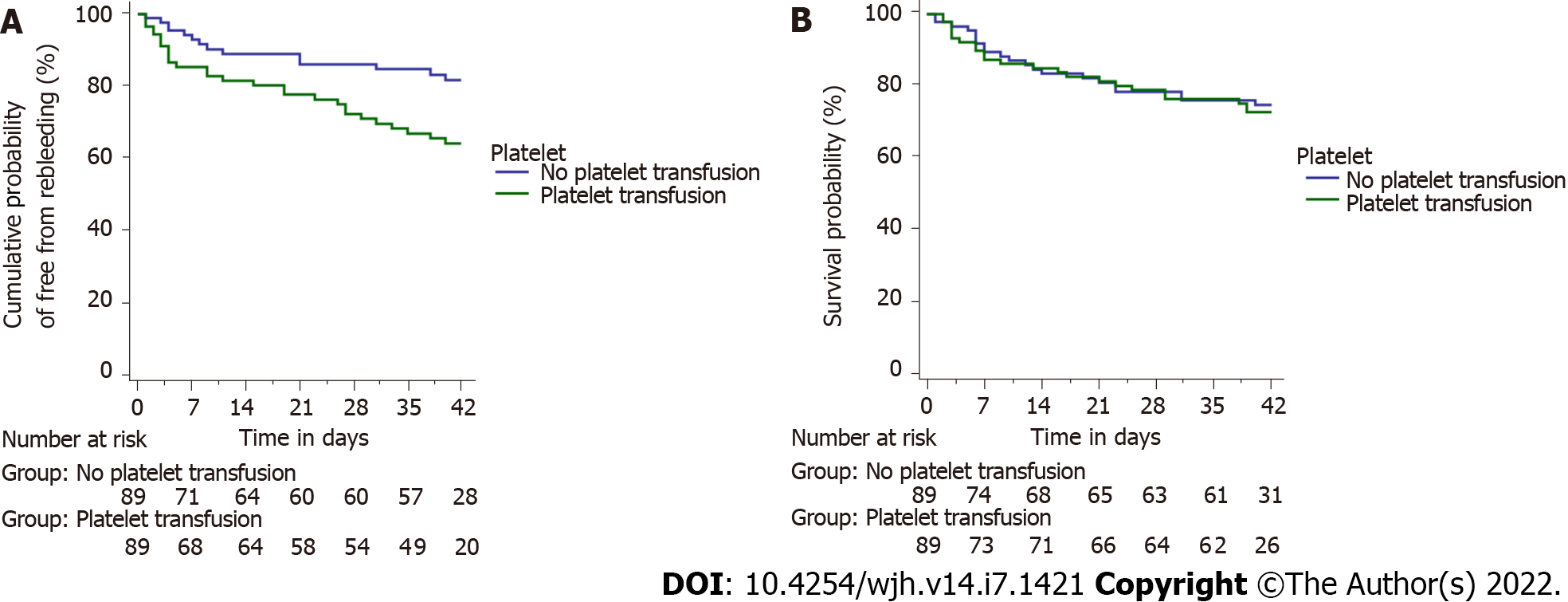
In the matched cohort (n = 89), patients receiving platelets had significantly higher rebleeding rates at day 5, 13/89 (14.6%) as compared to those who did not 4/89 (4.5%) (P = 0.039). The rate of rebleeding among those receiving platelets was even higher 29/89 (32.6%) at day 42 as compared to those who did not 14/89 (15.7%) (P = 0.014) (Figure 5A). Patients receiving platelets had higher mortality rates overall 23/89 (25.8%) as compared to those who did not 21/89 (23.6%), although the difference was not significant (P = 0.862) (Figure 5B).
In the pre-matched group, univariate Cox-proportional hazard analysis identified lower mean arterial pressure (MAP) at presentation, elevated levels of INR, serum urea, serum bilirubin, and AST to be associated with a significantly higher risk of rebleeding at 42 d. Patients with higher CTP and MELD scores, those presenting with decompensation in the form of ascites and HE, and those receiving PRBCs, FFP or platelets transfusions were at a higher risk of experiencing a rebleed within 42 d of the index event. Platelet count at presentation was not associated with rebleeding at 42 d. The Hazard ratio of the relevant risk factors is provided in Table 4.
