Published online Jan 27, 2022. doi: 10.4254/wjh.v14.i1.260
Peer-review started: May 15, 2021
First decision: July 8, 2021
Revised: July 10, 2021
Accepted: December 31, 2022
Article in press: December 31, 2021
Published online: January 27, 2022
Processing time: 250 Days and 17.7 Hours
Chronic viral B hepatitis (CHB) is a potentially life-threatening liver disease that may progress to liver failure and cirrhosis. Currently, although combinations of different laboratory methods are used in the follow-up and treatment of CHB, the failure of these procedures in some cases has led to the necessity of developing new approaches. In CHB, the intrahepatic expression pattern of viral antigens, including hepatitis B surface antigen (HBsAg), is related to different phases of inflammation. However, many studies have focused on the intracytoplasmic properties of HBsAg staining, and HBsAg positivity in liver tissue has not been evaluated by objective quantitative methods.
To investigate the relationship of image analysis-based quantitative HBsAg expression and its staining patterns with clinicopathological factors and treatment in CHB.
A total of 140 liver biopsies from treatment-naïve cases with CHB infection were included in this study. Following diagnosis, all patients were treated with entecavir (0.5 mg) and followed up at three-month intervals. The percentage of immunohistochemical HBsAg (p-HBsAg) expression in the liver was determined in whole tissue sections of biopsies from each case by image analysis. The immunohistochemical staining pattern was also evaluated separately according to 3 different previously defined classifications.
A positive correlation between p-HBsAg and serum levels of hepatitis B virus (HBV) DNA and HBsAg was observed (P < 0.001). The p-HBsAg value was significantly higher in younger patients than in older patients. When the groups were categorized according to the hepatitis B e antigen (HBeAg) status in HBeAg-positive cases, p-HBsAg was correlated with HBV DNA, hepatitis activity index (HAI) and fibrosis scores (P < 0.001). In this group, p-HBsAg and HBsAg expression patterns were also correlated with the viral response (VR) and the serological response (SR) (P < 0.001). Multivariate analysis revealed that p-HBsAg was an independent predictor of either VR or SR (P < 0.001). In HBeAg-negative patients, although HBsAg expression patterns were correlated with both HAI and fibrosis, no relationship was observed among p-HBsAg, clinicopathological factors and VR.
In pretreatment liver biopsies, the immunohistochemical determination of HBsAg expression by quantitative methods, beyond its distribution within the cell, may be a good predictor of the treatment response, especially in HBeAg-positive cases.
Core Tip: This report describes a study that investigated image analysis-based quantitative hepatitis B surface antigen (HBsAg) expression and its different staining patterns in liver biopsies from patients with chronic viral B hepatitis (CHB) and correlated them with clinicopathological factors and treatment. Our findings confirmed the association of cytoplasmic HBsAg staining patterns with disease activity. Besides, the determination of immunohistochemical HBsAg expression by image analysis may be an important predictor of the response to therapy, especially in hepatitis B e antigen-positive cases. Accordingly, evaluation of the percentage of HBsAg expression by objective methods in liver tissues from treatment-naïve CHB patients might provide a useful tool in the follow-up and treatment of this disease.
- Citation: Alpsoy A, Adanir H, Bayramoglu Z, Elpek GO. Correlation of hepatitis B surface antigen expression with clinicopathological and biochemical parameters in liver biopsies: A comprehensive study. World J Hepatol 2022; 14(1): 260-273
- URL: https://www.wjgnet.com/1948-5182/full/v14/i1/260.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i1.260
Despite advances in its prevention, diagnosis and treatment, chronic viral B hepatitis (CHB) continues to be an important worldwide health problem with high morbidity and mortality due to its dramatic consequences, such as cirrhosis and hepatocellular carcinoma[1]. In recent years, researchers have suggested that the combination of various laboratory methods with histopathological findings may allow the assessment of the prognosis and treatment of patients with CHB[2-4]. High liver biopsy activity scores, low viremia and high serum alanine aminotransferase (ALT) levels are associated with hepatitis B e antigen (HBeAg) seroconversion, while a decrease in serum hepatitis B surface antigen (HBsAg) levels is among the factors associated with an effective response to therapy[5-7]. Unfortunately, the presence of these factors is not always adequate to determine the progression of the disease and the efficacy of treatment. The treatment response is not sufficient in some patients with these factors, which contrasts with the adequate response in other patients without these factors, suggesting that other viral and host-related parameters may also be involved in the progression and therapy response.
Recent studies have also provided evidence that in patients with CHB, the expression of viral antigens and their expression patterns detected by immunohistochemistry may be related to clinicopathological factors and might have prognostic implications for the disease process. The expression of hepatitis B core antigen (HBcAg) and its expression patterns were found to be associated with serum HBeAg, viral replication and the hepatitis activity index (HAI)[8-11]. In addition, the results of some studies have suggested the potential use of HBcAg expression as a marker of treatment response[12-14].
Regarding HBsAg, the results of many different studies investigating the relationships between the intrahepatic expression of this antigen revealed that differences in the intracytoplasmic HBsAg staining pattern were related to viremia and active inflammation[14-18]. In these investigations, HBsAg expression was evaluated semiquantitatively, and different classifications were used for the HBsAg staining pattern. On the other hand, HBsAg, beyond being the main diagnostic marker of the disease, is a very important tool in the follow-up of the course and treatment of CHB, since a decrease in its level reflects the low replicative phase[19-21]. Therefore, more objective methods are needed to determine whether the immunohistochemical expression of HBsAg in pretreatment-performed liver biopsies can be useful in monitoring the prognosis and treatment of CHB.
Therefore, this study was undertaken to investigate the relationship between image analysis-based quantitation of HBsAg expression and corresponding staining patterns with clinicopathological factors and treatment.
This retrospective study was conducted in accordance with the Declaration of Helsinki. Approval for this study was obtained from the Akdeniz University Faculty of Medicine Clinical Research Ethics Committee (Date: January 28, 2015; Approval number: 2015.01.211.016). All patients provided written informed consent for participation in the study.
The study group consisted of 140 patients who were followed up and treated after the diagnosis of CHB at Akdeniz University Medical School between 2015 and 2020. The inclusion criteria were the presence of positive serum HBsAg for at least 6 mo and the absence of coinfection with human immunodeficiency, hepatitis delta and hepatitis C viruses. None of these patients had end-stage liver failure, immunosuppressive therapy, malignancy, autoimmune hepatitis or alcoholic hepatitis.
Clinical and laboratory parameters were recorded from all cases on the day of liver biopsy. These included the age of the patients, sex, HBV DNA, HBsAg ALT, INR, and platelet count. Forty-nine cases with elevated ALT levels underwent antiviral therapy. Ninety-one patients with low ALT levels received this treatment based on the increase in ALT levels in the first three months of follow-up. Accordingly, all patients were treated with entecavir (0.5 mg) within three months after the liver biopsies and followed up at three-month intervals. An HBV DNA level below 20 IU/mL detected by real-time polymerase chain reaction (PCR) was defined as the viral response (VR). HBeAg seroclearance and a decrease in serum HBsAg level during follow-up > 0.5 log10 IU/mL were defined as the serological response (SR) to treatment[22].
