Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1210
Peer-review started: May 16, 2021
First decision: June 27, 2021
Revised: July 9, 2022
Accepted: May 14, 2022
Article in press: May 14, 2022
Published online: June 27, 2022
Processing time: 402 Days and 23.8 Hours
Gut dysbiosis and changes in body composition (i.e., a decrease in the proportion of muscle mass and an increase in extracellular fluid) are common in cirrhosis.
To study the relationship between the gut microbiota and body composition in cirrhosis.
This observational study included 46 patients with cirrhosis. Stool microbiome was assessed using 16S rRNA gene sequencing. Multifrequency bioelectrical impedance analysis was performed to assess body composition in these patients.
An increase in fat mass and a decrease in body cell mass were noted in 23/46 (50.0%) and 15/46 (32.6%) patients, respectively. Changes in the gut microbiome were not independently associated with the fat mass percentage in cirrhosis. The abundance of Bacteroidaceae (P = 0.041) and Eggerthella (P = 0.001) increased, whereas that of Erysipelatoclostridiaceae (P = 0.006), Catenibacterium (P = 0.021), Coprococcus (P = 0.033), Desulfovibrio (P = 0.043), Intestinimonas (P = 0.028), and Senegalimassilia (P = 0.015) decreased in the gut microbiome of patients with body cell mass deficiency. The amount of extracellular fluid increased in 22/46 (47.6%) patients. Proteobacteria abundance (P < 0.001) increased, whereas Firmicutes (P = 0.023), Actinobacteria (P = 0.026), Bacilli (P = 0.008), Anaerovoraceceae (P = 0.027), Christensenellaceae (P = 0.038), Eggerthellaceae (P = 0.047), Erysipelatoclostridiaceae (P = 0.015), Erysipelotrichaceae (P = 0.003), Oscillospiraceae (P = 0.024), Rikenellaceae (P = 0.002), Collinsella (P = 0.030), Hungatella (P = 0.040), Peptococcaceae (P = 0.023), Slackia (P = 0.008), and Senegalimassilia (P = 0.024) abundance decreased in these patients. Patients with clinically significant ascites (n = 9) had a higher abundance of Proteobacteria (P = 0.031) and a lower abundance of Actinobacteria (P = 0.019) and Bacteroidetes (P = 0.046) than patients without clinically significant ascites (n = 37).
Changes in the amount of body cell mass and extracellular fluid are associated with changes in the gut microbiome in cirrhosis patients.
Core Tip: The abundance of Bacteroidaceae and Eggerthella increased, whereas that of Erysipelatoclostridiaceae, Catenibacterium, Coprococcus, Desulfovibrio, Intestinimonas, and Senegalimassilia decreased in the gut microbiome of patients with body cell mass deficiency. Proteobacteria abundance was increased, whereas Firmicutes, Actinobacteria, Bacilli, Christensenellaceae, Anaerovoraceceae, Eggerthellaceae, Erysipelatoclostridiaceae, Erysipelotrichaceae, Oscillospiraceae, Peptococcaceae, Rikenellaceae, Collinsella, Hungatella, Slackia, and Senegalimassilia abundance decreased in cirrhosis patients with excess extracellular fluid.
- Citation: Maslennikov R, Ivashkin V, Alieva A, Poluektova E, Kudryavtseva A, Krasnov G, Zharkova M, Zharikov Y. Gut dysbiosis and body composition in cirrhosis. World J Hepatol 2022; 14(6): 1210-1225
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1210.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1210
Cirrhosis is the final stage of chronic liver diseases. However, it is not limited to lesions in this organ but is also associated with a decrease in muscle mass (sarcopenia) and water accumulation in the body. The pathogenesis of sarcopenia in cirrhosis is complex, and it is assumed that changes in composition of the gut microbiota (gut dysbiosis) and small intestinal bacterial overgrowth (SIBO) play important roles in its development[1-5]. It is believed that these disorders of the gut microbiota promote bacterial translocation (the penetration of bacteria and their components into body tissues) and hyperammonemia, which increase protein catabolism and levels of myostatin, a protein that inhibits muscle growth[2].
Water retention in cirrhosis has also been suggested to be associated with disorders of the gut microbiota and occurs in response to bacterial translocation-induced vasodilation[6]. This leads to hypotension and compensatory fluid retention to maintain normal blood pressure levels. Although these relationships have been established with respect to SIBO[7,8], there are no studies on such associations with gut dysbiosis.
In addition, the gut microbiota status is known to be associated with disorders of lipid metabolism, leading to an increase in fat content in the body[9].
Bioelectrical impedance analysis is a method used for the complex assessment of body composition and is based on measurements of capacitive and active resistance of the human body. These can be used to identify fat and lean (free-fat) mass. The latter is represented by body cell mass, consisting mainly of musculoskeletal mass, and extracellular mass, comprised mainly of extracellular fluid. Although fat is located within cells, it and body cell mass are conditionally considered to be different components of the body in this analysis. Fat is practically non-conductive. Cells are capacitors (i.e., an electrolyte solution surrounded by a dielectric membrane) and give rise to the capacitive component of resistance, while free extracellular fluid contributes to the active resistance. Therefore, it is possible to assess body composition (amount of fat, body cell mass, and extracellular fluid) by analyzing the capacitive and active components of resistance of the body[10-13].
Although recent publications have reported the associations of some taxa of the gut microbiome with sarcopenia diagnosed by computed tomography[4,5], no studies have investigated the relations between the gut microbiome and all three main body components (fat, cells, and extracellular fluid) in cirrhosis.
The aim of the present study was to assess the relationship between the gut microbiota and body composition in cirrhosis.
In this observational study, 97 patients with cirrhosis were consecutively admitted to the Department of Hepatology of the Clinic for Internal Medicine, Gastroenterology and Hepatology at Sechenov University (Moscow, Russia) and screened for participation. The procedures were explained to potential participants, and written informed consent was obtained before enrollment. The study was approved by the Ethics Committee of Sechenov University in accordance with the Declaration of Helsinki.
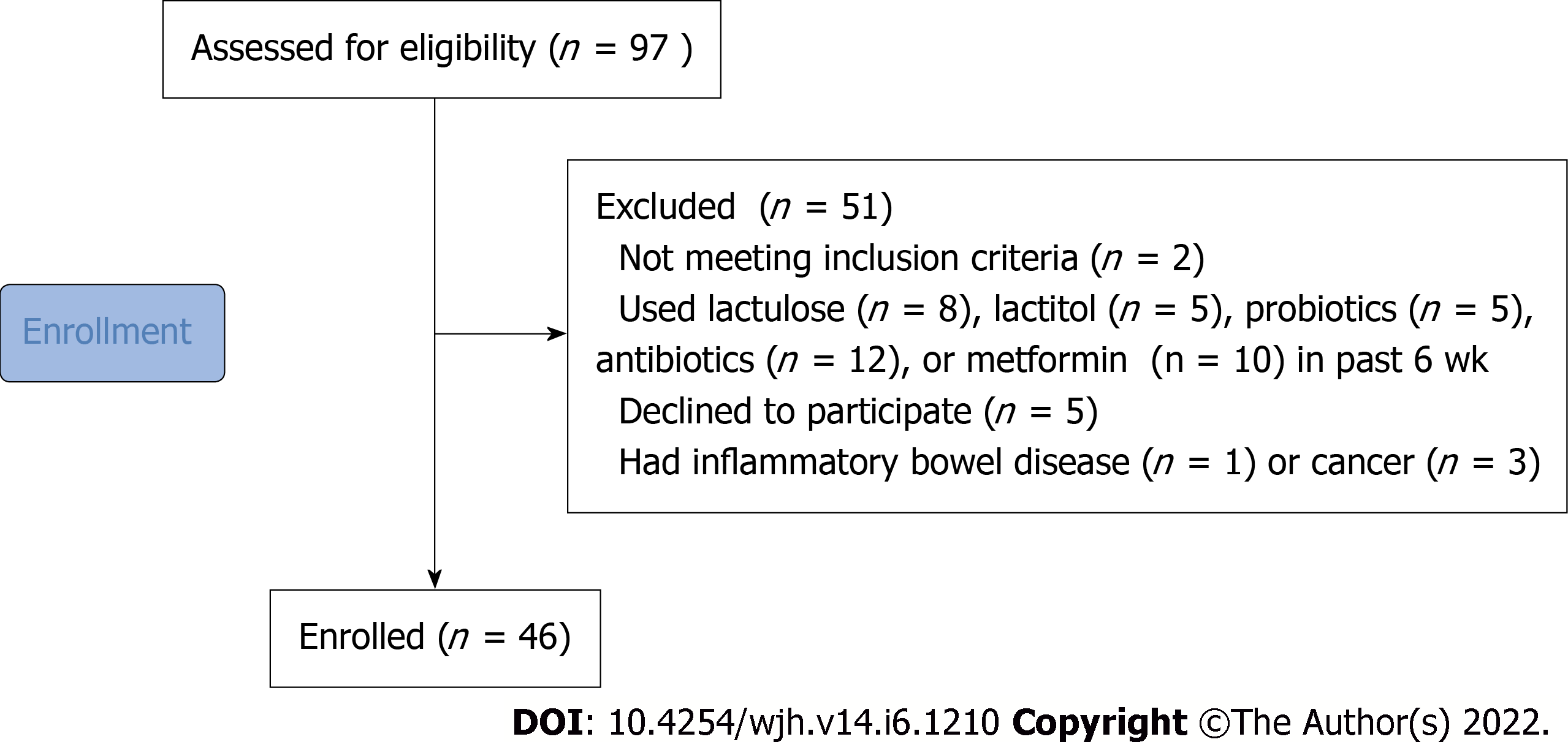
The inclusion criteria were diagnosis of cirrhosis verified by histological examination or clinical, biochemical, and ultrasound findings, and age between 18 and 70 years. The exclusion criteria included use of lactulose, lactitol, or other prebiotics, probiotics, antibiotics, or metformin in the past 6 wk, alcohol consumption in the past 6 wk, or diagnosis of inflammatory bowel disease, cancer, or any other serious disease. The exclusion criteria were specifically selected to remove the influence of these factors on the composition of the gut microbiota. Of the original 97 patients screened for inclusion, 46 were enrolled in the study and 51 were excluded (Figure 1).