| Whole cohort | After propensity score matching | |||||||
| Characteristics | Univariate analysis HR (95%CI) | P value | Univariate analysis HR (95%CI) | P value | Model 1 (Excluding CTP) | Model 2 (Including CTP) | ||
| Adjusted HR (95%CI) | P value | Adjusted HR (95%CI) | P value | |||||
| Age (yr) | 1.000 (0.987-1.013) | 0.973 | 1.007 (0.984-1.031) | 0.584 | ||||
| Sex | ||||||||
| Male | 1 | 1 | ||||||
| Female | 0.662 (0.393-1.114) | 0.120 | 1.330 (0.560-3.158) | 0.517 | ||||
| Heart rate (per minute) | 0.995 (0.986-1.004) | 0.298 | 1.011 (0.996-1.026) | 0.157 | ||||
| MAP (mm of Hg) | 0.979 (0.965-0.992) | 0.002 | 0.966 (0.938-0.996) | 0.024 | 9.972 (0.938-1.008) | 0.131 | 0.968 (0.936-1.001) | 0.057 |
| Hemoglobin (g/dL) | 0.969 (0.906-1.036) | 0.349 | 0.914 (0.800-1.045) | 0.189 | ||||
| TLC (× 109/L) | 1.023 (0.995-1.053) | 0.111 | 0.991 (0.933-1.052) | 0.761 | ||||
| Platelet count (× 109/L) | 0.999 (0.997-1.001) | 0.360 | 0.995 (0.989-1.001) | 0.129 | ||||
| INR | 1.528 (1.296-1.802) | < 0.001 | 1.427 (0.976-2.086) | 0.067 | 1.341 (0.784-2.295) | 0.284 | ||
| Serum urea (mg/dL) | 1.005 (1.001-1.008) | 0.012 | 1.004 (0.997-1.011) | 0.237 | ||||
| Creatinine (mg/dL) | 1.158 (1.021-1.314) | 0.023 | 1.031 (0.805-1.319) | 0.811 | ||||
| Sodium (meq/L) | 0.993 (0.966-1.022) | 0.647 | 0.980 (0.929-1.033) | 0.451 | ||||
| Bilirubin (mg/dL) | 1.065 (1.045-1.086) | < 0.001 | 1.016 (0.976-1.058) | 0.434 | ||||
| AST (IU/L) | 1.001 (1.000-1.001) | 0.005 | 1.001 (1.000-1.002) | 0.002 | 1.001 (1.000-1.002) | 0.284 | 1.001 (1.000-1.002) | 0.021 |
| ALT (IU/L) | 1.000 (0.998-1.002) | 0.920 | 1.000 (0.998-1.003) | 0.761 | ||||
| Albumin (g/dL) | 0.833 (0.672-1.031) | 0.093 | 0.543 (0.363-0.811) | 0.003 | 0.690 (0.431-1.105) | 0.122 | ||
| CTP | 1.255 (1.177-1.337) | < 0.001 | 1.169 (1.048-1.303) | 0.005 | - | 1.081 (0.959-1.220) | 0.203 | |
| MELD | 1.057 (1.038-1.077) | < 0.001 | 1.028 (0.995-1.063) | 0.102 | - | |||
| Ascites, yes | 2.525 (1.757-3.630) | < 0.001 | 1.906 (0.939-3.870) | 0.074 | 0.857 (0.376-1.953) | 0.713 | ||
| HCC, yes | 2.532 (1.367-4.690) | 0.003 | 0.370 (0.051-2.687) | 0.326 | ||||
| HE, yes | 3.969 (2.700-5.836) | < 0.001 | 2.489 (1.324-4.679) | 0.005 | 1.791 (0.836-3.836) | 0.134 | ||
| Endotherapy (yes) | 0.702 (0.480-1.027) | 0.069 | 0.999 (0.463-2.155) | 0.998 | ||||
| Child class | ||||||||
| A | 1 | 1 | ||||||
| B | 1.849 (1.187-2.879) | 0.007 | 1.811 (0.738-4.444) | 0.195 | ||||
| C | 4.653 (2.988-7.245) | < 0.001 | 3.695 (1.567-8.715) | 0.003 | ||||
| Etiology | ||||||||
| Alcohol | 1 | 0.095 | 1 | 0.124 | ||||
| Other | 0.753 (0.539-1.051) | 0.622 (0.339-1.140) | ||||||
| RBC (units) | ||||||||
| 0 | 1 | 1 | 1 | 1 | ||||
| 1 | 1.482 (0.944-2.327) | 0.087 | 1.162 (0.464-2.910) | 0.748 | 1.173 (0.460-2.992) | 0.738 | 1.253 (0.493-3.180) | 0.636 |
| ≥ 2 | 1.434 (0.979-2.098) | 0.064 | 2.571 (1.349-4.902) | 0.004 | 1.998 (0.962-4.152) | 0.064 | 1.900 (0.942-3.831) | 0.073 |
| FFP transfusion | 3.078 (2.096-4.518) | < 0.001 | 1.490 (0.777-2.858) | 0.220 | ||||
| Platelet transfusion | 2.613 (1.735-3.936) | < 0.001 | 2.204 (1.165-4.172) | 0.015 | 2.924 (1.448-5.903) | 0.003 | 2.702 (1.345-5.429) | 0.005 |
