Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1115
Peer-review started: May 4, 2020
First decision: May 24, 2020
Revised: June 1, 2020
Accepted: September 1, 2020
Article in press: September 1, 2020
Published online: November 27, 2020
Processing time: 204 Days and 9 Hours
Conventional coagulation tests are widely used in chronic liver disease to assess haemostasis and to guide blood product transfusion. This is despite the fact that conventional tests do not reliably separate those with a clinically significant coagulopathy from those who do not. Viscoelastic testing such as thromboelastography (TEG) correlate with bleeding risk and are more accurate in identifying those who will benefit from blood product transfusion. Despite this, viscoelastic tests have not been widely used in patients with chronic liver disease outside the transplant setting.
To assess the utility of Viscoelastic Testing guided transfusion in chronic liver disease patients presenting with bleeding or who require an invasive procedure.
PubMed and Google Scholar searches were performed using the key words “thromboelastography”, “TEG” or “viscoelastic” and “liver transplantation”, “cirrhosis” or “liver disease” and “transfusion”, “haemostasis”, “blood management” or “haemorrhage”. A full text review was undertaken and data was extracted from randomised control trials that evaluated the outcomes of viscoelastic test guided transfusion in those with liver disease. The study subjects, inclusion and exclusion criteria, methods, outcomes and length of follow up were examined. Data was extracted by two independent individuals using a standardized collection form. The risk of bias was assessed in the included studies.
A total of five randomised control trials included in the analysis examined the use of TEG guided blood product transfusion in cirrhosis prior to invasive procedures (n = 118), non-variceal haemorrhage (n = 96), variceal haemorrhage (n = 60) and liver transplantation (n = 28). TEG guided transfusion was effective in all five studies with a statistically significant reduction in overall blood product transfusion compared to standard of care. Four of the five studies reported a significant reduction in transfusion of fresh frozen plasma and platelets. Two studies showed a significant reduction in cryoprecipitate transfusion. No increased risk of bleeding was reported in the three trials where TEG was used perioperatively or prior to an invasive procedure. Two trials in the setting of cirrhotic variceal and non-variceal bleeding showed no difference in control of initial bleeding. In those with variceal bleeding, there was a statistically significant reduction in rate of re-bleeding at 42 d in the TEG arm 10% (vs 26.7% in the standard of care arm P = 0.012). Mortality data reported at various time points for all five trials from 6 wk up to 3 years was not statistically different between each arm. One trial in the setting of non-variceal bleeding demonstrated a significant reduction in adverse transfusion events in the TEG arm 30.6% (vs 74.5% in the control arm P < 0.01). In this study there was no significant difference in total hospital stay although length of stay in intensive care unit was reduced by an average of 2 d in the TEG arm (P = 0.012).
Viscoelastic testing has been shown to reduce blood product usage in chronic liver disease without compromising safety and may enable guidelines to be developed to ensure patients with liver disease are optimally managed.
Core Tip: Conventional coagulation tests do not predict bleeding or thrombosis risk in liver cirrhosis. Viscoelastic testing such as thromboelastography is a point of care test which can better predict clinically significant coagulopathy and the need for blood product transfusion compared to conventional coagulation tests. Randomized control trials have shown the clinical benefits of viscoelastic testing in liver cirrhosis in the perioperative setting and in those presenting acutely with bleeding. The primary aim of this systematic review is to verify the utility of viscoelastic testing guided transfusion in chronic liver disease patients presenting with bleeding or who require an invasive procedure.
- Citation: Wei H, Child LJ. Clinical utility of viscoelastic testing in chronic liver disease: A systematic review. World J Hepatol 2020; 12(11): 1115-1127
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1115.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1115
The liver plays a fundamental role in maintaining normal haemostasis. It synthesises the majority of clotting factors and anticoagulants and is involved in the regulation of platelet production through the synthesis of thrombopoietin (TPO). Chronic liver disease is associated with complex changes that result in a state of rebalanced homeostasis, where abnormalities in procoagulant factors are balanced by changes to anticoagulant factors[1,2]. The most significant changes to coagulation are summarised in Table 1[2-5].
| Procoagulant factors | Anticoagulant factors | |
| Primary haemostasis | Increased vWF | Thrombocytopenia |
| Reduced ADAMTS13 | +/- platelet dysfunction | |
| Secondary haemostasis/coagulation | High FVIII | Reduced synthesis of FII, FV, FVII, FIX and FXI |
| Reduced protein C, protein S and antithrombin | ||
| Dysfibrinogenaemia | ||
| Low fibrinogen (in end stage disease) | ||
| Fibrinolysis | Low plasminogen | Low antiplasmin |
| Low TAFI | ||
| High PAI-1 | High tPA |
Conventional coagulation tests such as prothrombin time (PT), international normalised ratio (INR) and activated partial thromboplastin time (APTT) are commonly used to assess haemostasis in patients with chronic liver disease. This is despite the fact these tests were never developed to provide information on complex haemostatic abnormalities and are not validated for predicting bleeding or thrombosis risk in cirrhosis[5]. Conventional coagulation tests only measure the first 5%-10% of fibrin formation and provide no information on clot strength and stability, in vivo activity of natural anticoagulants or the complex interaction between clotting factors, platelets and the endothelium[1]. While increases in PT and INR are associated with mortality in liver disease, there is no correlation between a raised PT, INR or APTT and risk of bleeding[3]. A systematic review published in 2005 found no correlation between a prolonged PT and bleeding risk in patients undergoing liver biopsy[6]. Pre-operative PT/INR is also not predictive of bleeding risk in those undergoing liver transplantation[5,7].