In addition, 14 healthy persons were examined.
The gold standard for studying the composition of the gut microbiota is analysis of the gut microbiome that is a cumulative genome of gut bacteria.
A stool sample was obtained from each patient and placed in a sterile disposable container the morning after admission and immediately frozen at -80°C[14].
Total DNA was isolated using the AmpliPrime DNA-sorb-AM kit (NextBio, Moscow, Russia) for clinical specimens, according to the manufacturer’s protocol. The isolated DNA was stored at -20°C. For qualitative and quantitative assessment of the isolated DNA we used NanoDrop 1000 equipment (Thermo Fisher Scientific, Waltham, MA, United States). The 16S library preparation was carried out according to the protocol of 16S Metagenomic Sequencing Library Preparation (Illumina, San Diego, CA, United States), which is recommended for Illumina MiSeq sample prep. The first round of amplification of V3-V4 16S rDNA variable regions was performed using the following primers: forward (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-CCTACGGGNGGCWGCAG) and reverse (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-GACTACHVGGGTATCTAATCC). These primers are aimed at the amplification of bacterial (more than 90%) but not archaeal (less than 5%) rRNA genes. The amplification program (Applied Biosystems 2720 Thermal Cycler, Foster City, CA, United States) was as follows: (1) 95°C for 3 min; (2) 30 cycles: 95°C for 30 s; 55°C for 30 s; 72°C for 30 s; (3) 72°C for 5 min; and (4) 4°C.
The derived amplicons were purified using Agencourt AMPure XP (Beckman Coulter, Brea, CA, United States) beads according to the manufacturer’s protocol. The second amplification round was used for double-indexing samples with a combination of specific primers. The amplification program was as follows: (1) 95°C for 3 min; (2) 8 cycles: 95°C for 30 s; 55°C for 30 s; 72°C for 30 s; (3) 72°C for 5 m; and (4) 4°C.
The purification of PCR products was also carried out using Agencourt AMPure XP. The concentration of the derived 16S rDNA libraries was measured using a Qubit® 2.0 fluorometer (Invitrogen, Carlsbad, CA, United States) using QuantiT™ dsDNA High-Sensitivity Assay Kit. The purified amplicons were mixed equimolarly according to the derived concentration values. Quality of the libraries was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, United States) and Agilent DNA 1000 Kit. Sequencing was carried out on a MiSeq machine (Illumina) using the MiSeq Reagent Kit v2 (paired-end reads, 2 × 300 nt).
First, forward and reverse reads were merged using MeFiT and CASPER[15]. For most samples more than 99% reads were successfully merged. Non-merged reads were excluded. Next, the merged reads were analyzed by the DADA2 package (a part of the Bioconductor project) for R[16] in order to infer RSV (ribosomal sequence variants). The analysis included the following steps: (1) Primer sequences were removed using Cutadapt; (2) Reads were filtered by quality; (3) Error distribution models were derived based on read quality profiles; (4) Sequencing errors were estimated and corrected; (5) RSV sequences were obtained; and (6) Chimeric RSVs were eliminated. Next, taxonomic annotation of the derived RSVs was performed with the DADA2 package using the Silva (version 138) 16S reference sequence database[17].
Bioelectrical impedance analysis was performed on the day after patient admission in the morning, to ensure that the patient had an empty stomach. The MEDASS device (Russia) was used for this purpose in accordance with the manufacturer's instructions.
The measurement was carried out by passing an alternating current with frequencies of 5 and 50 kHz through the patient. Conduction originates almost entirely due to the extracellular fluid in the presence of constant current. With an alternating current, the intracellular fluid also contributes to current conduction, depending on its frequency. The manufacturer’s software provides the values for fat and body cell mass and total and extracellular fluid based on these values of conduction and patient’s age, sex, height, and weight. This software also calculated individual norms for each patient based on his/her anthropometric data, age, sex, and the results of a local population study. Patients with a fat mass value above the upper limit of their individual norm were included in the group of patients with excess fat mass, and those with body cell mass below the lower limit of their individual norm were included in the group of patients with cell mass deficiency. Similarly, patients with an amount of extracellular fluid higher than the upper limit of their individual norm were included in the group of patients with excess extracellular fluid.
The underlying principle of this analysis and the methods used for calculating the indicators have been described in detail in previous publications[10,11].
We used the ratio of body cell mass to free-fat mass to assess body cell mass and the ratio of extracellular fluid to total fluid to quantitate the amount of extracellular fluid. This method ensured minimal influence of values of fat and body cell mass and extracellular fluid on each other.
Statistical analysis was performed with STATISTICA 10 (StatSoft Inc., Tulsa, OK, United States). The data are presented as medians (interquartile ranges). The abundance of taxa in the gut microbiome is presented as a percentage. Differences between continuous variables were assessed with the Mann-Whitney test. Fisher’s exact test was used to assess the differences between categorical variables. Correlations between variables were computed using Spearman’s rank correlation. If the compared groups differed in age, sex or severity of cirrhosis, multivariate regression analysis was performed. P values ≤ 0.05 were considered statistically significant.