| Grade of varices (high) | 0.829 (0.526-1.308) | 0.421 | 0.671 (0.311-1.446) | 0.308 | ||||
On PSM-analysis, the factors significant on univariate Cox-proportional hazard analysis are shown in Table 3. On multivariate analysis, platelet transfusion was independently associated with 42-d rebleeding (HR, 2.924, 95%CI, 1.448-5.903, P = 0.003) after adjusting for MAP, INR, AST, albumin, HE, and PRBC transfusion. In another multivariate model, platelet transfusion was also independently associated with 42-d rebleeding after adjusting for CTP score and other significant variables (Table 4).
The factors associated with 42-d mortality on univariate Cox-proportional hazard analysis are shown in Table 5. Platelet count/platelet transfusion was not associated with 42-d mortality in the PSM cohort. Presence of HE was independently associated with mortality after adjusting for INR, creatinine, bilirubin, AST, albumin, presence of ascites, endotherapy, etiology of chronic liver disease, and FFP transfusion.
| Whole cohort | After propensity score matching | |||||
| Characteristics | Univariate analysis HR (95%CI) | P value | Univariate analysis HR (95%CI) | P value | Adjusted HR (95%CI) | P value |
| Age (yr) | 1.010 (0.998-1.022) | 0.118 | 1.016 (0.993-1.039) | 0.174 | ||
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | 0.682 (0.427-1.088) | 0.108 | 0.889 (0.350-2.256) | 0.804 | ||
| Heart rate (per minute) | 0.999 (0.990-1.007) | 0.759 | 1.004 (0.989-1.021) | 0.581 | ||
| MAP (mm of Hg) | 0.988 (0.976-1.000) | 0.046 | 0.996 (0.967-1.027) | 0.816 | ||
| Hemoglobin (g/dL) | 0.949 (0.892-1.010) | 0.100 | 0.992 (0.872-1.128) | 0.903 | ||
| TLC (× 109/L) | 1.047 (1.023-1.071) | < 0.001 | 1.034 (0.986-1.084) | 0.172 | ||
| Platelet count (× 109/L) | 0.998 (0.995-1.000) | 0.053 | 0.998 (0.992-1.003) | 0.422 | ||
| INR | 1.903 (1.689-2.143) | < 0.001 | 1.656 (1.246-2.201) | 0.001 | 1.361 (0.825-2.244) | 0.228 |
| Serum urea (mg/dL) | 1.009 (1.006-1.012) | < 0.001 | 1.005 (0.998-1.012) | 0.142 | ||
| Creatinine (mg/dL) | 1.374 (1.263-1.495) | < 0.001 | 1.205 (1.004-1.446) | 0.046 | 0.985 (0.771-1.258) | 0.901 |
| Sodium (meq/L) | 0.991 (0.966-1.017) | 0.494 | 0.996 (0.943-1.052) | 0.876 | ||
| Bilirubin (mg/dL) | 1.077 (1.061-1.094) | < 0.001 | 1.040 (1.010-1.072) | 0.010 | 1.013 (0.967-1.061) | 0.588 |
| AST (IU/L) | 1.001 (1.001-1.002) | < 0.001 | 1.001 (1.001-1.002) | < 0.001 | 1.001 (1.000-1.002) | 0.113 |
| ALT (IU/L) | 1.002 (1.001-1.002) | < 0.001 | 1.001 (0.999-1.003) | 0.249 | ||
| Albumin (g/dL) | 0.671 (0.548-0.821) | < 0.001 | 0.641 (0.433-0.948) | 0.026 | 0.964 (0.619-1.501) | 0.871 |
| CTP | 1.369 (1.294-1.448) | < 0.001 | 1.239 (1.114-1.378) | < 0.001 | ||
| MELD | 1.097 (1.080-1.115) | < 0.001 | 1.060 (1.029-1.091) | < 0.001 | ||
| Ascites, yes | 2.673 (1.911-3.739) | < 0.001 | 1.876 (0.926-3.799) | 0.080 | 1.043 (0.431-2.525) | 0.925 |
| HCC, yes | 1.637 (0.836-3.206) | 0.150 | 1.258 (0.390-4.063) | 0.701 | ||
| HE, yes | 5.686 (4.102-7.881) | < 0.001 | 3.825 (2.014-6.953) | < 0.001 | 2.586 (1.260-5.307) | 0.010 |