Fresh frozen plasma (FFP) is often transfused in an attempt to normalise PT/INR in patients with liver disease[3,5]. Major societal guidelines differ in their recommendations on the management of cirrhotic patients with gastrointestinal bleeding and abnormal coagulation profiles. The American Association for the Study of Liver Disease does not recommend correcting an abnormal INR in cirrhotic patients with portal hypertensive bleeding[8]. In contrast, the British Society of Gastroenterology and the American Society for Gastrointestinal Endoscopy recommended correction of an abnormal INR in patients with acute variceal bleeding[9,10]. Despite these recommendations, there is a lack of data to support the use of FFP in this setting. Multiple studies have demonstrated that transfusion of FFP has minimal in vivo effect on a mildly prolonged PT/INR in patients with liver disease[3,5]. Other studies have demonstrated that thrombin generation, a dynamic and global measure of clot formation, remains normal in patients with cirrhosis despite a prolonged PT/INR and APTT[11-13]. The use of FFP in patients with cirrhosis is not without harm and epidemiological studies have shown an increased risk of transfusion associated acute lung injury (TRALI)[14]. FFP administration also results in volume expansion which can exacerbate portal hypertension, paradoxically increasing the risk of variceal bleeding[15].
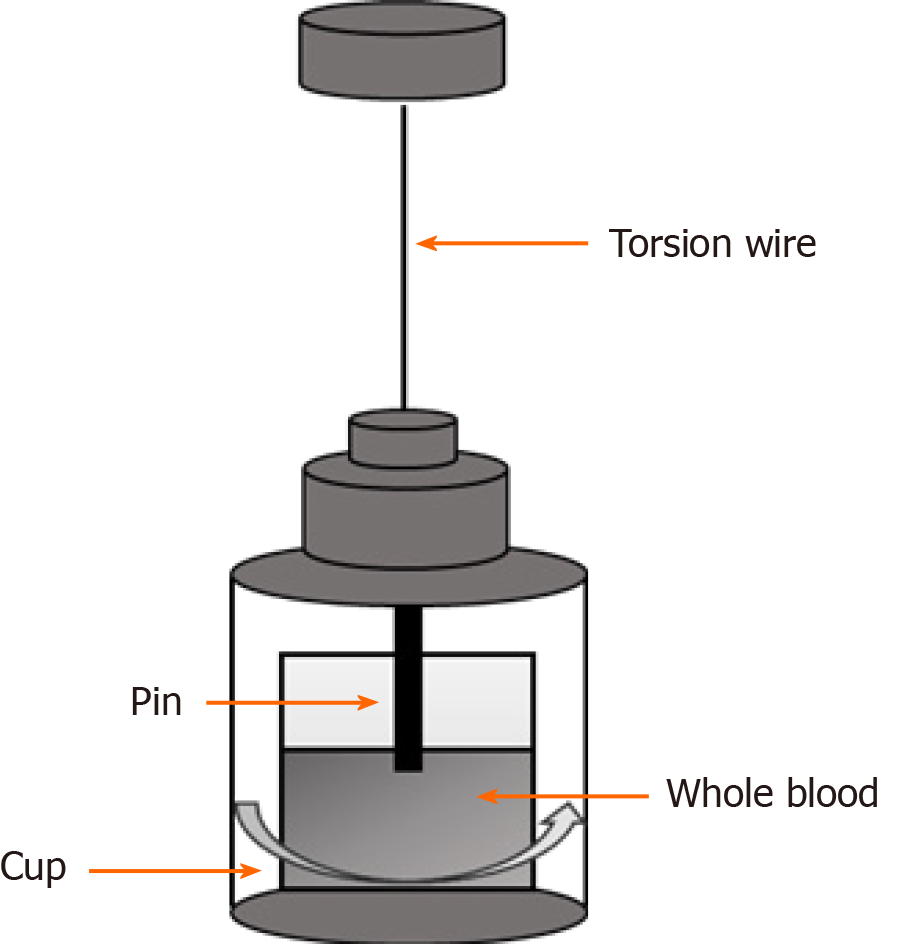
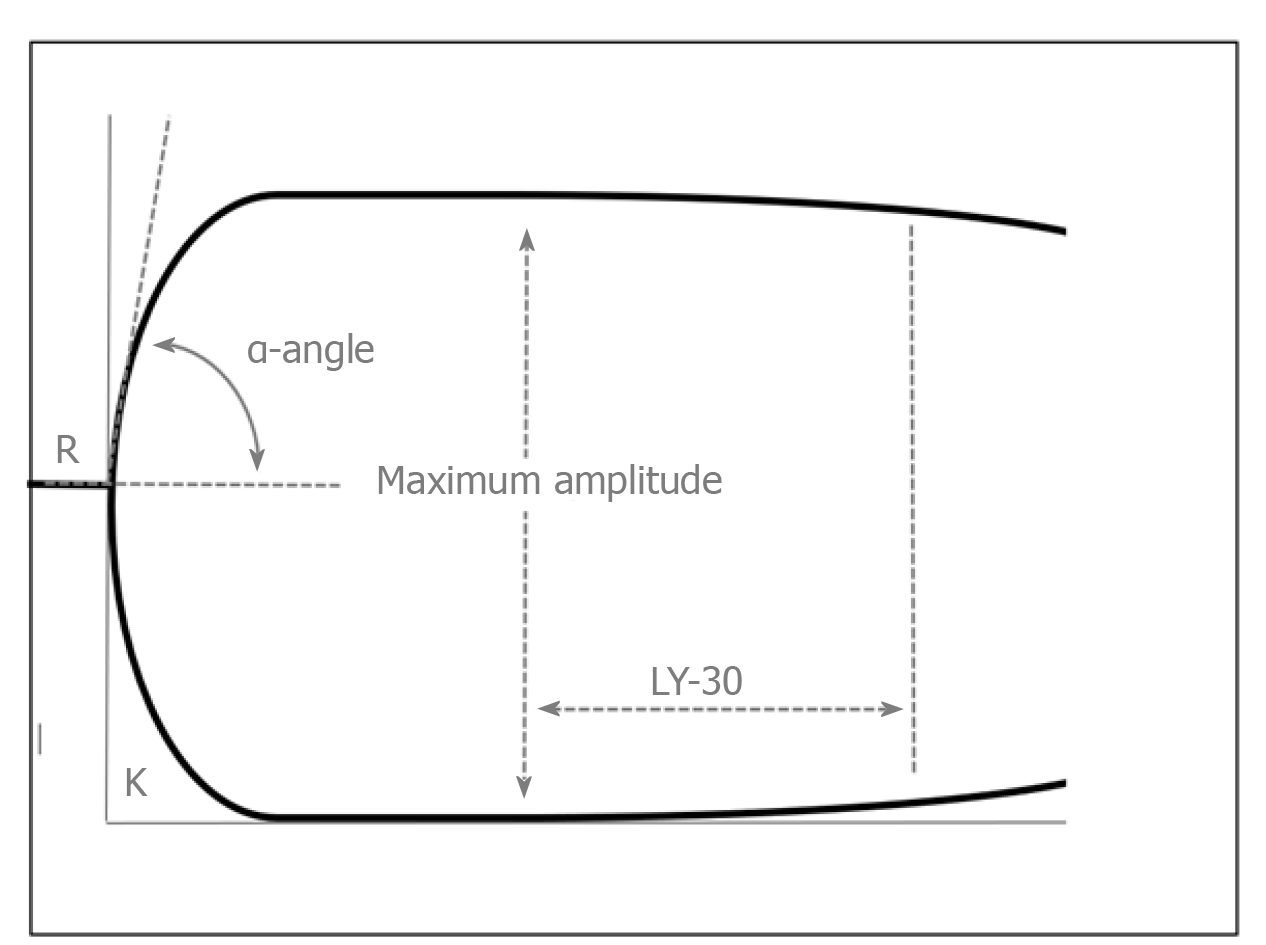
The concept of rebalanced haemostasis in liver disease and limitations of the conventional tests of coagulation has led to renewed interest in the use of global haemostatic assays including viscoelastic tests of coagulation (VETs) in patients with liver disease[2]. Compared to conventional tests, VETs such as thrombelastography (TEG) or rotational thrombelastometry (ROTEM) provide real time global assessment of clot formation in whole blood and information on the interaction between platelets and coagulation factors[3]. The general concept of TEG and an example of a normal trace are provided in Figures 1 and 2. Table 2 provides a comparison of TEG and ROTEM parameters.
| Measurement | TEG | ROTEM | |
| Period of initial fibrin formation | Time (min) to reach an amplitude of 2 mm | Reaction time (R) | Clotting time |
| Clot kinetics | Time (min) for clot amplitude to increase from 2 mm to 20 mm | Kinetics time (K) | Clot formation time |
| Clot kinetics | Angle of tangent line from clot initiation to the slope of the developing curve | Alpha angle (α) | Alpha angle (α) |
| Maximum clot strength | Peak amplitude (mm) | Maximum amplitude | Maximum clot firmness |
| Clot stability/fibrinolysis | Percent reduction in curve at 30 and 60 minutes | Lysis 30 (LY30) and lysis 60 (LY60) | Lysis index 30 (LI 30) |
The use of VETs to guide perioperative transfusion is well established and widely used in liver transplantation[2]. Despite this, VETs are not commonly used in patients with chronic liver disease outside the transplant setting. Observational and cohort studies in patients with liver disease have shown that alterations in TEG parameters correlate with bleeding risk. Pre-operative TEG MA is highly predictive of massive transfusion during liver transplantation[16]. Unlike conventional tests of coagulation, TEG has also been shown to predict re-bleeding in acute variceal haemorrhage[17]. In cirrhotic patients with an acute infection, TEG parameters become hypocoagulable suggesting that cirrhotic patients have little haemostatic reserve. These observations explain the established link between infection and variceal bleeding in chronic liver disease[17,18].