The characteristics of the patients enrolled in the study are listed in Table 1.
| Parameter | Value |
| Age, yr | 55 [43-61] |
| Male/Female | 18/28 |
| Etiology: | |
| Alcohol | 15 (32.6%) |
| Hepatitis C virus | 5 (10.9%) |
| Primary biliary cholangitis | 4 (8.7%) |
| Primary sclerosing cholangitis | 2 (4.3%) |
| Autoimmune hepatitis | 5 (10.9%) |
| Metabolic-associated liver disease | 4 (8.7%) |
| Wilson disease | 3 (6.5%) |
| Mixed | 3 (6.5%) |
| Cryptogenic | 5 (10.9%) |
| Red blood cells, 1012/L | 4.1 [3.5-4.8] |
| White blood cells, 109/L | 4.9 [3.1-6.3] |
| Platelets, 109/L | 105 [76-150] |
| Serum albumin, g/L | 37 [33-41] |
| Serum total bilirubin, μmol/L | 28 [16-56] |
| Prothrombin (Quick test), % | 70 [60-89] |
| Ascites: grade 2-3, n (%) | 9 (19.6%) |
| Esophageal varices: grade 2-3, n (%) | 17 (36.9%) |
| Spleen length, cm | 15.4 [13.1-17.1] |
| Portal vein diameter, mm | 13.0 [11.0-14.2] |
| Hepatic encephalopathy, n (%) | 15 (32.5%) |
| Child-Pugh class: A/B/C | 14/21/11 |
The amount of extracellular fluid and the fat mass were higher but the body cell mass was lower in patients with cirrhosis than in healthy individuals. The abundance of Bacteroidetes, Proteobacteria, and Bacilli was higher, but the abundance of Firmicutes and Clostridia was lower in the gut microbiome of these patients than in that of healthy individuals (Table 2).
| Patients with cirrhosis (n = 46) | Healthy persons (n = 14) | P | |
| Age, yr | 55 [43-61] | 51 [41-63] | 0.484 |
| Male/Female | 18/28 | 3/11 | 0.187 |
| Body mass index, kg/m2 | 27.0 [23.6-30.1] | 21.1 [19.7-26.0] | 0.002 |
| Fat mass, % | 34.7 [28.1-43.5] | 24.5 [20.7-31.2] | 0.002 |
| Free-fat mass, % | 65.3 [56.5-71.9] | 75.5 [68.8-79.3] | 0.002 |
| Body cell mass, % | 32.4 [28.0-36.5] | 44.5 [38.4-46.0] | < 0.001 |
| (Body cell mass)/(free-fat mass) | 0.50 [0.46-0.55] | 0.58 [0.55-0.60] | < 0.001 |
| Extracellular fluid, % | 20.1 [17.4-21.4] | 18.6 [16.8-19.3] | 0.044 |
| Total fluid, % | 47.9 [42.0-53.3] | 51.7 [46.8-52.9] | 0.238 |
| (Extracellular fluid)/(total fluid) | 0.41 [0.40-0.43] | 0.36 [0.36-0.37] | < 0.001 |
| Phase angle, ° | 5.3 [4.9-6.3] | 7.0 [6.2-7.3] | < 0.001 |
| Firmicutes | 38.6 [27.7-52.9] | 90.8 [85.7-94.1] | < 0.001 |
| Bacteroidetes | 38.6 [26.6-58.5] | 5.9 [4.7-8.1] | < 0.001 |
| Proteobacteria | 5.5 [2.3-10.6] | 0.4 [0.1-0.5] | < 0.001 |
| Clostridia | 36.2 [24.8-50.0] | 89.3 [86.7-91.0] | < 0.001 |
| Bacilli | 0.4 [0.2-1.1] | 0.1 [0.0-0.2] | < 0.001 |
The fat mass was increased in 23/46 (50.0%) patients. The abundance of Bacteroidetes, Desul
| Patients with excess fat mass (n = 23) | Patients without excess fat mass (n = 23) | P | |
| Age, yr | 58 [49-62] | 45 [39-59] | 0.033 |
| Male/Female | 3/20 | 15/8 | < 0.001 |
| Body mass index, kg/m2 | 29.7 [27.0-35.6] | 23.6 [22.0-27.1] | < 0.001 |
| Body fat, % | 43.5 [37.6-47.3] | 28.1 [23.1-31.2] | < 0.001 |
| Free-fat mass, % | 56.5 [52.7-62.4] | 71.9 [68.8-76.9] | < 0.001 |
| Body cell mass, % | 29.6 [25.6-33.9] | 34.9 [31.2-40.6] | 0.001 |
| (Body cell mass)/(free-fat mass) | 0.51 [0.45-0.56] | 0.49 [0.46-0.53] | 0.231 |
| Extracellular fluid, % | 17.6 [16.7-18.8] | 21.1 [20.4-22.4] | < 0.001 |
| Total fluid, % | 42.0 [38.5-45.8] | 52.6 [50.4-56.3] | < 0.001 |
| (Extracellular fluid)/(total fluid) | 0.42 [0.41-0.43] | 0.40 [0.39.2-0.41] | 0.005 |
| Phase angle, ° | 5.4 [5.2-6.6] | 5.1 [4.6-5.8] | 0.093 |
| Red blood cells, 1012/L | 4.5 [3.9-4.8] | 3.9 [3.3-4.6] | 0.097 |
| White blood cells, 109/L | 5.0 [3.6-6.3] | 4.7 [2.9-5.7] | 0.465 |
| Platelets, 109/L | 104 [77-150] | 106 [72-150] | 0.945 |
| Serum albumin, g/L | 37.6 [34.4-42.9] | 34.4 [31.3-38.1] | 0.030 |
| Serum total bilirubin, μmol/L | 20.1 [13.5-36.9] | 46.6 [19.1-77.9] | 0.037 |
| Prothrombin (Quick test), % | 75 [69-92] | 64 [40-80] | 0.022 |
| Ascites: grade 2-3 | 1 (4.3%) | 8 (34.8%) | 0.011 |
| Esophageal varices: grade 2-3 | 8 (34.8%) | 9 (39.1%) | 0.500 |
| Spleen length, cm | 15.3 [13.0-17.2] | 15.7 [13.1-17.1] | 0.759 |
| Portal vein diameter, mm | 12.7 [11.0-14.2] | 13.0 [11.0-14.4] | 0.803 |
| Hepatic encephalopathy | 4 (17.4%) | 9 (39.1%) | 0.095 |
| Child-Pugh score | 7 [6-9] | 9 [7-12] | 0.018 |
| Bacteroidetes | 46.1 [33.0-61.0] | 35.2 [18.3-44.1] | 0.039 |
| Barnesiellaceae | 0.92 [0.43-2.04 | 0.04 [0.00-0.11] | 0.016 |
| Marinifilaceae | 0.66 [0.21-1.03] | 0.17 [0.00-0.49] | 0.006 |
| Desulfobacteria | 0.55 [0.29-1.53] | 0.18 [0.01-0.45] | 0.032 |
| Bilophila | 0.36 [0.04-1.30] | 0.04 [0.00-0.18] | 0.016 |
| Coriobacteriaceae | 0.09 [0.04-0.56] | 0.03 [0.00-0.06] | 0.004 |
| Eggerthellaceae | 0.08 [0.03-0.20] | 0.03 [0.01-0.06] | 0.028 |
| Senegalimassilia | 0.01 [0.00-0.05] | 0.00 [0.00-0.00] | 0.040 |
| Slackia | 0.01 [0.00-0.05] | 0.00 [0.00-0.00] | 0.004 |
| Parasutterella | 0.01 [0.00-0.19] | 0.00 [0.00-0.00] | 0.015 |
| Odoribacter | 0.10 [0.03-0.24] | 0.26 [0.16-0.52] | 0.047 |
| Veillonella | 0.01 [0.00-0.07] | 0.16 [0.01-0.82] | 0.012 |
| Clostridiaceae | 0.01 [0.00-0.05] | 0.07 [0.00-0.29] | 0.041 |
The proportion of fat mass in total body mass showed a positive correlation with the abundance of Bacteroidetes, Desulfobacteria, Coriobacteriaceae, Barnesiellaceae, Bilophila, Collinsella, Megamonas, Parasutterella, and Slackia and a negative correlation with the abundance of Clostridiaceae, Campylobacter, and Veillonella (Table 4).