| Endotherapy, yes | 0.548 (0.394-0.760) | < 0.001 | 0.423 (0.226-0.790) | 0.007 | 0.589 (0.296-1.169) | 0.130 |
| Child class | ||||||
| A | 1 | 1 | ||||
| B | 1.771 (1.142-2.747) | 0.011 | 1.002 (0.395-2.538) | 0.997 | ||
| C | 6.785 (4.502-10.227) | < 0.001 | 3.759 (1.698-8.321) | 0.001 | ||
| Etiology | ||||||
| Alcohol | 1 | 1 | 1 | |||
| Other | 0.641 (0.473-0.870) | 0.004 | 0.600 (0.329-1.094) | 0.096 | 0.920 (0.470-1.799) | 0.808 |
| RBC | ||||||
| 0 | 1 | 1 | ||||
| 1 | 1 0.966 (0.629-1.484) | 0.874 | 1.158 (0.522-2.567) | 0.718 | ||
| ≥ 2 | 0.741 (0.505-1.086 | 0.125 | 1.024 (0.504-2.081) | 0.948 | ||
| FFP transfusion | 2.532 (1.762-3.637) | < 0.001 | 1.923 (1.040-3.555) | 0.037 | 1.066 (0.510-2.230) | 0.865 |
| Platelet transfusion | 1.489 (0.958-2.312) | 0.077 | 1.098 (0.608-1.984) | 0.757 | ||
| Grade of varices (high) | 1.348 (0.826-2.198) | 0.232 | 0.880 (0.392-1.975) | 0.757 | ||
Patients were also assessed for FFP transfusions received as part of management (details appended as Supplementary data). Patients who received FFP had significantly higher PRBC requirements (61.1% vs 37.9%; P < 0.001), with significantly more patients experiencing rebleed on day 5 (16.7% vs 3.7%; P < 0.001) and day 42 (32.4% vs 12.8%; P < 0.001) with higher mortality rates within 42 d of index bleeding (35.2% vs 15.9%; P < 0.001), as compared to those who did not receive transfusion (Supplemen
Kaplan Meier estimates revealed significantly higher rebleed rates at days 5 and 42 and higher 42-d mortality from index bleeding episode (P < 0.001) among patients who received FFP transfusions compared to those who did not (Supplementary Figure 1A and B).
A further subgroup analysis was done to assess outcomes of 177 patients who received either blood product (FFP or platelet) compared to 736 patients who received no transfusions (Supple
Cirrhosis-related coagulopathy is a topic of long-standing debate. Clinically, some patients demonstrate increased bleeding rates with invasive procedures. In contrast, others may develop spontaneous thrombosis of the main portal vein or its tributaries, indicating that the coagulation system in cirrhotics behaves differently in individual patients, demonstrating both pro- and anticoagulant tendencies[16-18]. Thus, coagulopathy in cirrhosis exists as a spectrum (“rebalanced hemostasis”) with anticoagulant and procoagulant nature being the two extreme endpoints. Recent evidence supports this approach to the management of bleeding risks in such patients[19].
Transfusion of blood products in cirrhotics is associated with several risks despite the apparent clinical benefits of correcting thrombocytopenia and deranged INR[20]. Prior studies have demonstrated rise portal pressures by 1.4 ± 0.7 mm of Hg for every 100 mL of blood product transfusion[21,22]. Overzealous resuscitative measures may predispose patients to a vicious cycle of rebleeding with higher transfusion requirements, extended hospital stays and poorer outcomes. This was demonstrated in the study by Villanueva et al[23], who reported that a restrictive transfusion strategy is beneficial in cirrhotics as compared to a more liberal transfusion strategy.