Serum HBsAg, HBeAg, antibodies against HBsAg (anti-HBs) and HBeAg were tested by using commercial kits (Abbott Laboratories, Abbott Park, IL, United States). Serum HBV-DNA was extracted from 200 μL of serum by a QIAmp DNA Blood Mini Kit (QIAGEN Inc., Valencia, CA, United States) and quantified by a real-time PCR amplification assay using a LightCycler (Roche Diagnostics, Basel, Switzerland). The detection sensitivity was 20 IU/mL[23]. Serum HBsAg was quantified with the ARCHITECT HBsAg assay (Abbott Laboratories) with a dynamic range from 0.05 IU/mL to 250 IU/mL.
Microscopic evaluation was performed on serial sections stained with hematoxylin and eosin from paraffin-embedded 10% formalin-fixed liver tissues. The HAI and fibrosis stages were determined according to the Ishak system[24]. HAI grading consists of the sum of necroinflammatory scores, including portal inflammation, periportal interface hepatitis, confluent necrosis and focal lytic necrosis, and each was given a score from 0 to 4-6 with a maximal HAI grade of 18. The fibrosis staging ranged from 0 to 6.
Immunohistochemical staining for HBsAg was performed by using a primary antibody against HBsAg (mouse monoclonal antibody, clone 3E7, Dako, Carpinteria, CA, United States). Staining was performed automatically with a BenchMark XT (Ventana Medical Systems, Tucson, AZ, United States) in the Department of Pathology, Akdeniz University Medical School.
Image analysis was performed using a SAMBA 2005 image processor (Alcatel-TITN, Grenoble, France). This system consists of a Leitz Diaplan Microscope connected to a personal computer through a Sony color camera and a data translation frame grabber board. For detection of the percentage of HBsAg immunostaining, the system was calibrated for a 20X objective. In each area, antigen immunoreactivity was measured in the entire tissue section. The results obtained were expressed as the percentage of the ratio of the HBsAg (p-HBsAg) immunoreactive area to the total scanned area.
The immunohistochemical staining pattern was evaluated separately according to 3 different previously defined classifications[15,18].
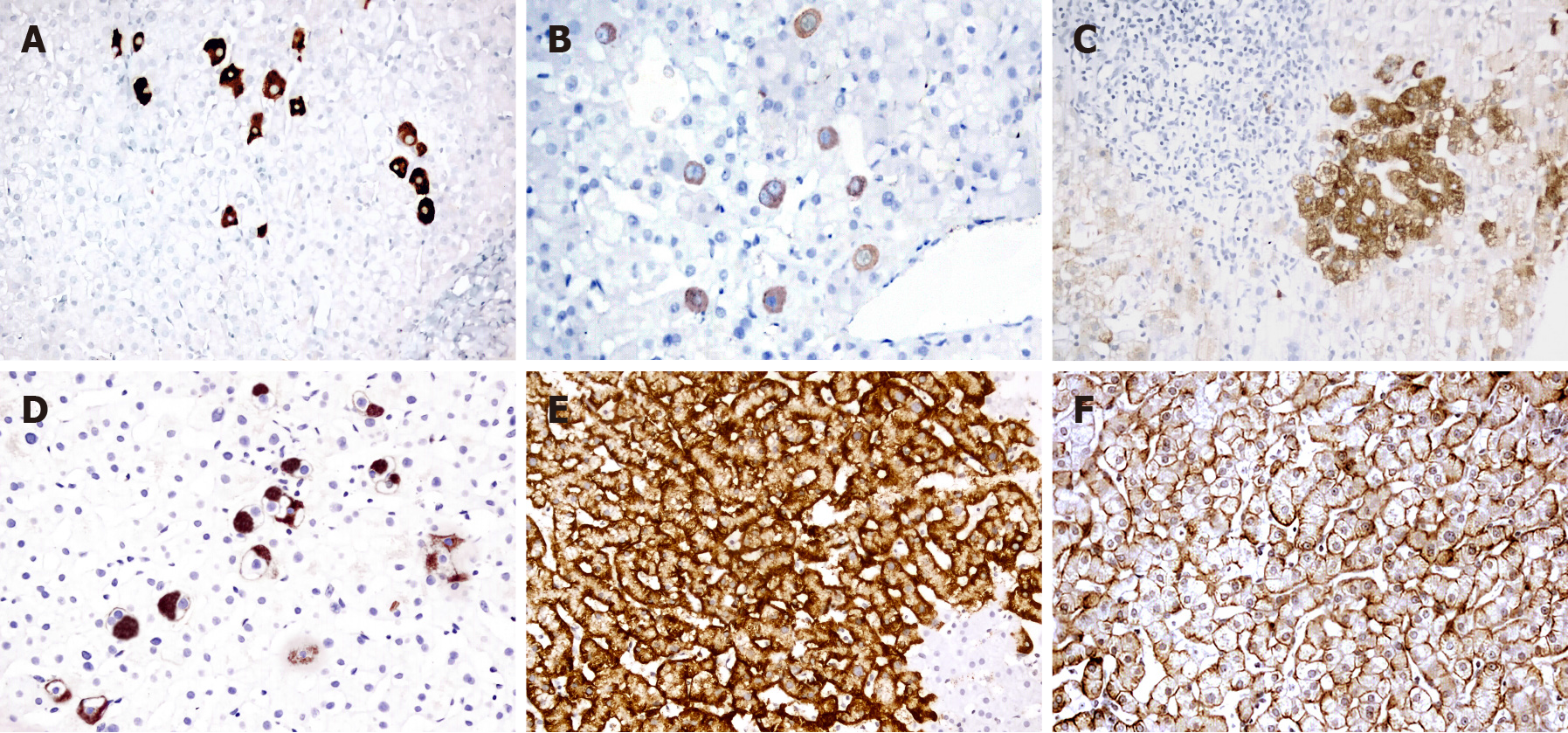
Staining pattern 1: The staining was classified according to the expression type and distribution into five major patterns. Patterns A and B were characterized by diffuse cytoplasmic staining in discrete hepatocytes (Figure 1A and B). While the intensity of staining was stronger in pattern A, it was faint in pattern B. In pattern C, cytoplasmic HBsAg was similar to that in pattern B but was distributed in clusters of hepatocytes (Figure 1C). Pattern D included globular and spotty cytoplasmic staining in either discrete or clustered hepatocytes (Figure 1D). Pattern E (marginal HBsAg) was characterized by submembranous staining of HBsAg beneath the cell membranes in the hepatocyte groups (Figure 1E).
Staining pattern 2: The expression of HBsAg was categorized according to its intracytoplasmic expression: 1: Diffuse (Figure 1A-C); 2: Globular (Figure 1D); and 3: Submembranous (Figure 1E).
Staining pattern 3: In this classification, HBs staining was categorized according to the presence or absence of membranous staining (Figure 1F).
Statistical analysis was performed using SPSS version 25.0 (IBM Corp., Armonk, NY, United States). Nominal and ordinal data are expressed as frequencies (percentages), and continuous variables are expressed as the mean (standard error). The χ2 test was employed to examine categorical data. Continuous variables between the groups and their relationship with the clinicopathological parameters were investigated by t tests. Univariate analysis, including response to therapy, was estimated with the Kaplan-Meier method. The log-rank test was employed for comparisons of response rates. A Cox proportional hazards regression model was applied for multivariate analysis. Spearman’s correlation test was used to determine relationships between p-HBsAg and the other continuous variables. The threshold P value accepted for statistical significance was < 0.05.
The mean age of the patients in the whole group was 42.79 ± 11.53 years, and the male to female ratio was 1.25 (Table 1). There was a positive correlation between p-HBsAg and the serum levels of HBV DNA and HBsAg (r: 0.490, r: 0.468 P < 0.001, respec
| Parameters | Total | HBeAg positive | HBeAg negative |
| n | 140 | 86 | 54 |
| Age (yr) | 42.75 ± 11.53 | 40.30 ± 11.03 | 46.74 ± 11.29a |
| Gender | |||
| Male | 78 | 48 (61.5) | 30 (38.5) |
| Female | 62 | 38 (61.3) | 24 (38.7) |
| HBV DNA (log10IU/mL) | 7.27 ± 2.17 | 7.67 ± 2.39 | 6.65 ± 1.58a |
| HBsAg (log10IU/mL) | 3.85 ± 1.04 | 4.01 ± 1.07 | 3.61 ± 0.94b |
| ALT IU/L | 7.27 ± 2.17 | 155.80 ± 114.32 | 134.54 ± 98.71 |
| ALT < 200 IU/L | 91 | 52 (51.7) | 39 (42.9) |
| ALT ≥ 200 IU/L | 49 | 34 (69.4) | 15 (30.6) |
| INR | 1.08 ± 0.21 | 1.04 ± 0.14 | 1.15 ± 0.27b |
| Platelets (× 103/μL) | 226.4 ± 73.43 | 224.54 ± 70.67 | 227.59 ± 75.53 |
| HAI score ≥ 9 | 61 | 38 (62.3) | 23 (37.7) |
| HAI score < 9 | 79 | 48 (60.8) | 31 (39.2) |
| Fibrosis ≥ 4 | 53 | 33 (62.3) | 20 (37.7) |
| Fibrosis < 4 | 87 | 53 (60.9) | 34 (39.1) |
| p-HBsAg | 41.05 ± 22.78 | 47.2 ± 13.98 | 31.26 ± 13.98a |
| HBsAg SP1 | |||
| A | 33 | 17 (51.5) | 16 (48.5) |
| B | 24 | 10 (41.7) | 14 (58.3) |
| C | 21 | 16 (76.2) | 5 (23.8) |
| D | 16 | 10 (62.5) | 6 (37.5) |
| E | 46 | 33 (71.7) | 13 (28.3) |
| HBsAg SP2 | |||
| Diffuse | 59 | 29 (49.2) | 30 (50.8) |
| Globular | 34 | 24 (70.6) | 10 (29.4) |
| Submembranous | 47 | 33 (70.2) | 14 (29.8) |
| Membranous expression | |||
| Present | 53 | 33 (63.3) | 20 (37.7) |
| Absent | 87 | 53 (60.9) | 34 (39.1) |
The relationship between viremia and histopathological findings in cases with positive HBeAg are summarized in Table 2. The average p-HBsAg level was found to be significantly higher in patients with higher HBV DNA values than in those with low HBV DNA levels. In contrast, an inverse relationship was noted between p-HBsAg and either HAI or the fibrosis score. Regarding staining pattern 1 (SP1), the number of cases with pattern A was higher among cases with higher HBV DNA levels. Their number decreased among cases with HAI ≥ 9, and it was not observed among cases with fibrosis. When staining pattern 2 (SP2) was analyzed, it was noted that the number of cases with diffuse staining was higher in the HAI ≥ 9 group. In contrast, among the cases with high HAI and fibrosis scores, the frequency of pattern E was observed in 42% and 75% of the cases, respectively.
| HBV DNA | HAI | Fibrosis | ||||
| Parameters | < 7.67 | ≥ 7.67 | < 9 | ≥ 9 | < 4 | ≥ 4 |
| n | 32 | 54 | 48 | 38 | 53 | 33 |
| Age (yr) | 48.28 ± 9.6 | 35.57 ± 8.93b | 37.79 ± 10.83 | 43.47 ± 10.59a | 36.06 ± 9.39 | 47.12 ± 10.11b |
| Gender | ||||||
| Male | 23 (71.9) | 25 (46.3)a | 23 (47.9) | 25 (65.8) | 27 (50.9) | 21 (63.6) |
| Female | 9 (28.1) | 29 (53.7) | 25 (52.1) | 13 (34.2) | 26 (49.1) | 12 (36.4) |
| HBV DNA (log10IU/mL) | - | - | 8.36 ± 2.26 | 6.79 ± 2.30a | 8.15 ± 2.32 | 6.89 ± 2.34 |
| HBsAg (log10IU/mL) | 3.02 ± 0.91 | 4.59 ± 0.64b | 4.28 ± 0.91 | 3.66 ± 1.61a | 4.19 ± 1.01 | 3.07 ± 1.10a |
| ALT IU/L | 106.97 ± 73.55 | 184.74 ± 124.85a | 148.97 ± 61.46 | 121.46 ± 50.84 | 167.74 ± 72.1 | 136.64 ± 56.2 |
| ALT< 200 IU/L | 26 (81.2) | 26 (48.1)a | 34 (70.8) | 18 (47.4)a | 32 (60.4) | 20 (60.6) |
| ALT ≥ 200 IU/L | 6 (18.8) | 28 (51.9) | 14 (29.2) | 20 (52.6) | 21 (39.6) | 13 (39.4) |
| INR | 1.03 ± 0.08 | 1.05 ± 0.16 | 1.03 ± 0.10 | 1.06 ± 0.17 | 1.04 ± 0.16 | 1.04 ± 0.09 |
| Platelets (× 103/μL) | 217.59 ± 66.85 | 233.98 ± 80.60 | 226 ± 74.74 | 228 ± 77.51 | 224.53 ± 74.72 | 232.61 ± 77.82 |
| HAI score ≥ 9 | 22 (68.8) | 16 (29.6)b | - | - | 17 (32.1) | 21 (63.6)b |
| HAI score < 9 | 10 (31.2) | 38 (70.4) | - | - | 36 (67.9) | 12 (36.4) |
| Fibrosis ≥ 4 | 19 (59.4) | 14 (25.9)a | 12 (25) | 21 (55.3)b | - | - |
| Fibrosis < 4 | 13 (40.6) | 40 (74.1) | 36 (75) | 17 (44.7) | - | - |
| p-HBsAg | 29.92 ± 10.84 | 57.44 ± 25.50b | 53.53 ± 25.12 | 39.21 ± 22.85a | 54.49 ± 26.02 | 35.49 ± 18.34b |
| HBsAg SP1 | ||||||
| A | 2 (6.2) | 15 (27.8)a | 11 (22.9 | 6 (15.8)a | 17 (32.1) | 0b |
| B | 4 (12.5) | 6 (11.1) | 1 (2.1) | 9 (23.7) | 6 (11.3) | 4 (12.1) |
| C | 6 (18.8) | 10 (18.5) | 12 (25) | 4 (10.5) | 14 (26.4) | 2 (6.1) |
| D | 4 (12.5) | 6 (11.1) | 7 (14.6) | 3 (7.9) | 8 (15.1) | 2 (6.1) |
| E | 16 (50) | 17 (31.5) | 17 (35.4) | 16 (42.1) | 8 (15.1) | 25 (75.8) |
| HBsAg SP2 | ||||||
| Diffuse | 8 (25) | 21 (38.9) | 12 (25) | 17 (44.7)a | 23 (43.4) | 6 (18.2)b |
| Globular | 8 (25) | 16 (29.6) | 19 (39.6) | 5 (13.1) | 22 (41.5) | 2 (6.1) |
| Submembranous | 16 (50) | 17 (31.5) | 17 (35.4) | 16 (42.1) | 8 (15.1) | 25 (75.8) |
| Membranous expression | ||||||
| Present | 5 (15.6) | 28 (51.9)b | 21 (43.8) | 12 (31.6) | 29 (54.7) | 4 (12.1)b |
| Absent | 27 (84.4) | 26 (48.1) | 27 (56.3) | 26 (68.4) | 24 (45.3) | 29 (87.9) |
The relationship between viremia and histopathological findings in cases with negative HBeAg is presented in Table 3. In this group, no correlation was observed between either the p-HBsAg or the membranous staining pattern and HBV DNA, HAI, or fibrosis. In the evaluation of patients according to SP1, in cases with HAI ≥ 9, the number of patients with pattern A was lower than that of patients with HAI < 9. In the latter, pattern E was rarely observed (12% of cases). SP2 was found to be related to HBV DNA levels, and diffuse staining was more frequent in cases with a higher level of HBV DNA. However, globular staining was rarely observed in these cases.
| HBV DNA | HAI | Fibrosis | ||||
| Parameters | < 6.65 | ≥ 6.65 | < 9 | ≥ 9 | < 4 | ≥ 4 |
| n | 23 | 31 | 31 | 23 | 34 | 20 |
| Age (yr) | 48.13 ± 10.34 | 45.71 ± 12.02 | 46.52 ± 11.73 | 47.04 ± 10.92 | 45.82 ± 11.63 | 48.30 ± 10.81 |
| Gender | ||||||
| Male | 10 (43.5) | 20 (64.5) | 19 (61.3) | 11 (47.8) | 23 (67.6) | 7 (35)a |
| Female | 13 (56.5) | 11 (35.5) | 12 (38.7) | 12 (52.2) | 11 (32.4) | 13 (65) |
| HBV DNA (log10IU/mL) | - | - | 4.97 ± 1.49 | 7.56 ± 1.21a | 4.16 ± 0.97 | 3.98 ± 0.93 |
| HBsAg (log10IU/mL) | 3.19 ± 0.84 | 3.92 ± 0.90 | 3.30 ± 0.8 | 3.86 ± 0.91a | 3.46 ± 0.95 | 4.03 ± 0.98 |
| ALT IU/L | 96.09 ± 33.55 | 163.06 ± 50.85a | 102.55 ± 41.36 | 177.65 ± 80.65 | 126.18 ± 64.87 | 148.75 ± 58.6 |
| ALT < 200 IU/L | 21 (91.3) | 18 (58.1)b | 26 (83.9) | 13 (56.5)a | 25 (73.5) | 14 (70) |
| ALT ≥ 200 IU/L | 2 (8.7) | 13 (41.9) | 5 (16.1) | 10 (43.5) | 9 (26.5) | 6 (30) |
| INR | 1.13 ± 0.27 | 1.17 ± 0.28 | 1.15 ± 0.3 | 1.16 ± 0.24 | 1.22 ± 0.32 | 1.04 ± 0.10b |
| Platelets (× 103/μL) | 234.91 ± 80.6 | 216.31 ± 61.29 | 237.67 ± 73.34 | 206.64 ± 73.34 | 218.21 ± 71.85 | 236.5 ± 68.79 |
| HAI score ≥ 9 | 2 (8.7) | 21 (67.7)b | - | - | 14 (26.5) | 9 (70)b |
| HAI score < 9 | 21 (91.3) | 10 (32.3) | - | - | 20 (73.5) | 11 (30) |
| Fibrosis ≥ 4 | 4 (17.4) | 16 (51.6)a | 6 (19.4) | 14 (60.9)b | - | - |
| Fibrosis < 4 | 19 (82.6) | 15 (48.4) | 25 (80.6) | 9 (39.1) | - | - |
| p-HBsAg | 30.39 ± 14.18 | 31.91 ± 14.04 | 28.68 ± 11.16 | 34.75 ± 16.71 | 33.21 ± 15.32 | 27.96 ± 10.93 |
| HBsAg SP1 | ||||||
| A | 6 (26.1) | 10 (32.3) | 13 (41.9) | 3 (13)a | 13 (38.2) | 3 (15) |
| B | 5 (21.7) | 9 (29) | 5 (16.1) | 9 (39.1) | 7 (20.6) | 7 (35) |
| C | 4 (17.4) | 1 (3.2) | 4 (12.9) | 1 (4.3) | 5 (14.7) | 0 |
| D | 5 (21.7) | 1 (3.2) | 5 (16.1) | 1 (4.3) | 3 (8.8) | 3 (15) |
| E | 3 (13) | 10 (32.3) | 4 (12.9) | 9 (39.1) | 6 (17.6) | 7 (35) |
| HBsAg SP2 | ||||||
| Diffuse | 11(47.8) | 19 (61.3)b | 18 (58.1) | 12 (52.2)a | 20 (58.8) | 10 (50) |
| Globular | 9 (39.1) | 2 (3.2) | 9 (29) | 1 (4.3) | 7 (20.6) | 3 (15) |
| Submembranous | 3 (13.1) | 10 (31.5) | 4 (12.9) | 10 (43.5) | 7 (20.6) | 7 (35) |
| Membranous expression | ||||||
| Present | 7 (30.4) | 13 (41.9) | 10 (32.3) | 10 (43.5) | 14 (41.2) | 6 (30) |
| Absent | 16 (69.6) | 18 (58.1) | 21 (67.7) | 13 (56.5) | 20 (58.8) | 14 (70) |
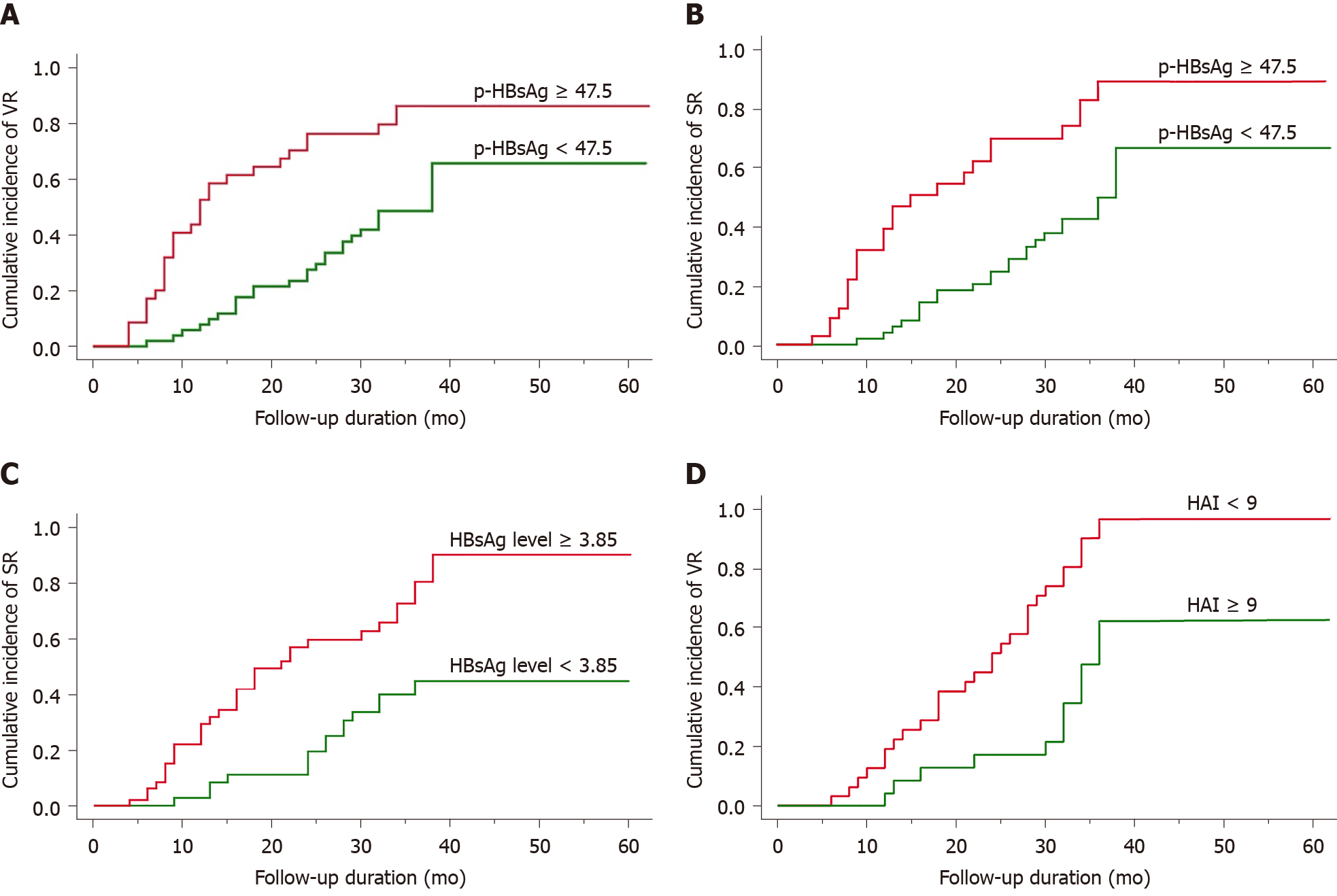
Patients were treated for a median duration of 28 mo (range, 4 mo to 62 mo). VR was achieved in 54 of 86 HBeAg-positive patients (66.7%) and in all 54 HBeAg-negative patients (100%), with a median time to VR of 16 and 9 mo, respectively. Factors associated with VR are shown in Table 4. In HBeAg-positive patients, the p-HBsAg and HBsAg expression patterns were correlated with VR, along with age, fibrosis, HVDNA and HBsAg levels in the log-rank test (P < 0.05). VR was observed in 53.7% of the patients with a high p-HBsAg. Similarly, pattern A and the presence of membranous expression were frequently observed in patients with VR. In multivariate analysis, the sole independent factor associated with VR was p-HBsAg (P < 0.01)(Figure 2A and Table 5). Forty-seven (54.6%) of 86 HBeAg-positive patients exhibited HBeAg SR, with a median time of 22 mo (range, 4 mo to 60 mo). Univariate analysis revealed that the p-HBsAg and HBsAg expression patterns were correlated with SR together with HBsAg levels and ALT > 200 (P < 0.05) (Table 4). Multivariate analysis demonstrated that p-HBsAg and the HAI score were independent factors related to SR (Figure 2B and C and Table 5). On the other hand, in the HBeAg-negative group, the most valuable determinant of VR was found to be the HAI score (Figure 2D and Table 5).
| HBeAg positive | HBeAg negative | ||||
| VR | SR | VR | |||
| Parameters | Absent | Present | Absent | Present | Present |
| Age (yr) | |||||
| < Mean | 11 (34.4) | 39 (72.2)b | 19 (48.7) | 31 (66)b | 24 (44.4) |
| ≥ Mean | 21 (65.6) | 15 (27.8) | 20 (51.43) | 16 (34) | 30 (55.6) |
| Gender | |||||
| Male | 21 (34.4) | 27 (50) | 24 (61.5) | 24 (48.9) | 30 (55.6) |
| Female | 11 (34.4) | 27 (50) | 15 (38.5) | 23 (51.1) | 24 (44.4) |
| HBV DNA | |||||
| Low | 17 (53.1) | 39 (72.2)a | 16 (41) | 16 (34) | 23 (42.6) |
| High | 15 (45.9) | 15 (27.8) | 23 (59) | 31 (66) | 31 (57.4) |
| HBsAg | |||||
| Low | 21 (65.6) | 15 (27.8)a | 21 (53.8) | 15 (31.9)b | 30 (55.6) |
| High | 11 (34.4) | 39 (72.2) | 18 (46.2) | 32 (68.1) | 24 (44.4) |
| ALT | |||||
| < 200 IU/L | 23 (71.9) | 29 (53.7) | 29 (74.4) | 23 (48.9)a | 39 (72.2) |
| ≥ 200 IU/L | 9 (28.1) | 25 (46.3) | 10 (25.6) | 24 (51.1) | 15 (27.8) |
| INR | |||||
| Low | 15 (46.9) | 26 (48.1) | 19 (48.7) | 22 (46.8) | 39 (72.2) |
| High | 17 (53.1) | 28 (51.9) | 20 (51.3) | 25 (53.2) | 15 (27.8) |
| Platelets | |||||
| Low | 15 (46.9) | 31 (57.4) | 20 (51.3) | 26 (55.3) | 31 (57.4) |
| High | 17 (53.1 | 23 (42.6) | 19 (48.7) | 21 (44.7) | 23 (42.6) |
| HAI score ≥ 9 | 13 (40.6) | 25 (46.3) | 11 (25) | 28 (59.6)a | 23 (42.6) |
| HAI score < 9 | 19 (59.4) | 29 (53.7) | 28 (75) | 19 (40.4) | 31 (57.4) |
| Fibrosis ≥ 4 | 17 (53.1) | 16 (29.6)a | 17 (43.6) | 16 (34) | 20 (37) |
| Fibrosis < 4 | 15 (46.9) | 10 (70.4) | 22 (56.4) | 31 (66) | 34 (63) |
| p-HbsAg | |||||
| Low | 26 (81.3) | 25 (46.3)b | 29 (74.4) | 24 (51.1)b | 21 (38.9) |
| High | 6 (18.8) | 29 (53.7) | 10 (25.6) | 23 (48.9) | 33 (61.1) |
| HBsAg SP1 | |||||
| A | 1 (3.1) | 16 (29.6)b | 2 (5.1) | 15 (31.9)b | 16 (29.6) |
| B | 0 | 10 (18.5) | 0 | 10 (21.3) | 14 (25.9) |
| C | 6 (18.8) | 10 (18.5) | 11 (28.2) | 5 (10.6) | 5 (9.3) |
| D | 6 (18.8) | 4 (7.4) | 6 (15.4) | 4 (8.5) | 6 (11.1) |
| E | 19 (59.4) | 14 (25.9) | 20 (51.3) | 13 (27.7) | 13 (24.1) |
| HBsAg SP2 | |||||
| Diffuse | 1 (3.1) | 28 (58.1)b | 2 (5.1) | 2 (57.4)b | 30 (55.6) |
| Globular | 12 (37.5) | 12 (22.2) | 17 (43.6) | 7 (14.9) | 10 (18.5) |
| Submembranous | 19 (59.4) | 14 (25.9) | 20 (51.3) | 13 (27.7) | 14 (25.9) |
| Membranous expression | |||||
| Absent | 27 (84.4) | 26 (48.1)b | 29 (74.4) | 24 (51.1)b | 21 (38.9) |
| Present | 5 (15.6) | 28 (51.9) | 10 (25.6) | 23 (48.9) | 33 (61.1) |
| HBeAg positive (n = 86) | HBeAg negative (n = 54) | ||||||||
| Parameters | VR | SR | VR | ||||||
| HR (95%CI) | P value1 | P value2 | HR (95%CI) | P value1 | P value2 | HR (95%CI) | P value2 | ||
| Age (yr) | 0.685 (0.228-2.055) | 0.001 | 0.449 | 0.892 (0.301-2.644) | 0.007 | 0.837 | 0.936 (0.460-1.904) | 0.855 | |
| Gender: male vs female | 0.581 (0.301-1.121) | 0.72 | 0.105 | 0.516 (0.251-1.062) | 0.79 | 0.072 | 2.410 (0.986-5.985) | 0.054 | |
| HBV DNA (log10IU/mL) | 0.782 (0.275-2.224) | 0.003 | 0.645 | 0.707 (0.246-2.029) | 0.40 | 0.519 | 0.620 (0.255-1.508) | 0.292 | |
| HBsAg (log10IU/mL) | 1.931 (0.757-4.923) | 0.001 | 0.168 | 2.252 (0.878-5.773) | 0.001 | 0.091 | 0.700 (0.294-1.669) | 0.422 | |
| ALT ≥ 200 IU/L vs < 200 IU/L | 0.992 (0.476-2.068) | 0.90 | 0.982 | 1.037 (0.436-2.471) | 0.018 | 0.934 | 0.854 (0.372-1.962) | 0.710 | |
| INR | 0.539 (0.271-1.072) | 0.751 | 0.078 | 0.493 (0.222-1.092) | 0.901 | 0.081 | 1.815 (0.791-4.161) | 0.159 | |
| Platelets (× 103/μL) | 0.619 (0.309-1.240) | 0.194 | 0.176 | 0.538 (0.243-1.189) | 0.389 | 0.125 | 0.925 (0.452-1.892) | 0.831 | |
| HAI score ≥ 9 vs < 9 | 1.566 (0.692-3.546) | 0.282 | 0.282 | 2.881 (1.170-7.094) | 0.132 | 0.021 | 6.876 (2.219-21.309) | 0.001 | |
| Fibrosis ≥ 4 vs < 4 | 1.245 (0.517-2.999) | 0.021 | 0.625 | 1.079 (0.427-2.730) | 0.092 | 0.872 | 0.833 (0.326-2.125) | 0.702 | |
| p-HBsAg | 3.178 (1.582-6.387) | 0.001 | 0.001 | 3.990 (1.843-8.638) | 0.001 | 0.001 | 1.089 (0.575-2.063) | 0.793 | |
| SP1 | 0.899 (0.495-1.634) | 0.001 | 0.727 | 3.760 (1.742-7.861) | 0.001 | 0.531 | 0.959 (0.443-2.167) | 0.990 | |
| SP2 | 0.727 (0.240-2.200) | 0.001 | 0.573 | 0.721 (0.216-2.410) | 0.001 | 0.595 | 0.996 (0.552-1.798) | 0.990 | |
| Membranous expression | 1.085 (0.513-2.294) | 0.001 | 0.831 | 1.082 (0.472-2.482) | 0.001 | 0.952 | 0.692 (0.246-1.944) | 0.485 | |
The few previous studies that evaluated HBsAg expression by semiquantitative methods have shown that serum HBsAg levels are correlated with the number of intrahepatic HBsAg-positive cells[19,20]. This observation was interpreted as serum and hepatic HBsAg being interrelated. On the other hand, in our study, we determined the percentage of intrahepatic HBsAg expression in the whole tissue section of each case by image analysis. Our results showed that in HBeAg-positive cases, p-HBsAg was associated not only with serum HBsAg but also with serum HBV DNA levels, indicating that intrahepatic HBsAg in liver tissues is closely related to viremia. Recently, Su et al[25] investigated HBsAg expression in the liver and its relationship with both serum HbsAg and interferon therapy using morphometric methods in treatment-naïve HbeAg-positive patients. Parallel to the findings of the present study, they observed a strong correlation between hepatic HbsAg expression and serum HbsAg levels. In addition, the relationship between HbsAg level and HbeAg loss after interferon therapy observed in this study suggested that the HbsAg expression level may be a useful marker to determine the response to therapy.
Therefore, in our study, we also aimed to elucidate the relationship between p-HbsAg levels and the treatment response in patients receiving entecavir treatment together with other HbsAg expression patterns. In the HbeAg-positive group, VR and SR were observed more frequently in cases with pretreatment values of p-HbsAg levels above the group average. In the HbeAg-positive group, the pretreatment values of p-HbsAg were strongly related to either VR or SR, suggesting the clinical significance of p-HbsAg in the management of HbeAg-positive CHB patients with antiviral treatment. Moreover, multivariate analysis revealed that in this group, p-HbsAg was an independent factor associated with VR and SR, warranting further investigation to evaluate the potential role of p-HbsAg as a tool to predict the response to therapy.
Another interesting result of this study is the presence of an inverse relationship between p-HbsAg and HAI and fibrosis scores in the HbeAg-positive group. Parallel to this finding, in cases with high HAI and/or fibrosis scores, the serum HBV DNA and HbsAg levels were significantly lower, suggesting that in the liver, the replacement of hepatocytes by both increased inflammatory infiltration and increased connective tissue can lead to this situation.
Our study demonstrated that HbsAg expression patterns, evaluated by various immunohistochemical methods, have different relationships with clinicopathological parameters and treatment responses in patients with CHB. Many years ago, following the detection of ground-glass hepatocytes by immunohistochemical methods in the liver tissues of patients with anti-Hbe positivity in the serum and a low HBV DNA level, the relationship of HbsAg expression patterns in hepatocytes with clinicopathological factors was investigated.Some studies have shown that patterns A and B, as opposed to those of C and D, are more common in younger subjects[15,18]. However, no similar age-related difference finding in HbsAg staining patterns was observed in other studies[16]. In our study, most of the patients with patterns A and B were younger than those with patterns C and D.
When the relationship of these staining patterns with disease activity and viremia has been investigated in recent studies, most of the data indicate that patterns A and B are associated with viremia[15,18,21,26,27]. In parallel with this finding, in our study, these patterns were associated with high HBV DNA and HAI in the HbeAg-positive group and were rarely observed in cases with high fibrosis scores, supporting the hypothesis that both are associated with active disease. Interestingly, the frequent detection of pattern B in the HbeAg-negative group, especially in cases with high HAI scores, also points to a relationship with active disease in these cases[15]. While the preliminary results indicate that the marginal staining pattern (pattern E) may be a determinant of inactive virus replication, other studies concluded that pattern C may be a marker of this situation[15,28]. In our cases, pattern E was more frequent in cases with low levels of HBV DNA and more frequent in cases with high fibrosis scores in the HbeAg-positive group, supporting data about its relationship with inactive disease reported by previous studies. Similar to recent findings in HbeAg-positive cases, membranous expression was absent in 84% and 87% of patients with lower HBV DNA values and fibrosis scores, respectively[18]. These findings suggest that this pattern of staining reflects active liver disease.
In an elegant study, Yim et al[26] investigated the relationship between these staining patterns and antiviral treatment response in patients with CHB. Diffuse staining patterns were associated with SR in HBeAg-positive patients. However, multivariate analysis revealed that this was not an independent predictor. In our study, although HBsAg expression patterns were associated with VR and SR in univariate analysis, they were not found to be independent factors. In contrast, p-HBsAg was a good predictor of the response to therapy in HBeAg-positive cases. This finding emphasizes that the determination of HBsAg expression by quantitative methods, beyond its distribution within the cell, may be an objective marker that can be used during the course and treatment of the disease.
In recent years, there has been growing interest in developing various noninvasive methods for monitoring the course and treatment of CHB. On the other hand, although it is an expensive and invasive method, liver biopsy remains the gold standard in determining liver injury and fibrosis during the follow-up of the disease. However, beyond these parameters, our results emphasize that the HBsAg expression levels measured in pretreatment liver biopsies via quantitative methods may be a useful marker for determining the treatment response.
This study demonstrated that HBsAg expression patterns, evaluated by various immunohistochemical methods, have different relationships with clinicopathological parameters and the treatment response in patients with CHB. Although the expression pattern of HBsAg differs significantly according to the disease status, it shows lower clinical significance in predicting the response to therapy than the percentage of HBsAg expression. Therefore, the quantitative evaluation of HBsAg expression in pretreatment-naïve liver biopsies of patients with CHB may provide a prediction of the response to antiviral therapy, especially in cases with HBeAg positivity. However, our findings should be supported by additional large studies.
The combination of various laboratory methods with histopathological findings is not always adequate to predict the progression and treatment response in patients with chronic viral B hepatitis (CHB). Therefore, efforts are being made to determine new parameters that may be useful in the follow-up of the disease.
It has been reported that differences in intracytoplasmic hepatitis B surface antigen (HBsAg) staining patterns are associated with viremia and active inflammation in CHB. However, HBsAg expression was evaluated semiquantitatively in these studies, and different classifications were used for the HBsAg staining pattern.
To investigate the relationship between image analysis-based quantitation of HBsAg expression and staining patterns with clinicopathological factors and treatment.
The study group consisted of 140 patients who were followed up and treated after the diagnosis of CHB. All cases were treated with entecavir. The evaluation of immunohistochemical HBsAg staining was performed in whole tissue sections by image analysis and expressed as a percentage of HBsAg staining (p-HBsAg). The staining pattern was also evaluated separately according to 3 different previously defined classifications. The correlations among immunohistochemical and clinicopathological findings were analyzed by χ2 and t-tests. The log-rank test was employed for comparisons of response rates. A Cox proportional hazards regression model was applied for multivariate analysis. Spearman’s correlation test was used to determine relationships between p-HBsAg and the other continuous variables.
There was a positive correlation between p-HBsAg and the serum levels of hepatitis B virus (HBV) DNA and HBsAg. The p-HBsAg was significantly higher in younger patients than in older patients, and the mean p-HBsAg level was significantly higher in the HBeAg-negative group than in the HBeAg-positive group. In the HBeAg-positive group, the average p-HBsAg level was significantly higher in patients with higher HBV DNA values than in those with low HBV DNA levels. Furthermore, an inverse relationship was noted between p-HBsAg and either hepatitis activity index (HAI) or the fibrosis score. Regarding staining pattern, diffuse cytoplasmic staining in many discrete hepatocytes was higher among cases with higher HBV DNA levels, and their number decreased among patients with HAI ≥ 9. However, submembranous staining was more frequent in subjects with higher HAI and fibrosis scores. In the same group, the p-HBsAg and HBsAg expression patterns were correlated with the viral response (VR) and serological response (SR). Multivariate analysis revealed that p-HBsAg was an independent factor associated with VR and SR. In the HBeAg-negative group, no correlation was observed between either the p-HbsAg or the membranous staining pattern and HBV DNA, HAI, or fibrosis. Diffuse staining was more frequent in cases with a higher level of HBV DNA. On the other hand, globular staining was rarely observed in these cases. In this group, the most valuable determinant of VR was the HAI score.
We have demonstrated that the expression pattern of HBsAg shows lower clinical significance in predicting the response to therapy than the percentage of HBsAg expression. Therefore, the quantitative evaluation of HBsAg expression in pretreatment-naïve liver biopsies of patients with CHB may predict the response to antiviral therapy, especially in cases with HBeAg positivity.
The evaluation of HBsAg expression by image analysis in pretreatment-naïve liver biopsies may be a valuable tool to predict the response to antiviral therapy. However, our findings should be supported by additional large-scaled studies.
| 1. | Yim HJ, Kim JH, Park JY, Yoon EL, Park H, Kwon JH, Sinn DH, Lee SH, Lee JH, Lee HW. Comparison of clinical practice guidelines for the management of chronic hepatitis B: When to start, when to change, and when to stop. Clin Mol Hepatol. 2020;26:411-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Park SH, Kim CH, Kim DJ, Suk KT, Cheong JY, Cho SW, Hwang SG, Lee YJ, Cho M, Yang JM, Kim YB. Usefulness of multiple biomarkers for the prediction of significant fibrosis in chronic hepatitis B. J Clin Gastroenterol. 2011;45:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293-1302.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 456] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 4. | Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: an update on serum and elastography markers. Expert Rev Gastroenterol Hepatol. 2019;13:361-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (12)] |
| 5. | Yuan H, Lee WM. Update of chronic hepatitis B. Curr Opin Gastroenterol. 2011;27:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Moucari R, Martinot-Peignoux M, Mackiewicz V, Boyer N, Ripault MP, Castelnau C, Leclere L, Dauvergne A, Valla D, Vidaud M, Nicolas-Chanoine MH, Marcellin P. Influence of genotype on hepatitis B surface antigen kinetics in hepatitis B e antigen-negative patients treated with pegylated interferon-alpha2a. Antivir Ther. 2009;14:1183-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Marcellin P, Bonino F, Yurdaydin C, Hadziyannis S, Moucari R, Kapprell HP, Rothe V, Popescu M, Brunetto MR. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int. 2013;7:88-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Bonino F, Brunetto MR. Chronic hepatitis B e antigen (HBeAg) negative, anti-HBe positive hepatitis B: an overview. J Hepatol. 2003;39 Suppl 1:S160-S163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Hsu HC, Su IJ, Lai MY, Chen DS, Chang MH, Chuang SM, Sung JL. Biologic and prognostic significance of hepatocyte hepatitis B core antigen expressions in the natural course of chronic hepatitis B virus infection. J Hepatol. 1987;5:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Kim TH, Cho EY, Oh HJ, Choi CS, Kim JW, Moon HB, Kim HC. The degrees of hepatocyte cytoplasmic expression of hepatitis B core antigen correlate with histologic activity of liver disease in the young patients with chronic hepatitis B infection. J Korean Med Sci. 2006;21:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Son MS, Yoo JH, Kwon CI, Ko KH, Hong SP, Hwang SG, Park PW, Park CK, Rim KS. Associations of Expressions of HBcAg and HBsAg with the Histologic Activity of Liver Disease and Viral Replication. Gut Liver. 2008;2:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Uzun Y, Bozkaya H, Erden E, Cinar K, Idilman R, Yurdaydin C, Uzunalimoglu O. Hepatitis B core antigen expression pattern reflects the response to anti-viral treatment. J Gastroenterol Hepatol. 2006;21:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Shi KD, Hwang SG, Choi JH, Hwang IJ, Yoon JH, Kim KI, Kwon CI, Hong SP, Park PW, Rim KS. [Hepatitis B core antigen expression pattern predicts response to lamivudine therapy in patients with chronic hepatitis B]. Korean J Hepatol. 2008;14:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Lee JG, Hwang SG, Yoon H, Son MS, Kim DY, Yoo JH, Kim KI, Rim KS. Hepatitis B core antigen expression in hepatocytes reflects viral response to entecavir in chronic hepatitis B patients. Gut Liver. 2013;7:462-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Hsu HC, Lai MY, Su IJ, Chen DS, Chang MH, Yang PM, Wu CY, Hsieh HC. Correlation of hepatocyte HBsAg expression with virus replication and liver pathology. Hepatology. 1988;8:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Ramakrishna B, Mukhopadhya A, Kurian G. Correlation of hepatocyte expression of hepatitis B viral antigens with histological activity and viral titer in chronic hepatitis B virus infection: an immunohistochemical study. J Gastroenterol Hepatol. 2008;23:1734-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Mukhopadhya A, Ramakrishna B, Richard V, Padankatti R, Eapen CE, Chandy GM. Liver histology and immunohistochemical findings in asymptomatic Indians with incidental detection of hepatitis B virus infection. Indian J Gastroenterol. 2006;25:128-131. [PubMed] |
| 18. | Chu CM, Liaw YF. Membrane staining for hepatitis B surface antigen on hepatocytes: a sensitive and specific marker of active viral replication in hepatitis B. J Clin Pathol. 1995;48:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology. 2011;53:2121-2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, Wong GL, Sung JJ. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5:1462-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 288] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, Slavin J, Bowden S, Gane EJ, Abbott W, Lau GK, Lewin SR, Visvanathan K, Desmond PV, Locarnini SA. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 342] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | Chuaypen N, Posuwan N, Chittmittraprap S, Hirankarn N, Treeprasertsuk S, Tanaka Y, Shinkai N, Poovorawan Y, Tangkijvanich P. Predictive role of serum HBsAg and HBcrAg kinetics in patients with HBeAg-negative chronic hepatitis B receiving pegylated interferon-based therapy. Clin Microbiol Infect. 2018;24:306.e7-306.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Payungporn S, Tangkijvanich P, Jantaradsamee P, Theamboonlers A, Poovorawan Y. Simultaneous quantitation and genotyping of hepatitis B virus by real-time PCR and melting curve analysis. J Virol Methods. 2004;120:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Petz D, Klauck S, Röhl FW, Malfertheiner P, Roessner A, Röcken C. Feasibility of histological grading and staging of chronic viral hepatitis using specimens obtained by thin-needle biopsy. Virchows Arch. 2003;442:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Su TH, Liu CJ, Yang HC, Jeng YM, Cheng HR, Liu CH, Tseng TC, Ling TY, Chen PJ, Chen DS, Kao JH. Clinical significance and evolution of hepatic HBsAg expression in HBeAg-positive patients receiving interferon therapy. J Gastroenterol. 2014;49:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Yim SY, Kim TH, Jun SS, Kim ES, Keum B, Seo YS, Yim HJ, Jeen YT, Chun HJ, Lee HS, Um SH, Kim CD, Won NH, Ryu HS. Expression of Hepatocyte Hepatitis B Core Antigen and Hepatitis B Surface Antigen as a Marker in the Management of Chronic Hepatitis B Patients. Gut Liver. 2017;11:417-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, Cornberg M. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 28. | Nakopoulou L, Adraskelas N, Stefanaki K, Zacharoulis D, Hadziyannis S. Expression of HBsAg and HBcAg in liver tissue: correlation with disease activity. Histol Histopathol. 1992;7:493-499. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dang SS S-Editor: Gao CC L-Editor: A P-Editor: Gao CC