As abnormalities on conventional tests of coagulation do not correlate with bleeding risk they cannot be used to distinguish between surgical or anatomic causes of bleeding such as portal hypertension and bleeding due to an underlying coagulopathy. Recent randomised control trials suggest that VETs have the potential to more accurately identify those who will benefit from blood product transfusion thereby avoiding unnecessary transfusions which has financial, resource and safety implications[19-23]. VETs may enable consistent and evidence-based guidelines to be developed to ensure that patients with liver disease, are optimally managed.
The aim of this systematic review is to assess the benefits and harms of using viscoelastic tests to guide blood product transfusion in patients with chronic liver disease who present with bleeding or require invasive procedures. To ensure that implementation of TEG and ROTEM is both safe and efficacious, this review will compare VETs with the conventional tests of coagulation and evaluate the implications of using TEG and ROTEM in patients with chronic liver disease.
This article adheres to the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) guideline[24].
Types of studies: We included original, randomised control trials that have been published in a medical journal in the English language irrespective of the blinding status. We excluded unpublished or observational trials from the analysis. Studies published in a language other than English were also excluded.
Types of participants: We included trials that examined the use of VETs in adult patients with chronic liver disease who presented with bleeding or required an invasive procedure. Trials involving children were excluded. There were no other subgroups of patient population that were excluded.
Types of interventions: We included trials comparing VET guided transfusion strategy with conventional tests of coagulation. Given the lack of consensus regarding transfusion strategies with conventional tests of coagulation, transfusion in the conventional care arm could be defined by the standard of care or guidelines that were in place at the centre performing the randomised control trial.
Primary outcome: Amount of blood products transfused. The amount of fresh frozen plasma, platelets and fibrinogen transfused were assessed. This includes the proportion of patients requiring transfusion of each blood product and the average amount of blood products transfused per patient.
Secondary outcomes: (1) Rates of bleeding in those undergoing an invasive procedure; (2) Rates of rebleeding in those presenting with bleeding; (3) Rates of adverse events related to blood transfusion; (4) Overall mortality using the longest follow-up data from each trial; (5) Length of stay in the intensive care unit; and (6) Number of days in hospital.
Electronic searches: A literature search was performed on MEDLINE (PubMed) and Google Scholar to identify original, English language articles assessing the use of VETs in patients with chronic liver disease. MEDLINE and Google Scholar were chosen primarily because of the large number of individual articles and range of journals that are included on these databases. In addition, MEDLINE targets healthcare professionals and researchers ensuring that the articles are relevant. It is authoritative and peer-reviewed so that the research included is well-designed and statistics are accurately represented. The search included articles published up until the 17th of November 2019.