There is a significant discrepancy between recommendations of major societies and actual clinical practice regarding transfusions in cirrhotics. A recent study from a tertiary healthcare center in India revealed that 40.5% of cirrhotics admitted over a 6 mo period for various indications received transfusions, 82.8% of which were prophylactic[13]. The American Gastroenterology Association (AGA, 2019), European Association for the Study of the Liver (EASL, 2018, 2022) and the American Association for the Study of Liver Diseases (AASLD, 2016) recommend against the use of FFP for prophylactic correction of deranged PT/INR levels during AVB[24-28]. The AGA 2019 guidelines suggest that platelets may be transfused to a target of 50 × 109/L based on low level of evidence while the other major societies (including the recent Baveno VII guidelines) cite insufficient evidence for recommending for or against transfusion of platelets in cirrhotics with AVB[24,28]. Studies have shown that platelet and FFP transfusion may increase procoagulant factor levels, endogenous thrombin potential and platelet counts in hemodynamically stable patients. However, the actual need for these transfusions and the clinical benefit during an episode of AVB remains uncertain[29]. Evidence for transfusion to correct thrombocytopenia is drawn from studies of prophylactic platelet transfusion to limit elective procedure related bleeding in CLD patients[30-32]. There is also a lot of scepticism associated with FFP transfusion in these patients based on the results of the retrospective study of 244 patients by Mohanty et al[11] which reported more severe episodes of bleeding along with higher rebleed rates at day 5, longer hospital stay and higher mortality at 42 d among 100 patients with AVB who received FFP. Even for patients undergoing prophylactic EVL of varices, higher rates of post EVL bleed were associated with advanced liver disease and not baseline INR or platelets as reported by Blasi et al[33] Thus baseline thrombocytopenia or deranged INR do not lead to higher post EVL bleeding rates in a prophylactic or emergent setting and attempting to correct it with transfusions may lead to more harm than good.
In our study, we identified 913 patients with cirrhosis experiencing AVB. Eighty percent of the study population were either Child-Pugh class A (374) or B (361). At baseline, 191 patients (20.9%) had a platelet count below 50 × 109/L, with 23 patients (2.5%) having platelets less than 20 × 109/L. There were no major statistically significant differences in clinical and biochemical parameters, CTP, or MELD score among the three groups. Patients with thrombocytopenia did not have higher PRBC requirements, rebleed rates or mortality post endotherapy. A point of clinical concern is the feasibility of endotherapy at platelet counts < 20 × 109/L, but our data (although limited by absolute numbers) demonstrates no increased risk of therapy failure in these patients[34]. Similar results were reported by Thinrungroj et al[35] in their cohort of 116 patients in which they demonstrated endotherapy to be safe at platelet counts as low as 30 × 109/L.
Overall, 91 patients (10%) received platelet transfusions. We used PSM analysis to adjust the baseline differences between the groups who received and did not receive platelet transfusion. Those receiving platelet transfusions had significantly higher rebleed rates within day 5 of transfusion (14.6%), which rose to 32.6% at day 42. Rebleeding rates were higher among patients with platelet counts > 20-50 × 109/L and > 50 × 109/L who received transfusions. Despite the higher rebleeding rates, there were no difference in PRBC requirements, indicating that the episodes did not result in a significant loss of blood volume. The mortality rates in those receiving transfusions were higher (25.8% vs 23.6%) but not statistically significant. Thus, patients with baseline platelets > 20 × 109/L are more likely to experience a rebleed if transfused platelets, but this does not translate to higher mortality rates at day 42. Hepatic encephalopathy was associated with poor outcomes in patients with cirrhosis and AVB[36].
Patients receiving FFP transfusion had significantly higher CTP and MELD scores than those who did not, indicating a sicker cohort. This is clinically expected as deranged INR occurs directly because of hepatic dysfunction. Significantly higher 5 and 42 d rebleed rates with higher 42-d mortality rates was noted among those receiving FFP. These patients also experienced higher blood volume loss with significantly higher PRBC requirement, lower hemoglobin level, and mean arterial pressures in this group. These results are in agreement with the recent study by Mohanty et al[11], who reported that bleeding in patients receiving FFP was more difficult to control and resulted in more extended hospital stays.