| Fat mass | Body cell mass | Extracellular fluid | |
| Bacteroidetes | r = 0.329; P = 0.026 | NS | NS |
| Desulfobacteria | r = 0.347; P = 0.018 | NS | NS |
| Firmicutes | NS | NS | r = -0.386; P = 0.008 |
| Proteobacteria | NS | NS | r = 0.320; P = 0.031 |
| Bacilli | NS | NS | r = -0.378; P = 0.009 |
| Clostridia | NS | NS | r = -0.305; P = 0.039 |
| Bacteroidaceae | NS | r = -0.294; P = 0.047 | NS |
| Barnesiellaceae | r = 0.291; P = 0.049 | r = 0.332; P = 0.024 | NS |
| Clostridiaceae | r = -0.326; P = 0.027 | NS | NS |
| Coriobacteriaceae | r = 0.319; P = 0.031 | NS | NS |
| Erysipelatoclostridiaceae | NS | r = 0.310; P = 0.036 | NS |
| Anaerotruncus | NS | r = 0.338; P = 0.022 | NS |
| Bilophila | r = 0.383; P = 0.009 | NS | r = 0.294; P = 0.048 |
| Campylobacter | r = -0.404; P = 0.005 | NS | NS |
| Catenibacterium | NS | r = 0.306; P = 0.040 | NS |
| Collinsella | r = 0.319; P = 0.031 | NS | NS |
| Megamonas | r = 0.337; P = 0.022 | NS | NS |
| Oscillospira | NS | r = 0.375; P = 0.010 | NS |
| Parasutterella | r = 0.365; P = 0.013 | NS | NS |
| Senegalimassilia | ns | r = 0.379; P = 0.009 | NS |
| Slackia | r = 0.439; P = 0.002 | NS | NS |
| Veillonella | r = -0.308; P = 0.037 | r = -0.294; P = 0.047 | NS |
Since the groups under comparison differed with respect to age, sex, and severity of cirrhosis, we performed a multivariate regression analysis and found that these changes in the gut microbiome were not independent factors affecting the percentage of fat mass in the total body mass of these patients.
The body cell mass was decreased in 15/46 (32.6%) patients. The abundance of Bacteroidaceae and Eggerthella increased in the gut microbiome of these patients, whereas that of Erysipelatoclostridiaceae, Catenibacterium, Coprococcus, Desulfovibrio, Intestinimonas, and Senegalimassilia decreased (Table 5).
| Patients with body cell mass deficiency (n = 15) | Patients without body cell mass deficiency (n = 31) | P | |
| Age, yr | 56 [46-63] | 49 [39-61] | 0.331 |
| Male/Female | 4/11 | 14/17 | 0.190 |
| Body mass index, kg/m2 | 27.0 [23.8-29.0] | 27.1 [23.2-30.9] | 0.806 |
| Body fat, % | 33.6 [29.8-42.7] | 36.3 [27.3-44.9] | 0.656 |
| Free-fat mass, % | 66.4 [57.3-70.2] | 63.7 [55.1-72.7] | 0.656 |
| Body cell mass, % | 29.0 [25.6-31.9] | 34.7 [30.0-37.1] | 0.002 |
| (Body cell mass)/(free-fat mass) | 0.45 [0.41-0.46] | 0.53 [0.49-0.56] | < 0.001 |
| Extracellular fluid, % | 20.4 [17.7-21.1] | 19.6 [17.0-21.8] | 0.648 |
| Total fluid, % | 48.6 [42.0-51.5] | 47.1 [41.0-53.3] | 0.926 |
| (Extracellular fluid)/(total fluid) | 0.42 [0.41-0.43] | 0.41 [0.39-0.43] | 0.223 |
| Phase angle, ° | 4.5 [4.2-4.6] | 5.8 [5.2-6.5] | < 0.001 |
| Red blood cells, 1012/L | 3.8 [3.5-4.6] | 4.2 [3.6-4.8] | 0.211 |
| White blood cells, 109/L | 4.1 [3.0-7.2] | 5.1 [3.3-5.9] | 0.159 |
| Platelets, 109/L | 116 [77-170] | 101 [72-142] | 0.211 |
| Serum albumin, g/L | 34.1 [29.3-37.3] | 37.6 [33.3-42.4] | 0.028 |
| Serum total bilirubin, μmol/L | 46.6 [18.7-66.2] | 22.3 [15.0--54.6] | 0.314 |
| Prothrombin (Quick test), % | 71 [54-92] | 70 [60-86] | 0.981 |
| Ascites: grade 2-3 | 4 (26.7%) | 5 (16.1%) | 0.320 |
| Esophageal varices: grade 2-3 | 4 (26.7%) | 13 (41.9%) | 0.251 |
| Spleen length, cm | 15.8 [13.4-17.0] | 15.3 [13.0-17.2] | 0.864 |
| Portal vein diameter, mm | 13.8 [11.0-14.4] | 12.7 [11.0-14.2] | 0.695 |
| Hepatic encephalopathy | 7 (46.7%) | 8 (25.8%) | 0.141 |
| Child-Pugh score | 9 [7-11] | 7 [6-9] | 0.092 |
| Bacteroidaceae | 22.7 [6.8-40.8] | 4.0 [1.4-20.5] | 0.041 |
| Eggerthella | 0.01 [0.00-0.03] | 0.00 [0.00-0.00] | 0.001 |
| Erysipelatoclostridiaceae | 0.02 [0.01-0.08] | 0.11 [0.04-0.25] | 0.006 |
| Coprococcus | 0.24 [0.06-0.68] | 0.68 [0.16-1.26] | 0.033 |
| Intestinimonas | 0.00 [0.00-0.03] | 0.03 [0.01-0.07] | 0.028 |
| Desulfovibrio | 0.00 [0.00-0.01] | 0.02 [0.00-0.38] | 0.043 |
| Catenibacterium | 0.00 [0.00-0.00] | 0.00 [0.00-0.20] | 0.021 |
| Senegalimassilia | 0.00 [0.00-0.00] | 0.00 [0.00-0.03] | 0.015 |
The proportion of body cell mass correlated positively with the abundance of Barnesiellaceae, Erysipelatoclostridiaceae, Anaerotruncus, Catenibacterium, Oscillospira, and Senegalimassilia, whereas a negative correlation with abundance of Bacteroidaceae and Veillonella was observed (Table 4).
The amount of extracellular fluid increased in 22/46 (47.6%) patients. The abundance of Proteobacteria was increased in the gut microbiome of these patients. However, the abundance of Firmicutes, Bacilli, Anaerovoraceceae, Christensenellaceae, Eggerthellaceae, Erysipelatoclostridiaceae, Erysipelotrichaceae, Oscillospiraceae, Peptococcaceae, Rikenellaceae, Actinobacteria, Collinsella, Hungatella, Slackia, and Senegalimassilia was decreased in the gut microbiome of these patients (Table 6).
