Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1128
Peer-review started: July 12, 2020
First decision: August 9, 2020
Revised: August 23, 2020
Accepted: September 15, 2020
Article in press: September 15, 2020
Published online: November 27, 2020
Processing time: 124 Days and 2.9 Hours
Hepatocellular carcinoma (HCC) is the most important primary malignant liver disease. A large proportion of patients with advanced HCC have macrovascular invasion. HCC tends to infiltrate vascular structures, particularly the portal vein and its branches, and more rarely, the hepatic veins. The intravascular tumor thrombus can affect the inferior vena cava (IVC) or even the right atrium (RA), the latter having a poor prognosis.
HCC is one of the most aggressive malignant tumors. Tumor thrombus (TT) formation in advanced HCC stages is common and usually involves the hepatic or portal veins. Herein, we report a 69-year-old woman who presented with dyspnea to the emergency department. A ventilation/perfusion lung scan was performed, ruling out pulmonary embolism. Hepatopulmonary syndrome and portopulmonary hypertension were discarded with contrasted echocardiography, but a mass in the RA was detected and confirmed by cardiac magnetic resonance imaging. Abdominal computed tomography showed a liver mass with a dynamic enhancement pattern compatible with HCC and an intraluminal IVC mass extending from the hepatic vein into the RA. HCC with TT expansion to IVC and RA is rare and indicates poor prognosis.
HCC with TT expansion to IVC and RA is rare and indicates poor prognosis. There is no consensus about anticoagulation or other interventions in these patients.
Core Tip: Hepatocellular carcinoma (HCC) with tumor thrombus (TT) in the right atrium (RA) is an unusual but critical condition. There is no standard treatment strategy or consensus. Alpha-fetoprotein is reportedly a new alternative biomarker to RECIST in order to detect tyrosine kinase inhibitors response in HCC, and our case supports this hypothesis. We report this HCC case due to the exceptionality of a TT extending into the RA in a patient with stable cirrhosis. We believe this case will warn professionals when facing similar cases, so systemic treatment can be started in a timely fashion and the treatment response can be evaluated with a serial blood test in patients suffering from advanced HCC.
- Citation: Gomez-Puerto D, Mirallas O, Vidal-González J, Vargas V. Hepatocellular carcinoma with tumor thrombus extends to the right atrium and portal vein: A case report. World J Hepatol 2020; 12(11): 1128-1135
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1128.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1128
Hepatocellular carcinoma (HCC) is the most common primary malignant liver disease and the fourth most common cause of cancer-related deaths, resulting in a global incidence of approximately 800000 cases per year[1]. Currently, liver cancer is the second most lethal tumor, after pancreatic cancer, with a 5-year survival rate of 18%[2]. The most common cause of HCC worldwide is hepatitis B virus chronic infection, but in Western countries, hepatitis C virus infection, chronic alcohol consumption and non-alcoholic steatohepatitis predominate.
HCC primarily develops in cirrhotic livers, which is the major cause of death in this population[3]. HCC also tends to infiltrate vascular structures, particularly the portal vein and its branches, and more rarely, the hepatic veins. Intravascular tumor thrombus (TT) can progress and extend to the inferior vena cava (IVC) or even to the right atrium (RA), the latter having a worse prognosis compared with patients with TT in the portal or hepatic vein[4,5]. IVC/RA tumor thrombus is an infrequent event of patients with HCC, with a reported incidence of 3% to 4%. Its diagnosis is challenging due to the lack of specific clinical signs[6-8].
A 69-year-old woman complained about experiencing asthenia and dyspnea with dizziness and diaphoresis.
A 69-year-old woman arrived at the emergency department with a 15-d history of progressive asthenia, dyspnea on moderate exertion and progressive worsening shortness of breath associated with dizziness and diaphoresis.
The patient was diagnosed in 2005 with alcoholic cirrhosis, without any decompensation since 2011. She followed an adequate follow-up in the hepatology department of the hospital and she had been abstinent since the last decompensation. Her last abdominal Doppler-ultrasonography from May 2019 showed liver cirrhosis with signs of portal hypertension, mild ascites and cholelithiasis. Furthermore her last upper gastrointestinal endoscopy from June 2019 showed esophageal varices and portal gastropathy. She also received treatment with beta-blockers and diuretics with good tolerance and adherence. Thereafter, she had a biological aortic valve replacement for severe aortic stenosis in December 2018, and since then, she remained asymptomatic.
The vital signs were within range and the physical examination was fairly normal. She had no chest discomfort or palpitations, orthopnea or an increase of the peripheral edema. She also denied having fever, chills, cough or expectoration, abdominal distention or any weight loss. In addition, she had a regular cardiac rhythm without cardiac murmur or rub noted, and the jugular venous pressure was not elevated. Her lungs were clear on auscultation and the oxygen saturation was correct without oxygen therapy. Her abdomen was also soft, non-tender and had no hepatosplenomegaly or clear signs of ascites. Moreover, she had no leg swelling, bruising or petechiae. There was no clubbing or cyanosis of her feet or hands. Signs of liver cirrhosis were found on examination, such as spider angioma and mild palmar erythema.
The blood test at admission revealed an impaired renal function with a creatinine of 1.82 mg/dL and a glomerular filtration rate of 28 mL/min/1.73 m2, mild hyponatremia with sodium of 132.3 mmol/L, elevated pro-brain natriuretic peptide (923 pg/mL) and high D-dimer (917 ng/mL).
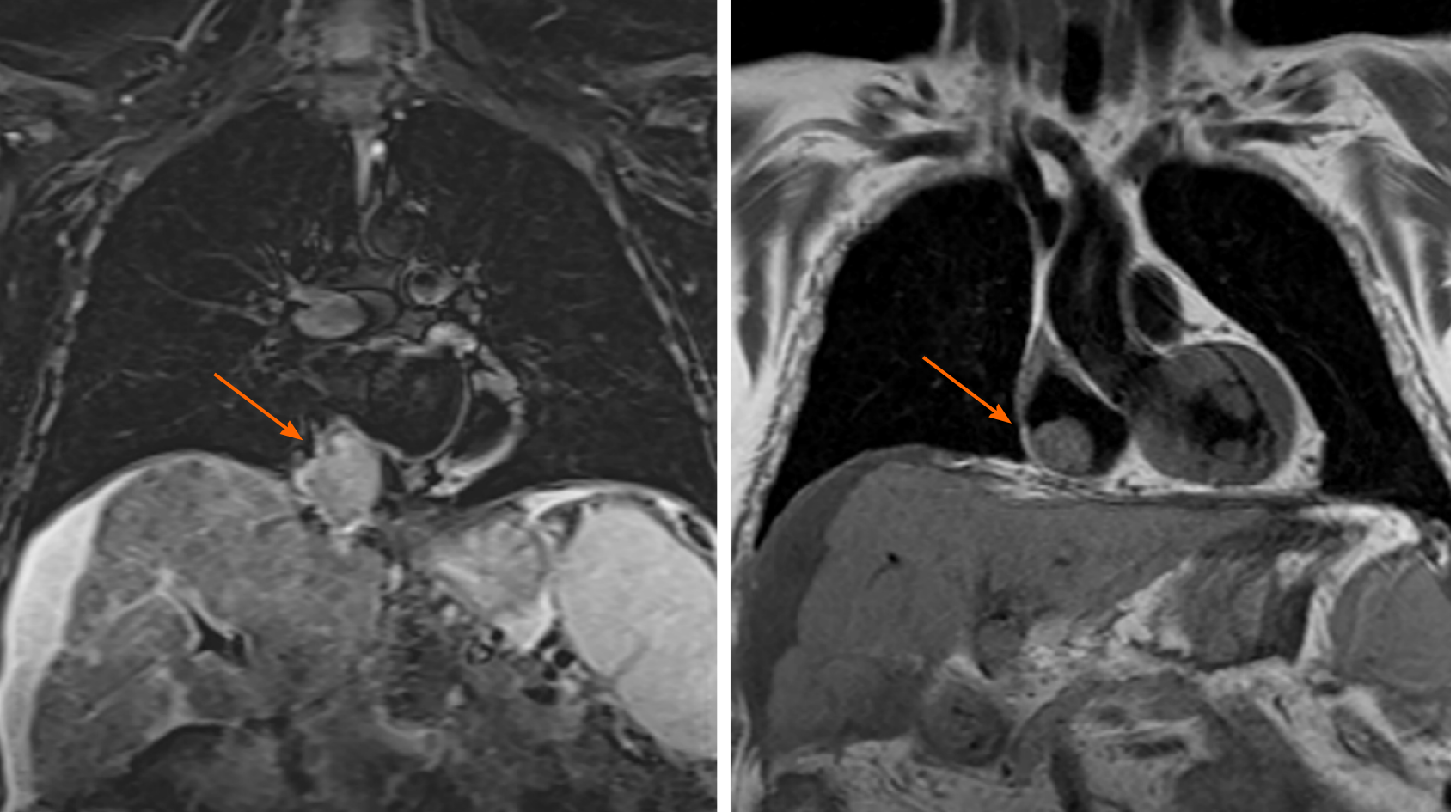
Her chest X-ray showed pathological signs. Due to the high suspicion of pulmonary embolism (PE) in a patient allergic to iodine, a ventilation/perfusion lung scan was performed, which did not exhibit any signs of PE. Contrasted echocardiography was requested to rule out portopulmonary hypertension or hepatopulmonary syndrome as the cause of the dyspnea of the patient. The test did not reveal signs of pulmonary hypertension nor shunts and showed a preserved systolic function (left ventricular ejection fraction [LVEF]: 58%) and a normally functioning prosthetic aortic valve. Unexpectedly, a mass at the posterior wall of the RA of 32 by 20 mm suggestive of an intracavitary thrombus was detected.
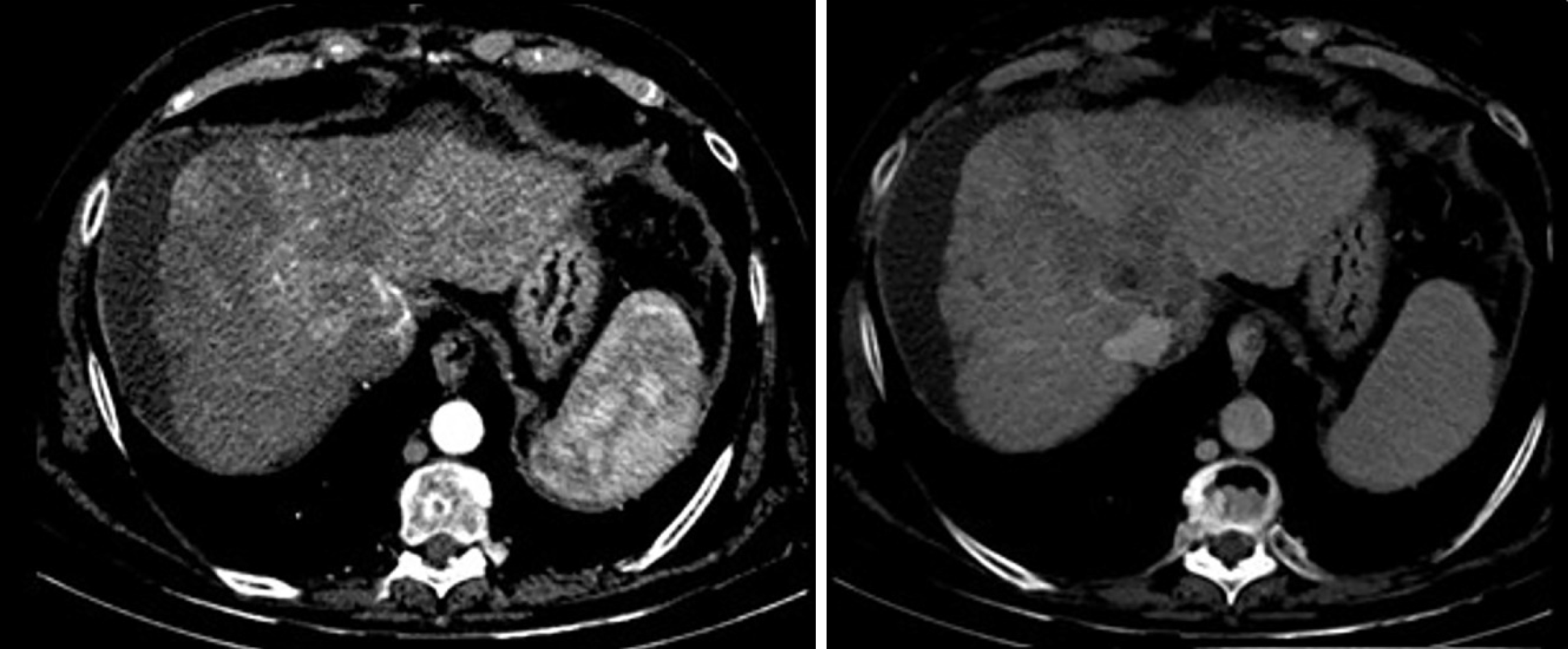
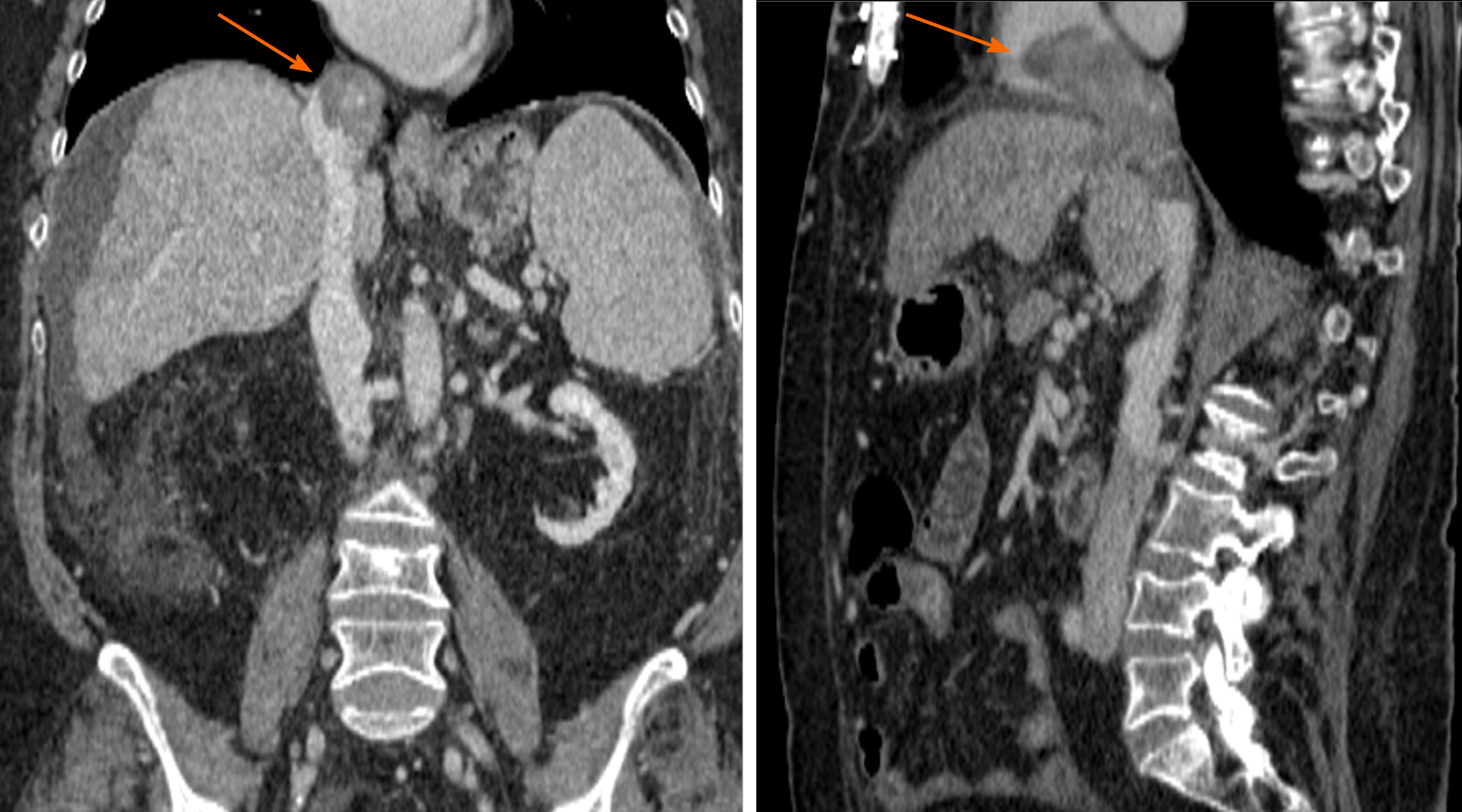
Magnetic resonance imaging was performed to confirm this finding due to its higher sensitivity and specificity. The mass inside the RA coming from the liver through the IVC was noted. This image was suggestive of an infiltrating HCC tumor thrombus (TT) (Figure 1). With the previous premedication, an abdominal multiphase CT was finally performed, thereby confirming the presence of a lesion in segment VIII with extension to segments V and I, suggestive of hepatocarcinoma (Figure 2). Left suprahepatic vein thrombosis with extension to the IVC until the RA and thrombosis in the left portal vein was noted, suggestive of tumor infiltration (Figure 3). On further work-up, her alpha-fetoprotein level was 6856.1 ng/mL.
A multidisciplinary committee was held to decide treatment and further work-up.
The final diagnosis of the presented case is HCC with TT extended to the RA and portal vein.
A multidisciplinary committee evaluated the patient and determined that she was not a candidate for surgical or ablative therapies. Thus, systemic treatment with sorafenib was started at 400 mg every 12 h and required dose reduction for asthenia. Given that the patient presented an extended TT, anticoagulation was introduced with low molecular weight heparin at a dose of 1 mg/kg every 12 h.
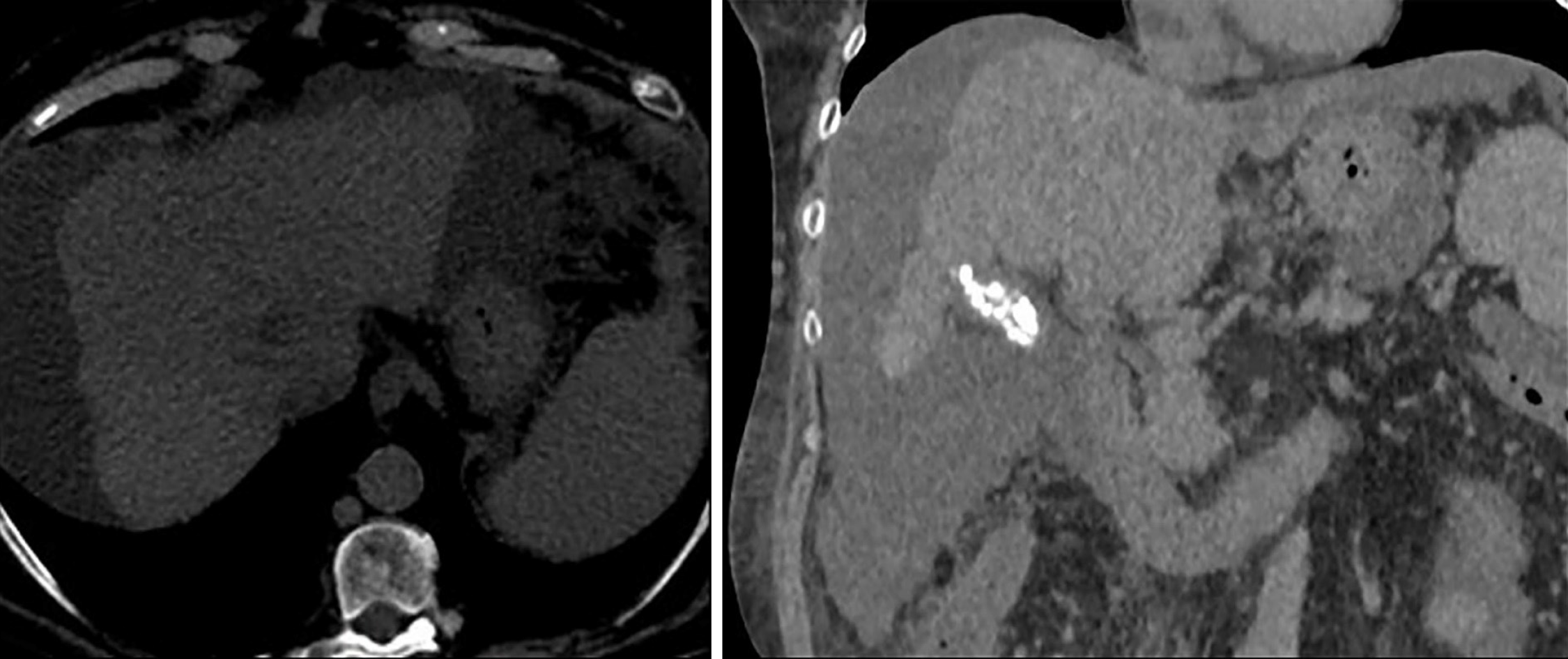
The patient was seen after 1 mo of being discharged, and she remained stable. She also showed an improved capacity for physical activities without any signs of embolism and diminished hepatomegaly in physical exploration. The dose of sorafenib was increased to a standard dose with positive tolerance. A non-contrast CT scan was completed 3 mo later (due to impaired kidney function). The limited evaluation presumed apparent stability of the tumoral mass (Figure 4). The alpha-fetoprotein level was 4156.1 ng/mL, thereby indicating a response to sorafenib. After 5 mo after receiving systemic therapy, the patient sought evaluation in the emergency department for being in a critical condition and was diagnosed with septic shock related to spontaneous bacterial peritonitis that ended fatally.
HCC is the most common primary liver neoplasm. It is the fifth most frequently diagnosed cancer in men, and it is the ninth most commonly diagnosed cancer in women worldwide[9]. The development of HCC is intently related to the presence of chronic liver diseases.
The diagnosis of HCC can be difficult and often requires the use of one or more imaging approaches. Ideally, tumors should be detected when they are ≤ 2 cm so that all treatment options can be offered. Vascular invasion and tumor thrombosis formation are commonly seen in cases of advanced HCC due to the activation of hemostasis[10]. The incidence of vascular invasion increases with a larger tumor size, and it has been reported to be present in 82% of patients with serum AFP levels > 1000 µg/L and a tumor diameter of > 5 cm[11]. IVC thrombosis is often asymptomatic, but symptoms related to portal hypertension can develop, for example, upper gastrointestinal bleeding, abdominal pain and ascites. Contrarily, the intra-atrial growth of HCC may not cause any symptoms per se but may lead to pulmonary thrombosis and pulmonary metastasis[12]. Advanced HCC with the invasion of the IVC and RA is exceptional, but it has been associated with poor prognosis and limited treatment options. In a retrospective study of 50 patients with advanced HCC with RA involvement, the median survival was only 2 mo with supportive care and only marginally improved to 4 mo with aggressive therapies[13].
The European Association for the Study of the Liver (EASL) recommends patients with preserved liver function and minimally affected performance status (PS 0-2), stage C according to the Barcelona Clinic Liver Cancer Group (BCLC) and intermediate-stage (BCLC B) not eligible for locoregional therapies to undergo treatment with systemic therapy. Sorafenib, a multi-kinase inhibitor (TKI) was introduced in the EASL guidelines in 2008. Sorafenib showed a survival benefit, documenting prolonged median survival and a nearly 3-mo extension of time to radiologic progression, which represented a breakthrough in the management of HCC[14]. Alpha-fetoprotein has been reported as a new alternative biomarker to RECIST to detect a sorafenib response in HCC, with the optimum cut-off points varying in published literature but suggesting that higher pre-treatment (AFP > 200 ng/mL) is associated with earlier recurrence and poorer overall survival[15,16]. Other experimental treatments were dismissed due to the age and medical history of the patient. Although sorafenib is the standard of care in these patients for more than a decade, further treatments and approaches have been undertaken in several reports as the resection of TT, the simultaneous resection of liver tumor and TT, external beam radiation therapy (EBRT), thalidomide treatment and transarterial chemoembolization (TACE). The postoperative survival period of patients with HCC and tumor thrombus in the RA varies from 18 d to 56 mo, with a mean survival of 20 mo[17]. In a retrospective cohort study of patients with HCC extending into the IVC/RA, treated with hepatectomy and thrombectomy, TACE and symptomatic treatment, the median survival was 19, 4.5 and 5 mo, respectively[17]. All these data indicate that removing the thrombus surgically combined with hepatectomy, or only TT extraction, might result in an improvement of survival in selected patients compared with other non-surgical procedures.
Radiofrequency ablation (commonly referred to as RFA) can result in long-term remission in small HCC. Nonetheless, performing RFA near the major vessels or diaphragm is difficult and challenging[18]. Several studies have validated that EBRT alone or combined with non-surgical treatment might achieve an excellent intrahepatic tumor control and a potential survival benefit of advanced HCC EBRT, presenting objective response rates ranging from 39% to 62% in patients with an associated macrovascular invasion[19]. The combination of TACE and RT has been beneficial compared with TACE alone for unresectable HCC in two meta-analyses[20,21]. Moreover, for major vessel invasion cases, combined TACE and RT had better OS and PFS than sorafenib, with a liver function not significantly worsened after treatment[22]. Further studies are crucial to accurately establish the incidence of adverse events and confirm these findings.
In our case, the patient was not a candidate for an invasive procedure; hence, systemic therapy with sorafenib was initiated. No predictive biomarkers of response to sorafenib or other TKI have been identified, and objective responses have been uncommon with the RECIST and modified RECIST criteria[23]. At a 3-mo follow-up, the patient improved symptoms with less dyspnea and asthenia, diminished hepatomegaly at physical exploration, a lower AFP levels and reached stable disease by CT scan.
HCC is one of the most common and aggressive malignant tumors, being the TT formation in advanced stages a relatively common complication usually involving the portal vein, and less frequently, the hepatic veins. Hepatopulmonary syndrome and portopulmonary hypertension are the two main causes of dyspnea that must be discarded in a cirrhotic patient.
Non-invasive imaging is an essential part of HCC diagnosis and contributes to primary liver tumor staging. When dynamic explorations demonstrate a typical diagnostic pattern, no further diagnostic invasive procedures are necessary. HCC TT expansion to IVC and RA is rare and indicates poor prognosis. There is no consensus on anticoagulation or other interventions in these patients. Treatment response has formerly been evaluated using the RECIST criteria by CT scan, but alpha-fetoprotein may be employed to consider sorafenib or any other TKI’s activity in HCC. Other treatment approaches could have been feasible as EBRT alone or combined with non-surgical treatment, but more data are essential to further establish the status of EBRT for the management of HCC.
The authors would like to acknowledge the important contribution by Dr. Alberto Escudero Rodriguez in the diagnostic, investigation and follow-up of the case reported.
| 1. | International Agency for Research in Cancer. Liver Cancer [Internet]. 2018. Available from: https://gco.iarc.fr/today/home. |
| 2. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1164] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 3. | Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 4. | Pesi B, Giudici F, Moraldi L, Montesi G, Romagnoli S, Pinelli F, Stefano P, Batignani G. Hepatocellular carcinoma on cirrhosis complicated with tumoral thrombi extended to the right atrium: results in three cases treated with major hepatectomy and thrombectomy under hypothermic cardiocirculatory arrest and literature review. World J Surg Oncol. 2016;14:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Luo X, Zhang B, Dong S, Zhang B, Chen X. Hepatocellular Carcinoma With Tumor Thrombus Occupying the Right Atrium and Portal Vein: A Case Report and Literature Review. Medicine (Baltimore). 2015;94:e1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lee IJ, Chung JW, Kim HC, Yin YH, So YH, Jeon UB, Jae HJ, Cho BH, Park JH. Extrahepatic collateral artery supply to the tumor thrombi of hepatocellular carcinoma invading inferior vena cava: the prevalence and determinant factors. J Vasc Interv Radiol. 2009;20:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol. 2013;20:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Numan L, Asif S, Abughanimeh OK. Hepatocellular Carcinoma with Tumor Thrombus Extending from the Portal Vein to the Right Atrium. Cureus. 2019;11:e4689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20722] [Article Influence: 1883.8] [Reference Citation Analysis (23)] |
| 10. | Connolly GC, Chen R, Hyrien O, Mantry P, Bozorgzadeh A, Abt P, Khorana AA. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb Res. 2008;122:299-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Sakata J, Shirai Y, Wakai T, Kaneko K, Nagahashi M, Hatakeyama K. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Huang J, Pan ZY, Li L, Jiang BG, Gu FM, Wang ZG, Wang ZH, Zhou WP. Hepatocellular carcinoma with inferior vena caval and right atrial tumor thrombi and massive pulmonary artery embolism: A case report. Mol Clin Oncol. 2017;6:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Chun YH, Ahn SH, Park JY, Kim DY, Han KH, Chon CY, Byun SJ, Kim SU. Clinical characteristics and treatment outcomes of hepatocellular carcinoma with inferior vena cava/heart invasion. Anticancer Res. 2011;31:4641-4646. [PubMed] |
| 14. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6423] [Article Influence: 802.9] [Reference Citation Analysis (9)] |
| 15. | Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V, Giordano L, Santoro A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Sánchez AIP, Roces LV, García IZ, López EL, Hernandez MAC, Parejo MIB, Peña-Díaz J. Value of α-fetoprotein as an early biomarker for treatment response to sorafenib therapy in advanced hepatocellular carcinoma. Oncol Lett. 2018;15:8863-8870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Miyazawa M, Torii T, Asano H, Yamada M, Toshimitsu Y, Shinozuka N, Koyama I. Does a surgery for hepatocellular carcinoma with tumor thrombus highly occupying in the right atrium have significance? Hepatogastroenterology. 2005;52:212-216. [PubMed] |
| 18. | Fu C, Liu N, Deng Q, Li X, Ma K, Bie P. Radiofrequency ablation vs. surgical resection on the treatment of patients with small hepatocellular carcinoma: a system review and meta-analysis of five randomized controlled trials. Hepatogastroenterology. 2014;61:1722-1729. [PubMed] |
| 19. | Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 610] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 20. | Hou JZ, Zeng ZC, Wang BL, Yang P, Zhang JY, Mo HF. High dose radiotherapy with image-guided hypo-IMRT for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombi is more feasible and efficacious than conventional 3D-CRT. Jpn J Clin Oncol. 2016;46:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 22. | Han B, Li C, Meng H, Gomes Romeiro F, Mancuso A, Zhou Z, Levi Sandri GB, Xu Y, Han T, Han L, Shao L, Qi X. Efficacy and safety of external-beam radiation therapy for hepatocellular carcinoma: An overview of current evidence according to the different target population. Biosci Trends. 2019;13:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, Ikeda M, Lim HY, Ho GF, Choo SP, Ren Z, Malhotra H, Ueno M, Ryoo BY, Kiang TC, Tai D, Vogel A, Cervantes A, Lu SN, Yen CJ, Huang YH, Chen SC, Hsu C, Shen YC, Tabernero J, Yen Y, Hsu CH, Yoshino T, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31:334-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qi XS S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Li JH