Comparing patients who receive any transfusion (FFP or platelets or both) vs. those who received none demonstrated the same trend of results, with those receiving transfusions being more likely to be decompensated clinically (elevated bilirubin, ascites and HE) with significantly higher rebleed rates on day 5 and 42 with higher 42-d mortality.
Our findings support the current evidence that both FFP and platelet transfusions lead to greater rebleed rates at 5 d, with FFP transfusions also adding to the mortality at 42 d. This highlights the fact that correction of coagulopathy in an attempt to control variceal bleeding is a futile target in the management of AVB. Thrombin generation assays may be helpful to guide transfusion practices and prevent unnecessary transfusions[37-39]. In recent times, two RCTs have demonstrated that TEG based transfusions have a role in restricting transfusions both in cirrhotics with AVB as well as those undergoing invasive procedures without compromising hemostasis[36,40].
Our study has certain limitations. The number of patients with platelet counts less than 20 × 109/L were few; hence our conclusions on endotherapy in this group are statistically underpowered. Being a tertiary care centre, we receive more sick patients with a poorer hemodynamic profile than other centres. The decision to transfuse blood products and the number of units was subjective and based on the treating physician’s discretion. Being a high-volume centre, we are not able to admit all patients and some patients are sent to other centres for admission post-endotherapy. We do not have data regarding the length of the hospital stay and intensive care unit requirement in these patients. However, despite these limitations, a key strength of our study is that we had several patients with varying severity of illness as graded by the CTP and MELD scores, which is reflective of a real-world scenario. Adding to the pragmatism of the study was that the patients were initially stabilized in the casualty by a team of physicians which included specialists and trainees in emergency medicine and internists prior to review by gastroenterologists. Thus, the transfusion practices reflect both the permeation and dissemination of clinical recommendations by the major societies in gastroenterology among physicians involved in patient management and its acceptability and adoption in general practice.
In conclusion, platelet and FFP transfusions do not lead to improved hemostasis in patients with cirrhosis experiencing an AVB and are associated with higher rebleed rates at 5 and 42 d. Platelet transfusions lead to higher rebleed rates at day 5 and 42 but do not contribute to higher mortality rates, while FFP transfusions are associated with higher rebleed rates at 5 and 42 d and are also associated with higher mortality rates at 42 d from index bleeding episodes.
The most important question answered by this study is that platelet transfusions are not beneficial but harmful to chronic liver disease patients presenting with variceal bleeding. We clearly have shown that thrombocytopenia at baseline did not impact the rebleed rates or mortality. Higher rebleed rates were seen only in those receiving platelets and FFP while those receiving FFP also demonstrated higher mortality rates. Moving further a prospective study to compare the impact of transfusions may be contemplated, but considering the potential of harm to patients, it may not be ethically feasible.
Platelet transfusions increase the rebleed rate at days 5 and 42 but do not contribute to higher mortality rates at day 42. FFP transfusions lead to more severe rebleeds on days 5 and 42 with higher mortality among recipients on day 42.
The study included 913 patients. Rebleeding rates were similar between the three platelet groups (< 20 × 109/L, 20-50 × 109/L, and > 50 × 109/L) on days 5 and 42. On day 42, the mortality rates for the three platelet groups were also similar. On PSM analysis, patients receiving platelets transfusions (n = 89) had significantly higher rebleeding rates on day 5 and day 42 than those who didn't. The mortality rates were also higher among patients receiving platelets, although the difference was insignificant. However, patients who received FFP had higher rebleed rates on days 5 and 42, along with higher mortality rates on day 42, with higher packed red blood cell requirements, indicating a more severe bleed with greater blood loss. On multivariate analysis, platelet transfusion and not platelet count, was independently associated with 42-d rebleeding. Hepatic encephalopathy was independently associated with 42-d mortality.
All patients with chronic liver disease presenting with acute variceal bleed over 4 years period from 2017 to 2021 and giving consent were enrolled for the study. Demographic and clinical data were collected at baseline and the patients followed up till death or 42 days whichever was later. Patients were divided into 3 groups based on platelet counts- < 20 × 109/L, 20-50 × 109/L, and > 50 × 109/L for analysis. A subgroup analysis was done for those receiving fresh frozen plasma (FFP) and platelets and FFP.
Our objectives were to identify the impact of platelet count and platelet transfusions in patients with chronic liver disease presenting with an acute variceal bleed in terms of rebleed rates on days 5 and 42 and mortality rates on day 42.