| Patients with excess extracellular fluid (n = 22) | Patients without excess extracellular fluid (n = 24) | P | |
| Age, yr | 53 [39-61] | 57 [44-62] | 0.545 |
| Male/Female | 10/12 | 8/16 | 0.300 |
| Body mass index, kg/m2 | 28.1 [24.2-31.2] | 26.8 [22.9-29.1] | 0.129 |
| Body fat, % | 32.4 [24.5-44.9] | 36.7 [30.4-42.7] | 0.391 |
| Free-fat mass, % | 67.6 [55.1-75.5] | 63.3 [57.3-69.6] | 0.391 |
| Body cell mass, % | 33.6 [28.0-40.6] | 32.2 [28.2-35.2] | 0.545 |
| (Body cell mass)/(free-fat mass) | 0.50 [0.45-0.55] | 0.49 [0.47-0.55] | 0.921 |
| Extracellular fluid, % | 20.9 [17.7-22.6] | 18.5 [17.2-20.4] | < 0.001 |
| Total fluid, % | 50.8 [41.2-55.9] | 45.9 [42.0-50.9] | 0.169 |
| (Extracellular fluid)/(total fluid) | 0.43 [0.40-43.3] | 0.41 [0.39-0.42] | 0.042 |
| Phase angle, ° | 5.4 [4.6-6.3] | 5.2 [4.9-6.3] | 0.879 |
| Red blood cells, 1012/L | 4.2 [3.4-4.8] | 4.0 [3.6-4.6] | 0.991 |
| White blood cells, 109/L | 4.6 [2.4-6.3] | 5.2 [3.4-6.2] | 0.419 |
| Platelets, 109/L | 89 [72-113] | 124 [76-158] | 0.082 |
| Serum albumin, g/L | 34.5 [30.4-41.3] | 37.5 [34.3-40.7] | 0.113 |
| Serum total bilirubin, μmol/L | 39.4 [18.5-66.2] | 24.8 [15.4-44.5] | 0.684 |
| Prothrombin (Quick test), % | 61 [40-86] | 76 [70-91] | 0.012 |
| Ascites: grade 2-3 | 9 (40.9%) | 0 | 0.001 |
| Esophageal varices: grade 2-3 | 9 (40.9%) | 8 (33.3%) | 0.410 |
| Spleen length, cm | 16.7 [14.8-18.2] | 14.4 [12.4-16.4] | 0.021 |
| Portal vein diameter, mm | 13.5 [11.0-15.0] | 12.5 [11.0-14.0] | 0.180 |
| Hepatic encephalopathy | 10 (45.5%) | 5 (20.8%) | 0.050 |
| Child-Pugh score | 9 [6-12] | 7 [6-9] | 0.088 |
| Proteobacteria | 8.93 [5.58-22.60] | 2.84 [1.61-5.66] | < 0.001 |
| Firmicutes | 31.4 [26.6-44.6] | 43.6 [33.4-58.7] | 0.023 |
| Oscillospiraceae | 4.43 [1.57-8.46] | 8.33 [5.00-12.9] | 0.024 |
| Rikenellaceae | 0.98 [0.03-1.78] | 2.83 [0.85-5.51] | 0.002 |
| Actinobacteria | 0.56 [0.11-1.43] | 1.21 [0.42-5.93] | 0.026 |
| Bacilli | 0.24 [0.15-0.52] | 0.54 [0.34-2.1] | 0.008 |
| Christensenellaceae | 0.12 [000-0.43] | 0.43 [0.07-2.59] | 0.038 |
| Collinsella | 0.04 [0.01-0.05] | 0.10 [0.02-0.24] | 0.030 |
| Eggerthellaceae | 0.04 [0.01-0.06] | 0.08 [0.03-0.20] | 0.047 |
| Erysipelatoclostridiaceae | 0.04 [0.00-0.13] | 0.10 [0.05-0.41] | 0.015 |
| Erysipelotrichaceae | 0.01 [0.00-0.03] | 0.05 [0.01-0.11] | 0.003 |
| Anaerovoraceceae | 0.01 [0.00-0.06] | 0.04 [0.01-0.11] | 0.027 |
| Hungatella | 0.00 [0.00-0.00] | 0.01 [0.00-0.04] | 0.040 |
| Slackia | 0.00 [0.00-0.00] | 0.00 [0.00-0.04] | 0.008 |
| Peptococcaceae | 0.00 [0.00-0.00] | 0.00 [0.00-0.02] | 0.023 |
| Senegalimassilia | 0.00 [0.00-0.00] | 0.01 [0.00-0.04]; | 0.024 |
The proportion of extracellular fluid in total body fluid in these patients was positively correlated with the abundance of Proteobacteria and Bilophila, and negatively correlated with that of Firmicutes, Bacilli, and Clostridia in the gut microbiome (Table 4).
There was no significant difference between these groups of patients in terms of the indices of microbiota biodiversity (Shannon, Chao1, ACE—Figure 2)[18], and no significant correlation was found between the latter and indicators of body composition.
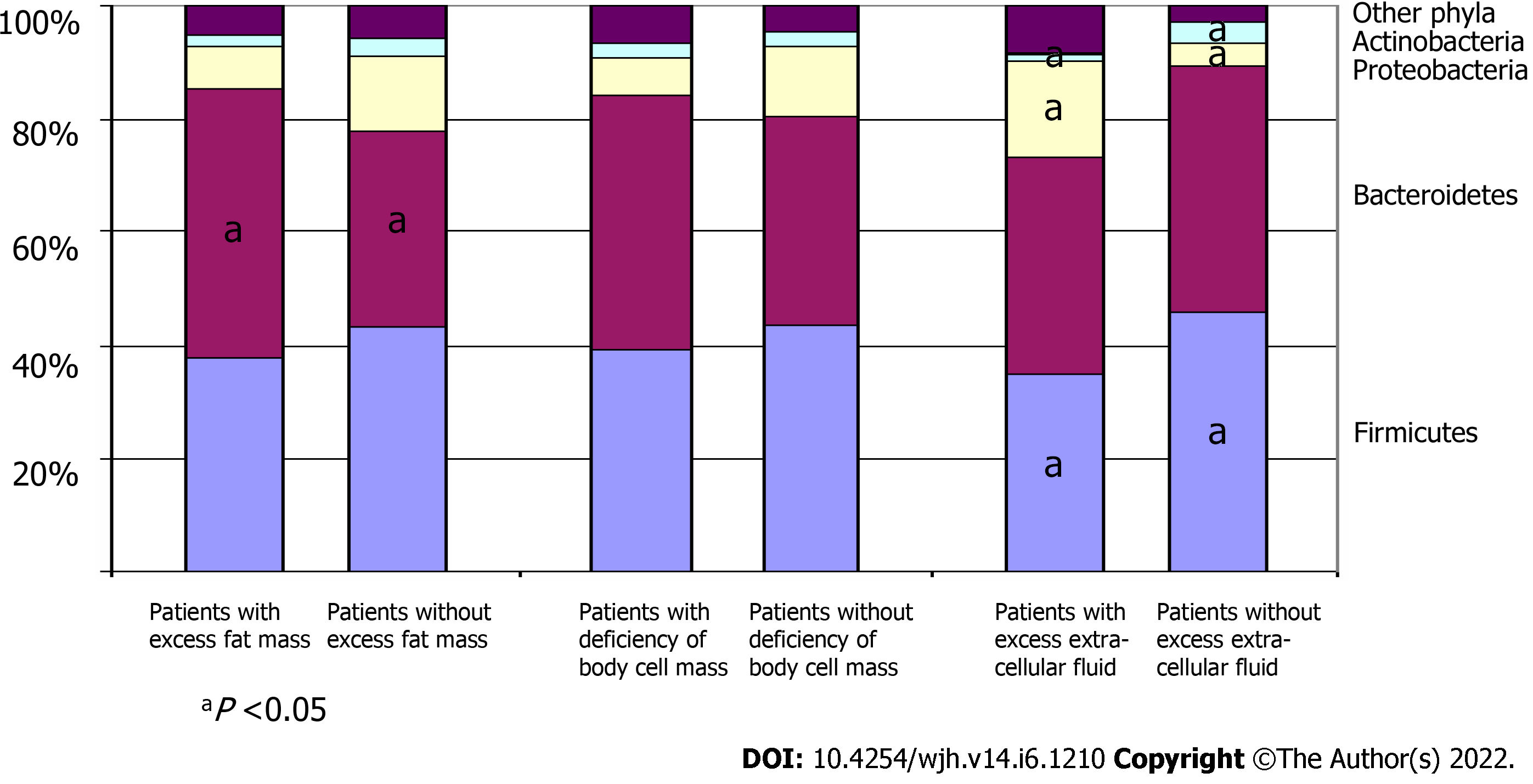
A comparison of the gut microbiome at the phylum level between patient groups is represented in Figure 3.