Selection of studies and data extraction: The following search string was used on PubMed: [(thromboelastography OR TEG OR viscoelastic) AND (“liver transplantation” OR cirrhosis OR liver disease) AND (transfusion OR haemostasis OR “blood management” OR haemorrhage)].
The following search string was used on Google Scholar: All of the words (thromboelastography, TEG, viscoelastic, liver transplantation, cirrhosis, liver disease, transfusion, haemostasis, blood management, haemorrhage).
The titles and abstracts of the articles were screened using the predefined inclusion and exclusion criteria detailed above. A full text review was then undertaken on the articles that met the inclusion criteria. Data was extracted from randomised control trials that evaluated the outcomes of VET guided transfusion in those with liver disease. The study subjects, inclusion and exclusion criteria, methods, outcomes and length of follow up were examined.
Data was extracted by two independent individuals using a data extraction form developed for this purpose. The risk of bias was assessed in the included studies by use of the Cochrane Collaboration tool for assessment of risk of bias[25].
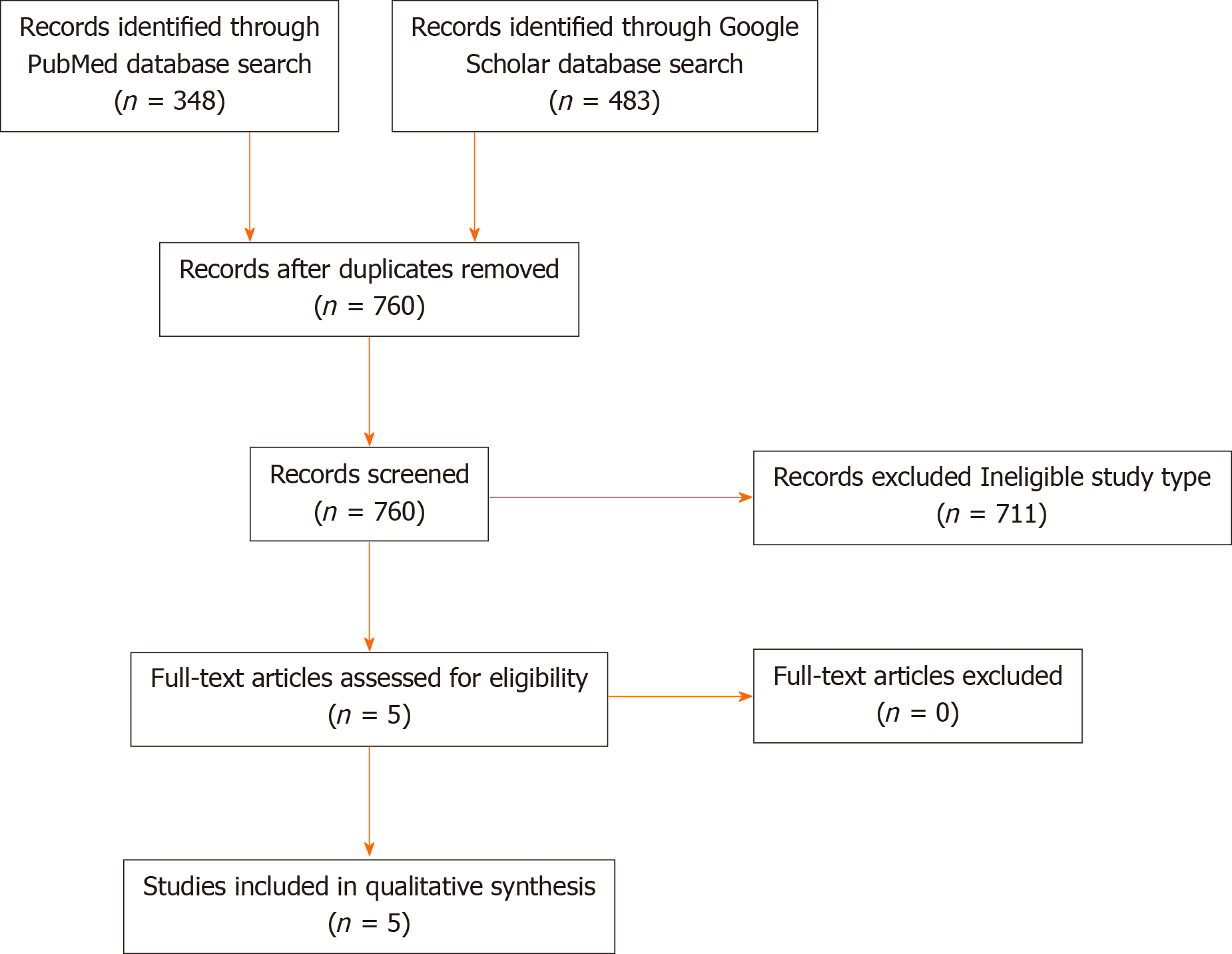
The MEDLINE search generated 348 results and the Google scholar search generated 483 results. There were 71 duplicates across the two databases which left a total of 760 results. Five articles met the eligibility criteria based on title and abstract review and were included in the analysis following full text review (Figure 3)[19-23].
Included studies: The five randomised control trials included in the analysis examined the use of TEG in guiding blood product transfusion[19-23]. None of the studies utilised ROTEM. A range of different TEG methods were used. One trial used the TEG5000 analyser with native blood[21] whereas another two trials used the TEG5000 analyser with a kaolin activator[20,23]. Two trials used the MonoTEM-A analyser on native whole blood[19,22]. As a consequence of the differing TEG analysers and methodology used, the thresholds for TEG guided transfusion differed significantly between the clinical trials (Table 3). The thresholds for transfusion were consistent across the studies utilising the same TEG analyser and method.
| Ref. | Year | No. of patients | Method of TEG | TEG thresholds for transfusion | SOC thresholds for transfusion | Outcomes: Blood product usage | Outcomes: Other |
| Wang et al[20] | 2010 | 28 | TEG 5000 | FFP titrated to maintain R time < 10 min | FFP titrated to maintain PT and APTT at less than one and a half times control | Statistically significant reduction in FFP use in TEG group (12.8 units in TEG group vs 21.5 units in control group, P < 0.05) | Trend towards reduction in blood loss in the TEG arm (not statistically significant) |
| 14 TEG | Kaolin activated | ||||||
| 14 SOC | |||||||
| SDAP when MA < 55 mm* | |||||||
| 5 pooled units of cryoprecipitate when alpha angle < 45 degrees** | Platelets to maintain a platelet count ≥ 50 × 109 | No reduction in RBC, Platelet or cryoprecipitate use | No statistically significant difference in mortality at 3 yr | ||||
| Cryoprecipitate to maintain fibrinogen > 1 g/L | |||||||
| De Pietri et al[21] | 2016 | 60 | TEG 5000 | FFP, 10 mL/kg*** when R time > 40 min1 | FFP, 10 mL/kg*** when INR > 1.8 | Statistically significant reduction in FFP use in TEG group. (Total amount of FFP transfused in those undergoing a low risk procedure: 4000 mL in TEG group vs 11050 mL in SOC group, P = 0.002) (Total amount of FFP transfused in those undergoing a high-risk procedure: 0 mL in TEG group vs 6500 mL in SOC group) | No statistically significant difference in periprocedural bleeding complications. |
| 30 TEG | Native blood (no activators) | ||||||
| 30 SOC | |||||||
| SDAP when MA < 30 mm* | SDAP when platelets < 50 × 109* | Statistically significant reduction in platelets transfused. (6.7% required a platelet transfusion in the TEG arm vs 33.3% in the SOC arm, P = 0.021) | Periprocedural bleeding events were rare with only one patient experiencing post procedure bleeding. | ||||
| Rout et al[22] | 2019 | 60 | MonoTEM-A® | FFP, 5 mL/kg*** when R time > 15 min | FFP, 5 mL/kg*** when INR > 1.8 | Statistically significant reduction in FFP use. (13.3% receiving FFP in the TEG group vs 46.7% in the SOC group P = 0.010. 1345 ml LFFP transfused in the TEG group vs 4605 mL in the SOC) | No difference in initial control of bleeding |
| 30 TEG | |||||||
| Native (no activators) | |||||||
| 30 SOC | |||||||
| No difference in rates of re-bleeding at 5 d | |||||||
| 3 pooled units of platelets when MA < 30 mm* | 3 pooled units of platelets when platelet count < 50 × 109* | ||||||
| Statistically significant reduction in rebleeding at 42 d (10% in the TEG group vs 36.7% in SOC, P = 0.012) | |||||||
| Statistically significant reduction in platelets transfused (10% in TEG group vs 70% SOC group P < 0.001. Total vol. of platelets transfused: 450 mL platelets in the TEG group vs 3450 mL in the SOC) | |||||||
| No difference in mortality at 6 wk (13.3% in TEG group vs 26.7% in SOC, P = 0.176) | |||||||
| No difference in RBC transfusion | |||||||
| Kumar et al[23] | 2019 | 96 | TEG 5000 | FFP, 10 mL/kg*** when R time > 10 min | FFP, 10 mL/kg*** if INR > 1.8 | Statistically significant reduction in FFP use (Total FFP transfused 440 mL in TEG vs 880 mL in SOC, P < 0.01) | Statistically significant reduction in transfusion related adverse events (30.6% in TEG group vs 74.5% in SOC P < 0.01)2 |
| 49 TEG | Kaolin activated | ||||||
| 47 SOC | |||||||
| SDAP when MA < 55 mm* | SDAP when platelets < 50 × 109* | Statistically significant reduction in platelets transfused (Average of 1 SDAP unit per patient in TEG group vs 2 SDAP units in SOC, P < 0.01) | |||||
| 5 pooled units of cryoprecipitate when alpha angle < 45 degrees** | 5 pooled units of cryoprecipitate if fibrinogen < 80 mg/dL** | ||||||
| Statistically significant reduction in ICU length of stay (median of 2 d in TEG arm vs 3 d in SOC. P = 0.012) | |||||||
| Statistically significant reduction in amount of cryoprecipitate used. (4 units in TEG group vs 16 in SOC group. P < 0.01) | No difference in failure to control bleeding at day 5 or rebleeding at day 42. | ||||||
| No difference in 5-d and 42-d mortality | |||||||
| Vuyyuru et al[19] | 2019 | 58 | MonoTEM-A® | FFP 5 mL/kg when R time > 14 min*** | FFP 5 mL/kg*** if INR ≥ 1.8 | No statistically significant difference in the amount of FFP transfused (24.1% requiring FFP in the TEG group vs 27.6% in the SOC, P = 0.764) | No difference in post procedure bleeding complications (0% in both groups) |
| 29 TEG | Native (no activators) | ||||||
| 29SOC | |||||||
| 3 pooled units of platelets when platelet count < 50 × 109* | |||||||
| 3 pooled units of platelets when MA < 32 mm* | |||||||
| No difference in pre and post procedure haemoglobin levels (TEG group: 11.3 ± 2.1 g/dL vs 11.2 ± 2.0 g/dL, P = 0.979; SOC group: 10.4 ± 2.1 g/dL vs 10.2 ± 2.0 g/dL, P = 0.205) | |||||||
| Statistically significant reduction in platelets transfused (10.3% requiring platelet transfusion in the TEG group vs 75.9% in the SOC group, P < 0.001) |
All clinical trials utilised conventional coagulation tests as the control. The thresholds for transfusion were based on major societal guidelines and the thresholds for transfusion of FFP and platelets were consistent across all five studies. FFP was administered when the INR was ≥ 1.8 (PT used in one study[20]) and platelets transfusion when the platelet count was < 50 × 109[19-23].
The included trials examined the utility of TEG in guiding blood product transfusion in a variety of settings. One randomised control trial examined the use of TEG in orthotopic liver transplantation[20]. Two trials examined the use of TEG prior to invasive procedures[19,21]. One trial examined the use of TEG in patients presenting with a variceal bleeding[22] and the final study examined the use of TEG in cirrhotic patients with non-variceal bleeding[23].
The study by Wang et al[20] in the setting of orthotopic liver transplantation was relevant to investigate the consistency of TEG to safely guide transfusion therapies in the setting of major surgery. This study included advanced liver disease patients with an overall model for end-stage liver disease score of 11.3 and deranged coagulation parameters considered to be at high risk of bleeding. These were patients who had similar baseline factors and definitions of coagulopathy compared to the two randomized trials examining the use of TEG prior to invasive procedures outside of the transplant setting[19,21].
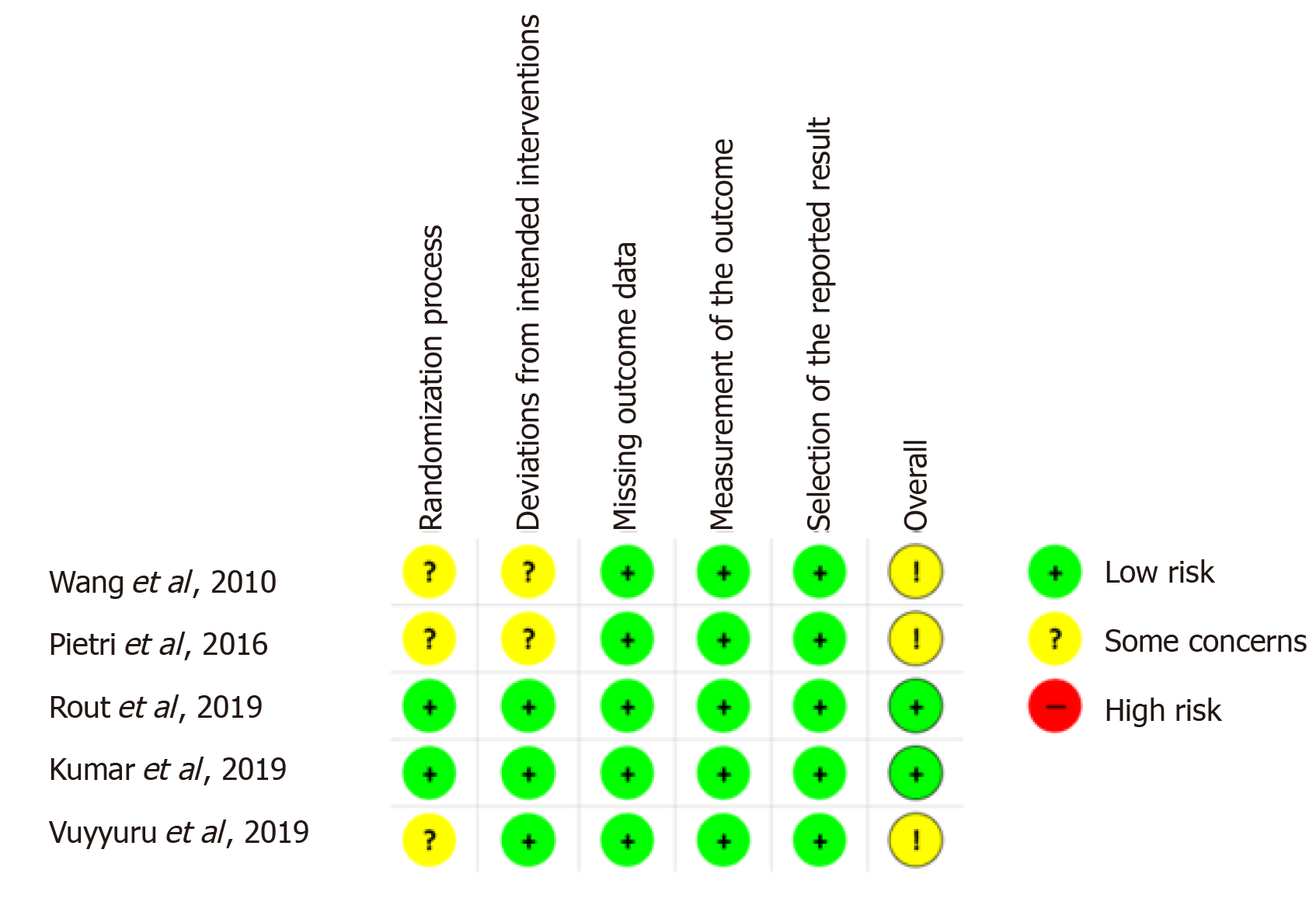
Risk of bias in the included studies: The overall methodologic quality of the studies was moderate to high (Figure 4) with an overall low risk of bias seen in 2 of the 5 studies (40%)[22,23] and no studies demonstrating an overall high risk of bias. The randomisation process was satisfactorily performed in 40% of the studies[22,23] and data regarding deviations from the pre-set protocol was satisfactorily reported in 60%[19,22,23]. None of the studies included had missing outcome data or selective outcome reporting.
All five studies reported a statistically significant reduction in overall blood product use with TEG guided transfusion[19-23]. The trials reported different outcomes with regards to the transfusion of specific blood products such as FFP, platelets, cryoprecipitate and red blood cells.
Four of the five studies reported a statistically significant reduction in FFP use[20-23]. In those presenting with variceal bleeding, 13.3% required FFP in the TEG arm compared to 46.7% in the conventional arm (P = 0.010)[22]. The absolute volume of FFP transfused was also markedly reduced in the TEG arm where 1345 mL of FFP was transfused compared with 4605 mL in the control arm[22]. The two studies examining the use of TEG prior to invasive procedures yielded different results with one trial showing a significant reduction in FFP use and the other showing no difference[19,21]. The two trials used different TEG analysers and methodology. As such, the transfusion thresholds in the TEG arm cannot be compared although the transfusion thresholds in the control group were identical. While not statistically significant, the trial which did not show a difference in FFP use had a higher number of patients with Childs Pugh B and C in the TEG arm than in the control arm (55.2% vs 31%)[19]. As the haemostatic rebalance is often lost in those with very advanced liver failure, this may have impacted on the results[26].
A statistically significant reduction in platelet transfusion was also reported in four of the five clinical trials[19,21-23] with no difference seen in the liver transplant trial[20]. Both trials that examined the use of TEG in cirrhotic patients requiring invasive procedures showed significantly lower rates of platelet use[19,21]. In one study, 13.3% required platelet transfusion in the TEG group versus 46.7% in the control group (P < 0.001)[22]. Again, the total volume transfused was markedly lower with 450mL transfused in the TEG group vs 3405 mL in the standard of care arm[22].
The amount of cryoprecipitate transfused was only measured in two trials[20,23]. In the liver transplant trial, there was no statistically significant difference in cryoprecipitate transfusion between the two groups[20]. In the study examining the use of TEG in those with non-variceal bleeding a statistically significant reduction was seen where 4 units of cryoprecipitate were used in the TEG group compared with 16 in the standard of care group (where each unit consisted of 5 pooled units of cryoprecipitate)[23].
Rates of bleeding in those undergoing an invasive procedure: There was no statistically significant difference in blood loss and/or bleeding events in the three trials which examined the use of TEG perioperatively or prior to an invasive procedure[19-21]. One trial measured the haemoglobin levels prior to and following each invasive procedure and found no significant difference in the levels between the TEG group and control group[19]. In all three studies, periprocedural bleeding rates were low in both [19-21]. Despite several patients having Childs-Pugh B and C disease, one study reported no bleeding complications in either arm following high-risk procedures including percutaneous liver biopsies[19].
Rates of rebleeding in those presenting with bleeding: Two trials examined the use of TEG in cirrhotic patients presenting with bleeding complications[22,23]. There was no difference in the ability to control initial bleeding between the TEG and conventional care groups. In those presenting with variceal bleeding, there was a statistically and clinically significant difference in the rate of re-bleeding at 42 d. 10% of those in the TEG group re-bled compared to 26.7% in the standard of care arm (P = 0.012)[22]. In the study of patients with non-variceal bleeding, no significant difference in rebleeding was seen at up to 42 d follow up[23].
Rates of adverse events related to blood transfusion: The rates of transfusion reactions and/or adverse events were reported in four out of the five studies[19,21-23] with no data available from the liver transplantation study[20]. Only one trial demonstrated a statistically significant reduction in adverse events related to transfusion where 30.6% had an adverse event in the TEG group versus 74.5% in the control arm[23]. These reaction rates are much higher than expected even in this high-risk population. The authors report a TRALI rate of 12.2% in the TEG arm versus 48.9% in the conventional arm[23]. The transfusion reactions were independently assessed by a panel of 3 experts to ensure appropriate classification. There is no mention of whether non-leucodepleted products were used in this trial which could potentially explain these unexpected results. In all other trials, transfusion reactions occurred infrequently with only two patients in all of the control groups and zero patients in the TEG groups experiencing an adverse transfusion event[19-22].
Overall mortality using the longest follow-up data from each trial: Mortality data was reported at various time points for all five trials with no statistically significant difference reported between the TEG group and control arm in any study[19-23]. In the liver transplant trial, there was no difference in overall survival at 3 years[20]. In the variceal bleeding trial, the mortality rate was high in both arms as one might expect in this high-risk population. The mortality rate was 13.3% at 6 wk in the TEG arm versus 26.7% in the control arm with a P value of 0.76[22]. The small number of participants included in each individual trial means that not all trials were adequately powered to assess a statistically significant difference in mortality.
Length of stay in the intensive care unit: Only one trial reported on length of stay in the intensive care unit. Following a presentation with non-variceal bleeding, there was a statistically significant reduction in the length of ICU stay. This was reported to be an average of 2 d in the TEG group versus 3 days in the control arm (P = 0.012)[23].
Number of days in hospital: Length of hospital stay was only reported in the study examining the use of TEG in non-variceal bleeding[23]. Length of hospital stay did not differ significantly between the two groups.
It is now widely accepted that chronic liver disease results in a state of rebalanced haemostasis where a reduction in procoagulant factors is balanced by a reduction in anticoagulant factors[3]. While conventional tests of coagulation are commonly used in patients with chronic liver disease, there is no correlation between a prolonged PT or INR and risk of bleeding in this patient group. While a minority of patients with liver disease are at an increased risk of bleeding, the conventional tests of coagulation do not reliably separate those who have a clinically significant coagulopathy from those who do not[3]. The haemostatic management of cirrhotic patients with a baseline coagulopathy on conventional testing remains difficult with a significant variation in clinical practice. The use of FFP to correct an abnormal PT or INR remains common practice despite a lack of evidence demonstrating clinical benefit[3,5]. The potential harms of transfusion in this patient group are well documented[5].
VETs have significant potential to inform and improve the haemostatic management of patients with chronic liver disease. Alteration in TEG parameters have been shown to correlate with bleeding risk in this patient group. TEG guided transfusion has been shown to reduce allogeneic blood product use in cirrhotic patients who require invasive procedures, including liver transplantation and in those presenting with variceal and non-variceal gastrointestinal bleeding[19-23]. The reduction in blood product use in five randomised control trials was not associated with an increased risk of bleeding, difference in the ability to control bleeding, morbidity or mortality when compared to standard care. In acute variceal haemorrhage, the rate of re-bleeding at 42 d was significantly lower with TEG guided transfusion[22]. Although the numbers included in each individual randomised control trial are small and there are differences in methodology and TEG cut-offs, the outcomes suggest a clinical benefit from TEG monitoring in chronic liver disease. The randomised control data available suggests that TEG provides a more accurate assessment of haemostasis, including bleeding risk and provides a more meaningful guide for blood product administration than conventional tests of coagulation in patients with chronic liver disease.
In conclusion, the poor predictive value of conventional coagulation tests in chronic liver disease has led to renewed interest in the use of global measures of haemostasis. Randomised control trials have confirmed earlier observations that VETs are more accurate in assessing bleeding risk and reduce blood product usage in chronic liver disease without compromising safety. While additional prospective randomised trials are needed to establish appropriate transfusion thresholds, VETs may enable consistent and evidence-based guidelines to be developed to ensure that patients with liver disease, are optimally managed.
Conventional coagulation tests do not predict bleeding or thrombosis risk in liver cirrhosis. Viscoelastic tests of coagulation (VETs) such as thrombelastography (TEG) is a point of care test that can predict clinically significant coagulopathy and the need for blood product transfusion. Despite this, VETs have not been widely used in patients with chronic liver disease outside the transplant setting.
The systematic review provides a summary and evaluation of existing clinical evidence for VET guided transfusion in chronic liver disease. This data will be important to improve the haemostatic management in these patients.
To verify the utility of VET guided transfusion in chronic liver disease patients presenting with bleeding or who require an invasive procedure.
A comprehensive systematic literature search was performed according to the methodology of evidenced-based medicine. We included randomized controlled trials that compared the use of VET guided transfusion to conventional coagulation tests in the setting of chronic liver disease who presented with bleeding or required an invasive procedure.
Five studies were included in the analysis examining the use of TEG guided blood product transfusion in cirrhosis prior to invasive procedures, non-variceal haemorrhage, variceal haemorrhage and liver transplantation. TEG guided transfusion reduced overall blood product utilization compared to standard of care in all five studies. No increase in length of stay, mortality or risk of bleeding was observed. In those presenting with variceal bleeding, there was a statistically significant reduction in rate of re-bleeding at 42 d in the TEG arm versus standard of care.
This systematic review highlights the role of VET in reducing blood product utilization in chronic liver disease without compromising safety and may enable guidelines to be developed to ensure patients with liver disease are optimally managed.
There is an urgent need to develop protocols utilizing VET to guide transfusion in liver cirrhosis outside of the transplant setting in order to optimize haemostatic management of these patients.
| 1. | Saner FH, Bezinover D. Assessment and management of coagulopathy in critically-ill patients with liver failure. Curr Opin Crit Care. 2019;25:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol (N Y). 2012;8:513-520. [PubMed] |
| 3. | Kujovich JL. Coagulopathy in liver disease: a balancing act. Hematology Am Soc Hematol Educ Program. 2015;2015:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 4. | Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell'Era A, Fabris F, Salerno F, Mannucci PM. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Mallett SV, Chowdary P, Burroughs AK. Clinical utility of viscoelastic tests of coagulation in patients with liver disease. Liver Int. 2013;33:961-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Segal JB, Dzik WH; Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 497] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 7. | Curry NS, Davenport R, Pavord S, Mallett SV, Kitchen D, Klein AA, Maybury H, Collins PW, Laffan M. The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol. 2018;182:789-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1503] [Article Influence: 167.0] [Reference Citation Analysis (3)] |
| 9. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, Christie JM; Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (2)] |
| 10. | Hwang JH, Shergill AK, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley KQ, Fonkalsrud L, Jue T, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2014;80:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 11. | Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, Mannuccio Mannucci P. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 503] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 12. | Gatt A, Riddell A, Calvaruso V, Tuddenham EG, Makris M, Burroughs AK. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J Thromb Haemost. 2010;8:1994-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Tripodi A, Chantarangkul V, Primignani M, Clerici M, Dell'era A, Aghemo A, Mannucci PM. Thrombin generation in plasma from patients with cirrhosis supplemented with normal plasma: considerations on the efficacy of treatment with fresh-frozen plasma. Intern Emerg Med. 2012;7:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Benson AB, Austin GL, Berg M, McFann KK, Thomas S, Ramirez G, Rosen H, Silliman CC, Moss M. Transfusion-related acute lung injury in ICU patients admitted with gastrointestinal bleeding. Intensive Care Med. 2010;36:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Mannucci PM, Tripodi A. Liver disease, coagulopathies and transfusion therapy. Blood Transfus. 2013;11:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 16. | Lawson PJ, Moore HB, Moore EE, Stettler GR, Pshak TJ, Kam I, Silliman CC, Nydam TL. Preoperative thrombelastography maximum amplitude predicts massive transfusion in liver transplantation. J Surg Res. 2017;220:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Chau TN, Chan YW, Patch D, Tokunaga S, Greenslade L, Burroughs AK. Thrombelastographic changes and early rebleeding in cirrhotic patients with variceal bleeding. Gut. 1998;43:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Vuyyuru SK, Singh AD, Gamanagatti SR, Rout G, Gunjan D, Shalimar. A Randomized Control Trial of Thromboelastography-Guided Transfusion in Cirrhosis for High-Risk Invasive Liver-Related Procedures. Dig Dis Sci. 2020;65:2104-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 20. | Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, Chan KH, Mandell S, Tsou MY. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42:2590-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 21. | De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B, Gerunda GE, di Benedetto F, Garcia-Tsao G, Villa E. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology. 2016;63:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 317] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 22. | Rout G, Shalimar, Gunjan D, Mahapatra SJ, Kedia S, Garg PK, Nayak B. Thromboelastography-guided Blood Product Transfusion in Cirrhosis Patients With Variceal Bleeding: A Randomized Controlled Trial. J Clin Gastroenterol. 2020;54:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 23. | Kumar M, Ahmad J, Maiwall R, Choudhury A, Bajpai M, Mitra LG, Saluja V, Mohan Agarwal P, Bihari C, Shasthry SM, Jindal A, Bhardwaj A, Kumar G, Sarin SK. Thromboelastography-Guided Blood Component Use in Patients With Cirrhosis With Nonvariceal Bleeding: A Randomized Controlled Trial. Hepatology. 2020;71:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 24. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123-e130. [PubMed] |
| 25. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18855] [Article Influence: 2693.6] [Reference Citation Analysis (0)] |
| 26. | De Pietri L, Bianchini M, Rompianesi G, Bertellini E, Begliomini B. Thromboelastographic reference ranges for a cirrhotic patient population undergoing liver transplantation. World J Transplant. 2016;6:583-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: New Zealand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adam EH, Hilmi I, Ruiz-Margáin A S-Editor: Gong ZM L-Editor: A P-Editor: Li JH