The lack of data on platelet transfusion often leads to unnecessary transfusions of high volumes of platelets or fresh frozen plasma to chronic liver disease patients with acute variceal bleeding. Transfusions lead to a rise in portal pressure and may precipitate a rebleed, leading to further transfusions and a vicious cycle. Thus patient outcomes may be potentially worsened by unnecessary and empiric transfusions.
There is a paucity of data on the impact of platelet transfusion on outcomes of patients of chronic liver disease presenting with acute variceal bleed. None of the major clinical guidelines provides definitive recommendations on transfusion of platelets during a variceal bleed to correct thrombocytopenia. Thus clinical management of such patients is guided by local policies rather than evidence-based.
| 1. | Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing Concepts of Cirrhotic Coagulopathy. Am J Gastroenterol. 2017;112:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 2. | Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 485] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 3. | Lisman T. Interpreting Hemostatic Profiles Assessed With Viscoelastic Tests in Patients With Cirrhosis. J Clin Gastroenterol. 2020;54:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 4. | Haq I, Tripathi D. Recent advances in the management of variceal bleeding. Gastroenterol Rep (Oxf). 2017;5:113-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Miller JB, Figueroa EJ, Haug RM, Shah NL. Thrombocytopenia in Chronic Liver Disease and the Role of Thrombopoietin Agonists. Gastroenterol Hepatol (N Y). 2019;15:326-332. [PubMed] |
| 6. | Razzaghi A, Barkun AN. Platelet transfusion threshold in patients with upper gastrointestinal bleeding: a systematic review. J Clin Gastroenterol. 2012;46:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Sugi MD, Albadawi H, Knuttinen G, Naidu SG, Mathur AK, Moss AA, Oklu R. Transplant artery thrombosis and outcomes. Cardiovasc Diagn Ther. 2017;7:S219-S227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Sharma M, Yong C, Majure D, Zellner C, Roberts JP, Bass NM, Ports TA, Yeghiazarians Y, Gregoratos G, Boyle AJ. Safety of cardiac catheterization in patients with end-stage liver disease awaiting liver transplantation. Am J Cardiol. 2009;103:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell'Era A, Fabris F, Salerno F, Mannucci PM. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, Mannuccio Mannucci P. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 504] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 11. | Mohanty A, Kapuria D, Canakis A, Lin H, Amat MJ, Rangel Paniz G, Placone NT, Thomasson R, Roy H, Chak E, Baffy G, Curry MP, Laine L, Rustagi T. Fresh frozen plasma transfusion in acute variceal haemorrhage: Results from a multicentre cohort study. Liver Int. 2021;41:1901-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Kumar R, Kerbert AJC, Sheikh MF, Roth N, Calvao JAF, Mesquita MD, Barreira AI, Gurm HS, Ramsahye K, Mookerjee RP, Yu D, Davies NH, Mehta G, Agarwal B, Patch D, Jalan R. Determinants of mortality in patients with cirrhosis and uncontrolled variceal bleeding. J Hepatol. 2021;74:66-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Kakkar B, Maiwall R, Bajpai M. Transfusion practices in cirrhotic patients at a tertiary liver care center from Northern India. Hematol Transfus Cell Ther. 2021;43:280-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Rout G, Shalimar, Gunjan D, Mahapatra SJ, Kedia S, Garg PK, Nayak B. Thromboelastography-guided Blood Product Transfusion in Cirrhosis Patients With Variceal Bleeding: A Randomized Controlled Trial. J Clin Gastroenterol. 2020;54:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 15. | de Franchis R; Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1047] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 16. | Khoury T, Ayman AR, Cohen J, Daher S, Shmuel C, Mizrahi M. The Complex Role of Anticoagulation in Cirrhosis: An Updated Review of Where We Are and Where We Are Going. Digestion. 2016;93:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Nery F, Chevret S, Condat B, de Raucourt E, Boudaoud L, Rautou PE, Plessier A, Roulot D, Chaffaut C, Bourcier V, Trinchet JC, Valla DC; Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 356] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 18. | Northup PG, Garcia-Pagan JC, Garcia-Tsao G, Intagliata NM, Superina RA, Roberts LN, Lisman T, Valla DC. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 430] [Article Influence: 86.0] [Reference Citation Analysis (1)] |