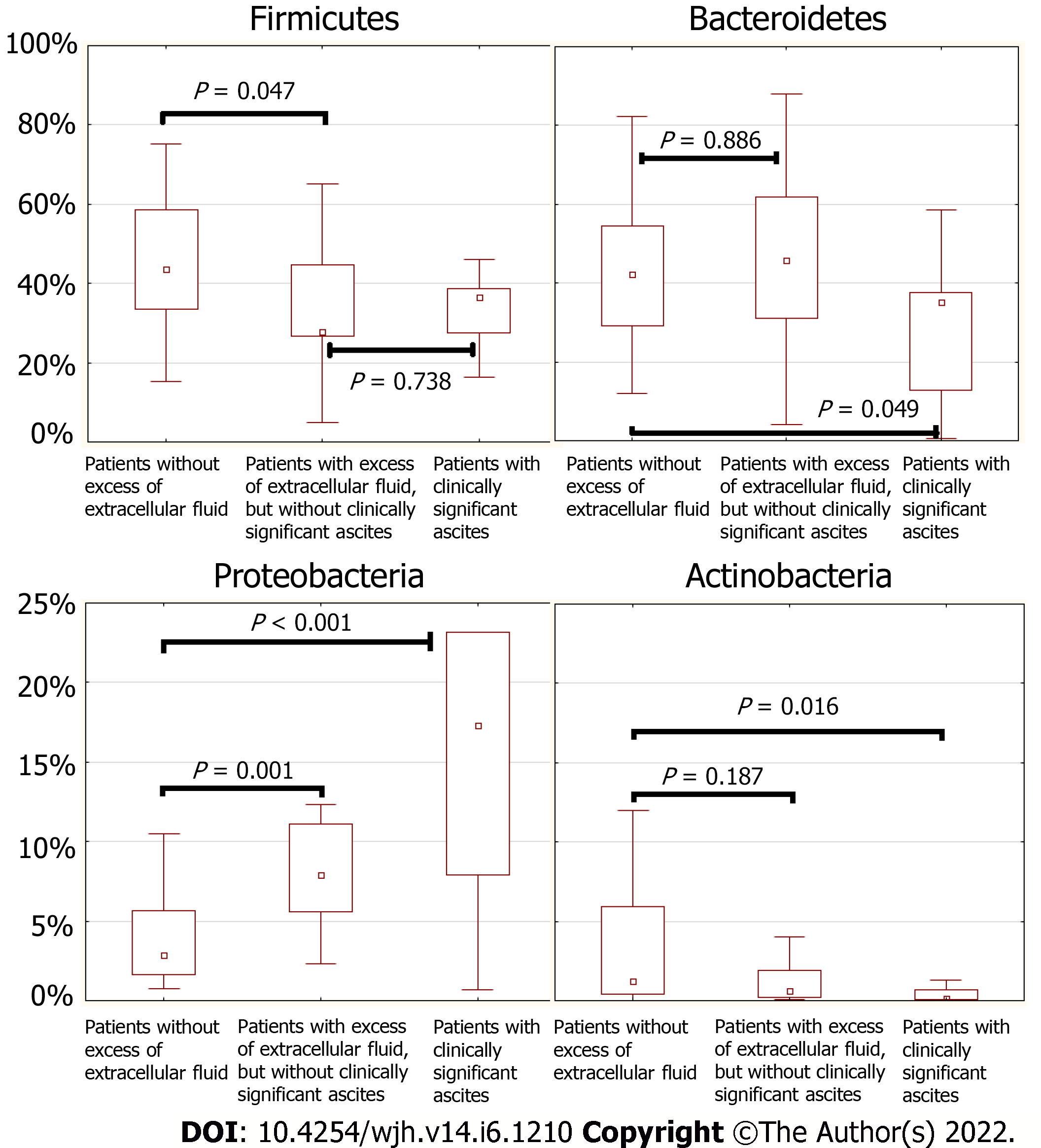
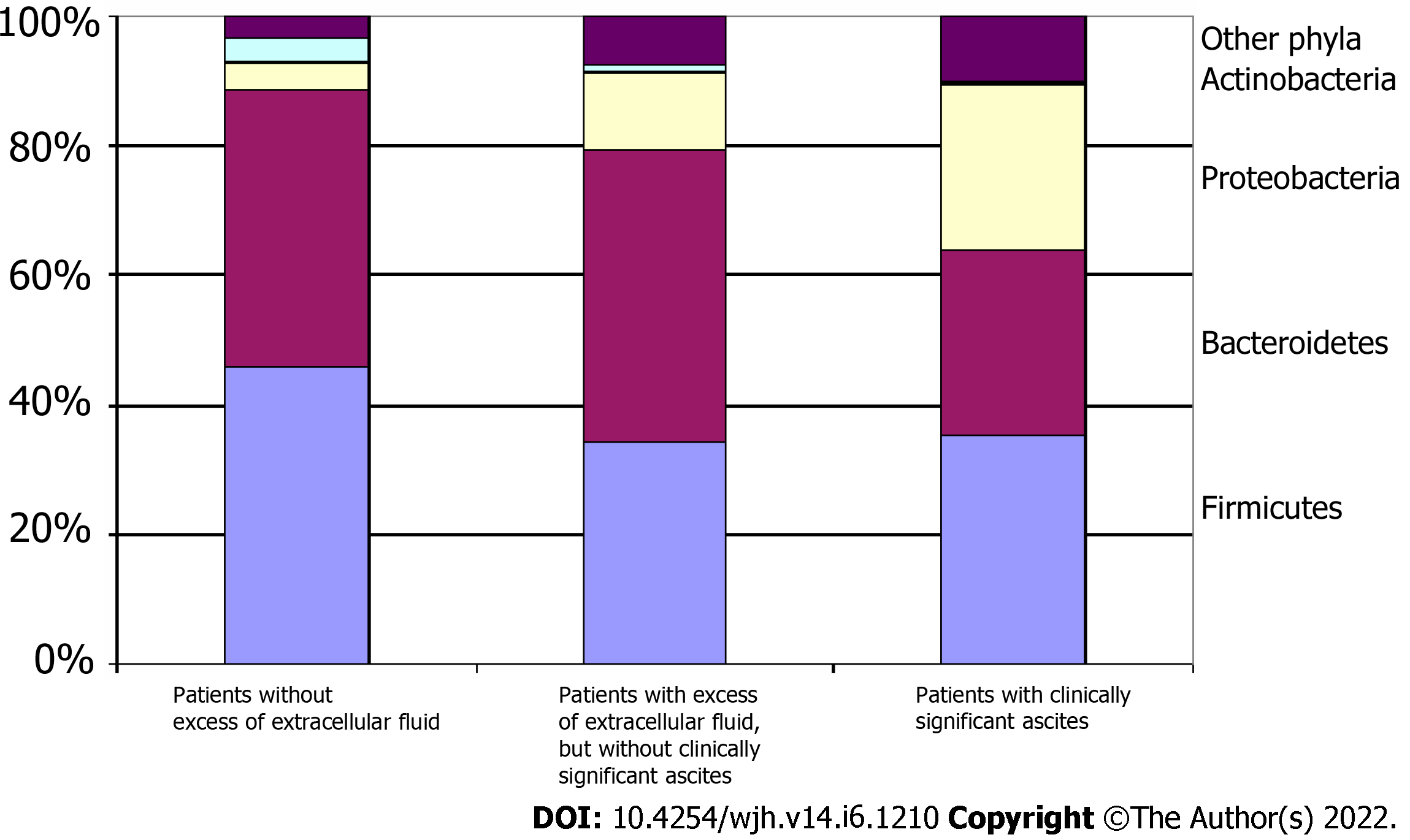
Patients with clinically significant ascites (stages 2 and 3 according to the classification of the International Club of Ascites; n = 9) had a higher abundance of Proteobacteria [17.3 (7.9-23.2)% vs 5.03 (2.26-7.93)%; P = 0.031] and a lower abundance of Actinobacteria [0.11 (0.09-0.66)% vs 1.04 (0.36-3.89)%; P = 0.019] and Bacteroidetes [35.2 (12.9-37.6)% vs 43.2 (29.4-60.3)%; P = 0.046] in their gut microbiome than patients without clinically significant ascites (n = 37).
The abundance of Firmicutes was decreased in patients with excess extracellular fluid regardless of the presence of clinically significant ascites, while a decrease in the abundance of Bacteroidetes occurred only in those patients with excess extracellular fluid who had clinically significant ascites (Figures 4 and 5). The abundance of Proteobacteria progressively increased, and the abundance of Actinobacteria progressively decreased in the transition from patients without excess extracellular fluid to patients with excess extracellular fluid but without clinically significant ascites, and further to patients with clinically significant ascites (Figures 4 and 5).
Gut dysbiosis is common in cirrhosis and is associated with the development of hepatic encephalopathy, lower serum albumin and cholinesterase levels, systemic inflammation, and poorer short- and long-term prognosis[19-21]. The aim of the present study was to assess the relationship between the gut microbiota and body composition in patients with cirrhosis.
The changes in body composition and the gut microbiome with cirrhosis in our study were mostly consistent with earlier findings[1,6,19-21].
Although malnutrition is typical in patients with cirrhosis, half of the patients enrolled in the present study had excess fat mass. This can be explained by the fact that 30% of the included patients had compensated cirrhosis (class A Child-Pugh score), while severe cirrhosis (Child-Pugh class C), for which malnutrition was most characteristic, was observed in less than a quarter of the patients. The inclusion of a small percentage of patients with severe cirrhosis is both a disadvantage and an advantage in our study, as we included patients with varying degrees of cirrhosis severity, which enabled a more generalized analysis.
Cirrhosis was less severe in patients with excess fat mass. In terms of the taxa of gut microbiota, the increased abundance of Bacteroidetes in these patients was the most significant change. However, obesity in patients without cirrhosis is associated with a decrease in the abundance of Bacteroidetes[22,23]. The change in abundance of Bacteroidetes in cirrhosis is controversial: studies have reported its decrease[24-26], increase[27], and non-significant changes[19]. One study reported an increase in Bacteroidetes abundance in compensated cirrhosis, which decreased further to attain normal levels with decompensation[28]. Patients with excess fat mass had less severe cirrhosis and were older than patients without excess fat mass. Multivariate regression analysis established that the age and Child-Pugh score, but not the gut microbiome status, significantly determined the level of fat mass in patients with cirrhosis, thereby resolving this contradiction.
Patients with body cell mass deficiency who were considered to have sarcopenia accounted for one third of the included patients. They also had another sign of malnutrition (namely, hypoalbuminemia), although they did not show significant differences in the values of other biomarkers of liver failure (serum bilirubin and prothrombin) and portal hypertension (clinically significant ascites and spleen length) compared to patients with normal body cell mass. Patients grouped with respect to body cell mass deficiency did not show significant differences in the gut microbiome at the level of higher taxa (phyla), although the abundance of Bacteroidaceae was higher in patients with body cell mass deficiency. These patients also had increased abundance of Eggerthella, which is considered a biomarker of fragility[29,30]. These findings are consistent with recent studies of the gut microbiome in cirrhosis patients with sarcopenia[4,5]. However, body cell mass deficiency in cirrhosis patients was found to be associated with a decrease in the abundance of Coprococcus, Intestinimonas, Catenibacterium, and Barnesiellaceae in our study, which was not reported in these earlier studies[4,5]. A decrease in the abundance of the butyrate-producing Coprococcus has been reported in hemodialysis patients with sarcopenia[31]. Intestinimonas produces butyrate and vitamin B12, and is involved in the metabolism of bile acids and glucose in hosts[32-34]. Catenibacterium is associated with the development of insulin resistance in morbid obesity[35]; thus, it is quite possible that a decrease in its content in the gut microbiome is associated with malnutrition. Decreased abundance of Barnesiellaceae and increased abundance of Veillonella in the gut microbiome, found in our study in patients with body cell mass deficiency, have previously been described in the general cohort of sarcopenic patients[36], but not in earlier investigations of sarcopenia in cirrhosis patients[4,5].
The pathophysiology of sarcopenia in cirrhosis has started to attract more attention[37]. The present study is the third publication to describe the changes in the gut microbiome in this condition. The major findings from all three publications partially correspond with each other, but there are also some differences between them, which highlights the need for further studies of these relationships. We did not obtain a significant correlation between the main taxa responsible for bacterial translocation (Proteobacteria and Bacilli) and a decrease in the body cell mass. This diminishes the plausibility of the hypothesis of their relationship. Unfortunately, we were unable to investigate blood levels of myostatin and ammonia and the correlations between them, the body cell mass, and taxa of the gut microbiome. Thus, the exact mechanisms of the effects of gut microbiota on muscle mass in cirrhosis should be established by further research.
An increase in the content of extracellular fluid in the body was accompanied by the frequent development of clinically significant ascites and splenomegaly. Among the large number of taxa that changes were associated with an increase in the content of extracellular fluid in patients with cirrhosis, the most important changes were an increase in the abundance of Proteobacteria and a decrease in that of Firmicutes.
An increase in the abundance of Proteobacteria and other taxa belonging to this phylum was previously described in patients with cirrhosis compared to healthy individuals in most studies[19,24,26-28,38-43]. Proteobacteria have an active endotoxin, which is believed to be associated with the development of systemic inflammation, vasodilation, and subsequent compensatory accumulation of extracellular fluid in cirrhosis[20]. Despite multiple reviews touching upon this aspect, the present study is the first to prove that an increased abundance of Proteobacteria in the gut microbiome is indeed associated with the accumulation of extracellular fluid in patients with cirrhosis.
Firmicutes are mainly represented by the class of autochthonous strict anaerobes Clostridia and the class of facultative anaerobes Bacilli. Among the Bacilli, there are many opportunistic species associated with endogenous infections in cirrhosis[44]. The abundance of Clostridia and Bacilli changes with the progression of cirrhosis: while the former decreases, the latter increases[19,28]. Therefore, the net change in the abundance of Firmicutes in cirrhosis has been reported to increase in some studies[25] and decrease in others[41]. The association of increased extracellular fluid content with decreased abundance of beneficial Clostridia was expected, but the observed association with the decreased abundance of harmful Bacilli was surprising. However, Bacilli, unlike Proteobacteria, do not produce endotoxin. Therefore, it seems that it is endotoxin, and not other factors of bacterial pathogenicity, that plays a major role in the accumulation of extracellular fluid in patients with cirrhosis.
Our study showed that fluid retention developed before the development of clinically significant ascites. At the same time, there was a further increase in the abundance of Proteobacteria with a decrease in the abundance of Bacteroidetes in patients with clinically significant ascites. We observed a stepwise change in the gut microbiome at the phylum level with an increase in the content of extracellular fluid in the body: First Proteobacteria displace Firmicutes, and then they override Bacteroidetes (Figure 5).
The strengths of the present study are that these findings represent the first comprehensive report on the relationship between gut microbiota and changes in body composition in cirrhosis, and the first confirmation that an increased abundance of Proteobacteria is associated with increased extracellular fluid in patients with cirrhosis. In addition, this study is one of the few works that have investigated the relationship between the gut microbiome and sarcopenia in patients with cirrhosis.
The limitation of our study lies in its small sample size, although this did not prevent us from obtaining significant results.
In conclusion, we have shown that the various body components are differently associated with changes in the gut microbiome in cirrhosis. The amount of fat mass does not depend on its composition, the amount of body cell mass is associated with changes in the abundance of its minor taxa, and the amount of extracellular fluid is associated with changes in the abundance of the main taxa of the gut microbiome (Proteobacteria, Firmicutes, and Bacteroidetes).
Gut dysbiosis and changes in body composition (i.e., a decrease in the proportion of muscle mass and an increase in extracellular fluid) are common in cirrhosis.
To study the relationship between the gut microbiota and body composition in cirrhosis.
To study the relationship between the gut microbiota and various body components in cirrhosis.
This observational study included 46 patients with cirrhosis. Stool microbiome was assessed using 16S rRNA gene sequencing. Multifrequency bioelectrical impedance analysis was performed to assess body composition in these patients.
The abundance of Bacteroidaceae and Eggerthella increased, whereas that of Coprococcus, Erysipelatoclostridiaceae, Intestinimonas, Desulfovibrio, Catenibacterium, and Senegalimassilia decreased in the gut microbiome of patients with body cell mass deficiency. Proteobacteria abundance was increased, whereas Firmicutes, Oscillospiraceae, Rikenellaceae, Actinobacteria, Bacilli, Christensenellaceae, Collinsella, Eggerthellaceae, Erysipelatoclostridiaceae, Erysipelotrichaceae, Anaerovoraceceae, Hungatella, Slackia, Peptococcaceae, and Senegalimassilia abundance decreased in cirrhosis patients with excess extracellular fluid.
Changes in the amount of body cell mass and extracellular fluid are associated with changes in the gut microbiome in cirrhosis patients.
Further studies are required to establish the mechanisms underlying the influence of the gut microbiota on the value of body cell mass.
The authors are grateful to the staff of the Department of Hepatology: Alexei Lapshin, Shauki Ondos, Petr Tkachenko, Igor Tikhonov and others.
| 1. | Ponziani FR, Gasbarrini A. Sarcopenia in Patients with Advanced Liver Disease. Curr Protein Pept Sci. 2018;19:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Yao J, Chang L, Yuan L, Duan Z. Nutrition status and small intestinal bacterial overgrowth in patients with virus-related cirrhosis. Asia Pac J Clin Nutr. 2016;25:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 4. | Ren X, Hao S, Yang C, Yuan L, Zhou X, Zhao H, Yao J. Alterations of intestinal microbiota in liver cirrhosis with muscle wasting. Nutrition. 2021;83:111081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Ponziani FR, Picca A, Marzetti E, Calvani R, Conta G, Del Chierico F, Capuani G, Faccia M, Fianchi F, Funaro B, Josè Coelho-Junior H, Petito V, Rinninella E, Paroni Sterbini F, Reddel S, Vernocchi P, Cristina Mele M, Miccheli A, Putignani L, Sanguinetti M, Pompili M, Gasbarrini A; GuLiver study group. Characterization of the gut-liver-muscle axis in cirrhotic patients with sarcopenia. Liver Int. 2021;41:1320-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Simbrunner B, Mandorfer M, Trauner M, Reiberger T. Gut-liver axis signaling in portal hypertension. World J Gastroenterol. 2019;25:5897-5917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int. 2018;12:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Maslennikov R, Pavlov C, Ivashkin V. Is small intestinal bacterial overgrowth a cause of hyperdynamic circulation in cirrhosis? Turk J Gastroenterol. 2019;30:964-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Overby HB, Ferguson JF. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota:Host Cross talk and Modulate Obesity and Hypertension. Curr Hypertens Rep. 2021;23:8. [PubMed] [DOI] [Full Text] |
| 10. | Cichoż-Lach H, Michalak A. A Comprehensive Review of Bioelectrical Impedance Analysis and Other Methods in the Assessment of Nutritional Status in Patients with Liver Cirrhosis. Gastroenterol Res Pract. 2017;2017:6765856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AM, Pichard C; Composition of the ESPEN Working Group. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1882] [Article Influence: 89.6] [Reference Citation Analysis (1)] |
| 12. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, Lilienthal Heitmann B, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, M W J Schols A, Pichard C; ESPEN. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1512] [Article Influence: 72.0] [Reference Citation Analysis (1)] |
| 13. | Fernandes SA, de Mattos AA, Tovo CV, Marroni CA. Nutritional evaluation in cirrhosis: Emphasis on the phase angle. World J Hepatol. 2016;8:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Fouhy F, Deane J, Rea MC, O'Sullivan Ó, Ross RP, O'Callaghan G, Plant BJ, Stanton C. The effects of freezing on faecal microbiota as determined using MiSeq sequencing and culture-based investigations. PLoS One. 2015;10:e0119355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Parikh HI, Koparde VN, Bradley SP, Buck GA, Sheth NU. MeFiT: merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinformatics. 2016;17:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18515] [Cited by in RCA: 19918] [Article Influence: 1991.8] [Reference Citation Analysis (14)] |
| 17. | Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590-D596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15104] [Cited by in RCA: 19665] [Article Influence: 1512.7] [Reference Citation Analysis (0)] |
| 18. | Kim BR, Shin J, Guevarra R, Lee JH, Kim DW, Seol KH, Kim HB, Isaacson R. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J Microbiol Biotechnol. 2017;27:2089-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 587] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 19. | Maslennikov R, Ivashkin V, Efremova I, Alieva A, Kashuh E, Tsvetaeva E, Poluektova E, Shirokova E, Ivashkin K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J Hepatol. 2021;13:557-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 21. | Bajaj JS, Vargas HE, Reddy KR, Lai JC, O'Leary JG, Tandon P, Wong F, Mitrani R, White MB, Kelly M, Fagan A, Patil R, Sait S, Sikaroodi M, Thacker LR, Gillevet PM. Association Between Intestinal Microbiota Collected at Hospital Admission and Outcomes of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:756-765.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Palmas V, Pisanu S, Madau V, Casula E, Deledda A, Cusano R, Uva P, Vascellari S, Loviselli A, Manzin A, Velluzzi F. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci Rep. 2021;11:5532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 23. | Gasmi Benahmed A, Gasmi A, Doşa A, Chirumbolo S, Mujawdiya PK, Aaseth J, Dadar M, Bjørklund G. Association between the gut and oral microbiome with obesity. Anaerobe. 2020;70:102248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 24. | Jin M, Kalainy S, Baskota N, Chiang D, Deehan EC, McDougall C, Tandon P, Martínez I, Cervera C, Walter J, Abraldes JG. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019;39:1437-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Liu Y, Jin Y, Li J, Zhao L, Li Z, Xu J, Zhao F, Feng J, Chen H, Fang C, Shilpakar R, Wei Y. Small Bowel Transit and Altered Gut Microbiota in Patients With Liver Cirrhosis. Front Physiol. 2018;9:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 823] [Article Influence: 54.9] [Reference Citation Analysis (3)] |
| 27. | Zeng Y, Chen S, Fu Y, Wu W, Chen T, Chen J, Yang B, Ou Q. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat. 2020;27:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 28. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 878] [Article Influence: 73.2] [Reference Citation Analysis (1)] |
| 29. | Margiotta E, Miragoli F, Callegari ML, Vettoretti S, Caldiroli L, Meneghini M, Zanoni F, Messa P. Gut microbiota composition and frailty in elderly patients with Chronic Kidney Disease. PLoS One. 2020;15:e0228530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O'Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 303] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 31. | Lin TY, Wu PH, Lin YT, Hung SC. Characterization of Gut Microbiota Composition in Hemodialysis Patients With Normal Weight Obesity. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Bui TP, Shetty SA, Lagkouvardos I, Ritari J, Chamlagain B, Douillard FP, Paulin L, Piironen V, Clavel T, Plugge CM, de Vos WM. Comparative genomics and physiology of the butyrate-producing bacterium Intestinimonas butyriciproducens. Environ Microbiol Rep. 2016;8:1024-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Vitale M, Giacco R, Laiola M, Della Pepa G, Luongo D, Mangione A, Salamone D, Vitaglione P, Ercolini D, Rivellese AA. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: Can SCFAs play a role? Clin Nutr. 2021;40:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 34. | Lin H, An Y, Tang H, Wang Y. Alterations of Bile Acids and Gut Microbiota in Obesity Induced by High Fat Diet in Rat Model. J Agric Food Chem. 2019;67:3624-3632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 35. | Moreno-Indias I, Sánchez-Alcoholado L, García-Fuentes E, Cardona F, Queipo-Ortuño MI, Tinahones FJ. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am J Transl Res. 2016;8:5672-5684. [PubMed] |
| 36. | Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho-Junior HJ, Gervasoni J, Primiano A, Putignani L, Del Chierico F, Reddel S, Gasbarrini A, Landi F, Bernabei R, Marzetti E. Gut Microbial, Inflammatory and Metabolic Signatures in Older People with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 37. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 748] [Article Influence: 106.9] [Reference Citation Analysis (2)] |
| 38. | Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 653] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 39. | Kajihara M, Koido S, Kanai T, Ito Z, Matsumoto Y, Takakura K, Saruta M, Kato K, Odamaki T, Xiao JZ, Sato N, Ohkusa T. Characterisation of blood microbiota in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2019;31:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Chen Z, Xie Y, Zhou F, Zhang B, Wu J, Yang L, Xu S, Stedtfeld R, Chen Q, Liu J, Zhang X, Xu H, Ren J. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front Microbiol. 2020;11:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Zheng R, Wang G, Pang Z, Ran N, Gu Y, Guan X, Yuan Y, Zuo X, Pan H, Zheng J, Wang F. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020;9:4232-4250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 42. | Lapidot Y, Amir A, Nosenko R, Uzan-Yulzari A, Veitsman E, Cohen-Ezra O, Davidov Y, Weiss P, Bradichevski T, Segev S, Koren O, Safran M, Ben-Ari Z. Alterations in the Gut Microbiome in the Progression of Cirrhosis to Hepatocellular Carcinoma. mSystems. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 43. | Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ, Sikaroodi M, Gillevet PM. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 44. | Bhattacharya C, Das-Mondal M, Gupta D, Sarkar AK, Kar-Purkayastha S, Konar A. Infection in cirrhosis: A prospective study. Ann Hepatol. 2019;18:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amedei A, Italy; Houri H, Iran S-Editor: Chang KL L-Editor: Webster JR P-Editor: Chang KL