| 19. | Intagliata NM, Caldwell SH. Management of disordered hemostasis and coagulation in patients with cirrhosis. Clin Liver Dis (Hoboken). 2014;3:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Liu P, Hum J, Jou J, Scanlan RM, Shatzel J. Transfusion strategies in patients with cirrhosis. Eur J Haematol. 2020;104:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Boyer JL, Chatterjee C, Iber FL, Basu AK. Effect of plasma-volume expansion on portal hypertension. N Engl J Med. 1966;275:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Zimmon DS, Kessler RE. The portal pressure-blood volume relationship in cirrhosis. Gut. 1974;15:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santaló M, Muñiz E, Guarner C. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1100] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 24. | O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology. 2019;157:34-43.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (1)] |
| 25. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1990] [Article Influence: 248.8] [Reference Citation Analysis (2)] |
| 26. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1506] [Article Influence: 167.3] [Reference Citation Analysis (3)] |
| 27. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on prevention and management of bleeding and thrombosis in patients with cirrhosis. J Hepatol. 2022;76:1151-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 28. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1868] [Article Influence: 467.0] [Reference Citation Analysis (3)] |
| 29. | von Meijenfeldt FA, van den Boom BP, Adelmeijer J, Roberts LN, Lisman T, Bernal W. Prophylactic fresh frozen plasma and platelet transfusion have a prothrombotic effect in patients with liver disease. J Thromb Haemost. 2021;19:664-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Maan R, de Knegt RJ, Veldt BJ. Management of Thrombocytopenia in Chronic Liver Disease: Focus on Pharmacotherapeutic Strategies. Drugs. 2015;75:1981-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Demetri GD. Targeted approaches for the treatment of thrombocytopenia. Oncologist. 2001;6 Suppl 5:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Terrault N, Chen YC, Izumi N, Kayali Z, Mitrut P, Tak WY, Allen LF, Hassanein T. Avatrombopag Before Procedures Reduces Need for Platelet Transfusion in Patients With Chronic Liver Disease and Thrombocytopenia. Gastroenterology. 2018;155:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (1)] |
| 33. | Blasi A, Machlab S, Risco R, Costa-Freixas JP, Hernández-Cely G, Horta D, Bofill A, Ruiz-Ramirez P, Profitos J, Sanahuja JM, Fernandez-Simon A, Gómez MV, Sánchez-Delgado J, Cardenas A. A multicenter analysis of the role of prophylactic transfusion of blood products in patients with cirrhosis and esophageal varices undergoing endoscopic band ligation. JHEP Rep. 2021;3:100363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Vieira da Rocha EC, D'Amico EA, Caldwell SH, Flores da Rocha TR, Soares E Silva CS, Dos Santos Bomfim V, Felga G, Barbosa WF, Kassab F, Polli DA, Carrilho FJ, Farias AQ. A prospective study of conventional and expanded coagulation indices in predicting ulcer bleeding after variceal band ligation. Clin Gastroenterol Hepatol. 2009;7:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Thinrungroj N, Pisespongsa P, Kijdamrongthum P, Leerapun A, Chitaparanux T, Thongsawat S, Praisontarangkul OA. Tu1277 Endoscopic Variceal Ligation (EVL) Is Safe in Cirrhotic Patients With Severe Thrombocytopenia. Gastrointest Endosc. 2013;77:AB484. [DOI] [Full Text] |
| 36. | Rout G, Sharma S, Gunjan D, Kedia S, Saraya A, Nayak B, Singh V, Kumar R, Shalimar. Development and Validation of a Novel Model for Outcomes in Patients with Cirrhosis and Acute Variceal Bleeding. Dig Dis Sci. 2019;64:2327-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Smith SA, Travers RJ, Morrissey JH. How it all starts: Initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015;50:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 38. | Salvagno GL, Berntorp E. Thrombin Generation Assays (TGAs). Methods Mol Biol. 2017;1646:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Shenoy A, Intagliata NM. Thromboelastography and Utility in Hepatology Practice. Clin Liver Dis (Hoboken). 2020;16:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Vuyyuru SK, Singh AD, Gamanagatti SR, Rout G, Gunjan D, Shalimar. A Randomized Control Trial of Thromboelastography-Guided Transfusion in Cirrhosis for High-Risk Invasive Liver-Related Procedures. Dig Dis Sci. 2020;65:2104-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lisman T, Netherlands; Wondmagegn H, Ethiopia; Yang ZG, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH