Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1020
Peer-review started: April 15, 2020
First decision: June 4, 2020
Revised: July 25, 2020
Accepted: October 12, 2020
Article in press: October 12, 2020
Published online: November 27, 2020
Processing time: 222 Days and 23.7 Hours
Combined hepatocellular and cholangiocarcinoma (HCC/CC) is a rare primary hepatic malignancy which carries a poor prognosis due to its aggressive nature. Few centers have enough cases to draw definitive conclusions and there is limited understanding of prognosis. Given the rarity of HCC/CC, an analysis of large national cancer database was needed to obtain larger number of HCC/CC cases.
To identify associated factors for 5-year survival of HCC/CC.
We conducted a retrospective study of The Surveillance, Epidemiology, and End Results (SEER) database obtained from SEER*Stat 8.3.6 software. Previously defined histology code 8180 for the International Classification of Disease for Oncology, 3rd edition was used to identify HCC/CC cases from 2004 to 2015. We collected demographics, American Joint Committee on Cancer (AJCC) stage, treatment, tumor size, and survival data. These data were converted to categorical variables. The Shapiro-Wilk normality test was used to assess normal distribution. Mann-Whitney U test was used to compare continuous variables without normal distribution, and t-test was used to compare continuous variables with a normal distribution. The Kaplan-Meier survival curve analyzed 5-year survival. Univariate and multivariate logistic regression model was used to analyze factors associated with 5-year survival. Multivariate Cox proportional hazard regression was done on 5-year survival. We defined P < 0.05 was statistically significant.
We identified 497 patients with the following characteristics: Mean age 62.4 years (SD: 11.3), 149 (30.0%) were female, racial distribution was: 276 (55.5%) white, 53 (10.7%) black, 84 (16.9%) Asian and Pacific Islander (API), 77 (15.5%) Hispanic, and 7 (1.4%) others or unknown. Stage I/II disease occurred in 41.5% and tumor size < 50 mm was seen in 35.6% of patients. Twenty-four (4.8%) received locoregional therapy (LRT), 119 (23.9%) underwent resection, and 50 (10.1%) underwent liver transplantation. The overall median survival was 6 mo [Interquartile range (IQR): 1-22]. After multivariate logistic regression, tumor size < 50 mm [Odds ratios (OR): 2.415, P = 0.05], resection (OR: 12.849, P < 0.01), and transplant (OR: 27.129, P < 0.01) showed significance for 5-year survival. Age > 60, sex, race, AJCC stages, metastasis, and LRT were not significant. However, API vs white showed significant OR of 2.793 (CI: 1.120-6.967). Cox proportional hazard regression showed AJCC stages, tumor size < 50 mm, LRT, resection, and transplant showed significant hazard ratio.
HCC/CC patients with tumor size < 50 mm, resection, and transplant were associated with an increase in 5-year survival. API showed advantageous OR and hazard ratios over white, black.
Core Tip: Combined hepatocellular and cholangiocarcinoma (HCC/CC) is a rare primary hepatic malignancy which carries a poor prognosis due to its aggressive nature. Few centers have enough cases to draw definitive conclusions and there is limited understanding of prognosis. This analysis of Surveillance, Epidemiology, and End Results database comprised of 497 patients. HCC/CC patients with tumor size < 50 mm, resection, and transplant were associated with an increase in 5-year survival. Asian and Pacific Islander (API) showed advantageous odds ratios and hazard ratios over white, black. Elucidation of better prognosis on API are needed in the future studies.
- Citation: Sempokuya T, Wien EA, Pattison RJ, Ma J, Wong LL. Factors associated with 5-year survival of combined hepatocellular and cholangiocarcinoma. World J Hepatol 2020; 12(11): 1020-1030
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1020.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1020
Malignancies of the liver are broadly grouped into hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). Combined hepatocellular and cholangiocarcinoma (HCC/CC) is a rare primary hepatic malignancy which has a distinct phenotype, shares characteristics of both HCC and ICC. HCC/CC carries a particularly poor prognosis due to its aggressive nature. HCC/CC has an estimated incidence between 1% and 14.2%[1-4]; however, this is likely an underestimation due to diagnostic inaccuracy. HCC/CC shows phenotypic characteristics of both HCC and CC with malignant differentiation of hepatocytes and biliary epithelial cells. While HCC/CC is thought to originate from a common hepatic stem cell[5,6], there has been much debate on whether HCC/CC shares more commonalities with HCC or CC[1,7-9]. Current knowledge suggests HCC/CC is a unique entity with a spectrum of clinical features between those of HCC and CC[10]. In addition to its aggressive nature, clinical and pathologic heterogeneity results in reduced survival compared to HCC or CC alone. Furthermore, treatment of HCC/CC is challenging as there are no broadly accepted guidelines other than recommendation for resection and possible liver transplantation in patients afflicted with this condition[11,12].
Because HCC/CC is a rare malignancy, there are no large studies or randomized clinical trials to compare diagnostic or treatment modalities. The Liver Imaging Reporting and Data System is a widely used criteria for imaging diagnosis of HCC[13], but its performance in differentiating HCC/CC from HCC by magnetic resonance imaging is much less reliable[14]. Prognosis of HCC/CC after resection compared to HCC or CC alone is controversial. While Zhang et al[15] showed better early survival of HCC/CC compared to ICC and worse outcome than HCC alone, this study only included 15 HCC/CC patients. On the other hand, Song et al[16] showed HCC/CC had a significantly shorter recurrence free survival after resection compare to ICC, 0.9 years and 1.3 years, respectively. Prognostic indicators beyond this are mostly unknown. The role and indications of liver transplantation in HCC/CC also remain equivocal at this time[12,17]. For HCC, downstaging can be employed for patients to be eligible for liver resection or transplantation with favorable outcomes. However, no established protocol is available for HCC/CC.
Despite the diagnostic and prognostic challenges, distinguishing this unique entity is crucial to its optimal management and outcomes. Few centers have enough cases to draw definitive conclusions, and there is a limited understanding of prognosis. This study attempts to further understand and identify factors associated with 5-year survival in patient with HCC/CC.
Population data from the Surveillance, Epidemiology, and End Results (SEER) database published by the National Cancer Institute were obtained through Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat/) version <8.3.6>[18]. SEER Registries are population-based registries that report cancer incidence, characteristics, treatment and, mortality on select U.S. states since 1973. Approximately 34.6 % of all cancer cases in the U.S. population are covered[19]. This study was conducted after complying with the SEER Research Data Use Agreement. As we utilized a publicly available, de-identified database, approval from an Institutional Review Board was not required to conduct this study.
We collected data on patients with a diagnosis of HCC/CC between 2004 to 2015 with the previously defined International Classification of Diseases for Oncology, 3rd Edition the histology code of 8180[4]. Variables collected included age at diagnosis, year at diagnosis, sex, race (Whites, blacks, Hispanics, Asians or Pacific Islanders (API), or others), marital status, stage by the American Joint Committee on Cancer (AJCC) Staging Manual, 6th edition[20], SEER Staging, presence of metastasis, state and county of residence, tumor sizes, treatment modality of primary site, and survival (mo). SEER data utilized in this study was based on information from 18 U.S. states and regions available to conduct survival analysis including: Alaska Native Tumor Registry, California (San Francisco-Oakland, San Jose-Monterey, Los Angeles, Greater California), Connecticut, Georgia (Atlanta, Greater Georgia, Rural Georgia), Hawaii, Iowa, Kentucky, Louisiana, Michigan (Detroit), New Jersey, New Mexico, Utah and Washington (Seattle-Puget Sound) (More details available at https://seer.cancer.gov/registries/terms.html).
We performed statistical analysis with R version 3.4.1 (The R foundation for Statistical Computing, Vienna, Austria), EZR version 1.36 (Division of Hematology, Saitama Medical Center, Jichi Medical University, Japan)[21], and SAS version 9.4 (SAS Institute Inc., Cary, NC, United States). χ2 test was used to compare categorical variables. The Shapiro-Wilk normality test was used to assess normal distribution. Mann-Whitney U test was used to compare continuous variables without normal distribution, and t-test was used to compare continuous variables with a normal distribution. The Kaplan-Meier survival curve with log-rank test was used to estimate overall survival probability and compare 5-year survival curves for risk factor groups. Continuous variables were converted to categorical variables for logistic regression models. A univariate and multivariate logistic regression model was used to analyze factors associated with 5-year survival. Exclusion of patients with unknown or other race and surgical status was done on this regression model due to small population size. Risk factor variables included in the logistic regression mode were sex (male and female), age (< 60 and ≥ 60-years old), race (White, black, Hispanic, and API), AJCC stages (I/II, III/IV, and unknown), metastasis (distant metastasis, none/unknown), tumor size (< 50 and ≥ 50 mm), surgical status [Locoregional therapy (LRT), resection, and transplant]. All of these variables were included in the multivariate model. The primary outcome variable of interest was 5-year overall survival, defined as the time from HCC diagnosis to death from any cause, with censoring if the patients were still alive after 5-years of follow-up. Hazard ratios (HR) for overall survival evaluated using multivariate Cox proportional hazard regressions mode by using the same variable as logistic regression. Logistic regression model allows prediction of variables with survival status and Cox proportional hazard regression model enables analysis of time dependent variables related to survival. P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Ma J from Department of Biostatistics, College of Public Health, University of Nebraska Medical Center.
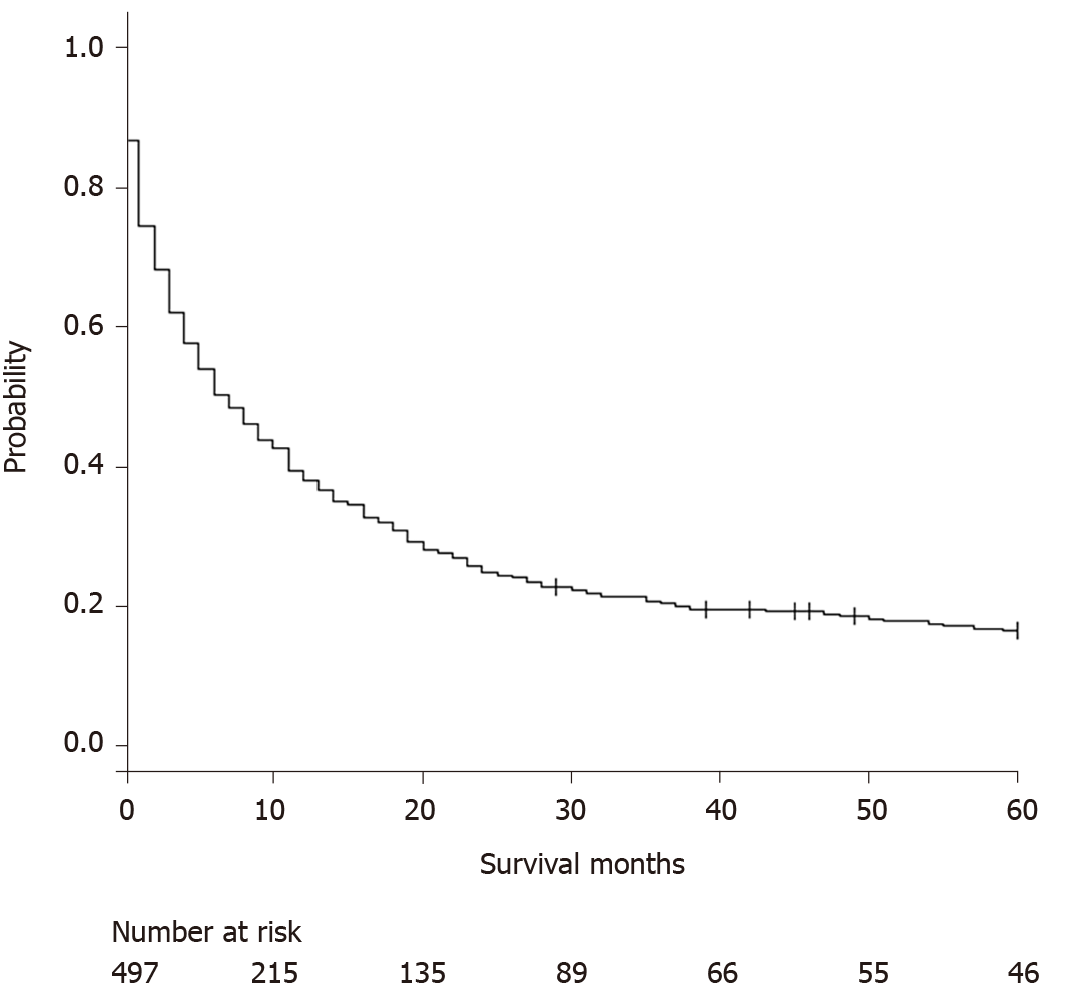
We identified 497 patients with the following characteristics: Mean age 62.4 years (SD: 11.3), 270 (54.3%) were age > 60 years old, and 149 (30.0%) were female. Racial distribution was as follows: 276 (55.5%) white, 53 (10.7%) black, 84 (16.9%) API, 77 (15.5%) Hispanic, and 7 (1.4%) others or unknown. Stage I/II disease occurred in 206 (41.5%), 128 (25.8%) had metastasis at the time of diagnosis, and tumor size < 50 mm was seen in 177 (35.6%) of patients. Twenty-four (4.8%) received LRT, 119 (23.9%) underwent resection, and 50 (10.1%) underwent liver transplantation. Detailed baseline characteristics are shown on Table 1. The overall median survival was 6 mo [Interquartile range (IQR): 1-22] (Figure 1). Age at diagnosis and survival months did not show a normal distribution by the Shapiro-Wilk normality test; therefore, comparison was made by the Mann-Whitney U test. There were significant differences for age at diagnosis, age > 60 years old, AJCC stages, SEER stages (localized, regional extension, lymph nodes involvement, distant metastasis), proportion of Stage I/II, positive lymph nodes, metastasis at the diagnosis, tumor size < 30 mm, < 40 mm, and < 50 mm, resection, and liver transplantation (all P < 0.01). There was no significant difference between 5-year survivors and non-5-year survivors for sex, race, state of residence, marital status, tumor size < 20 mm, LRT.
| Overall | 5-year survivor | Non-5-year survivor | P value | |
| Median age (IQR) | 62 (56-69) | 57 (51.25-63.75) | 62 (56-70) | < 0.01 |
| Sex (%Male) | 348 (70.0) | 36 (78.3) | 312 (69.2) | 0.27 |
| Race | 0.23 | |||
| Whites | 276 (55.5) | 23 (50.0) | 253 (56.1) | |
| Blacks | 53 (10.7) | 2 (4.3) | 51 (11.3) | |
| Hispanics | 77 (15.5) | 8 (17.4) | 69 (15.3) | |
| API | 84 (16.9) | 13 (28.3) | 71 (15.7) | |
| Others | 7 (1.4) | 0 (0) | 7 (1.6) | |
| AJCC stages | < 0.01 | |||
| I | 116 (23.3) | 19 (41.3) | 97 (21.5) | |
| II | 90 (18.1) | 19 (41.3) | 71 (15.7) | |
| III | 78 (15.7) | 5 (10.9) | 73 (16.2) | |
| IV | 139 (28.0) | 1 (2.2) | 138 (30.6) | |
| Unknown | 74 (14.9) | 2 (4.3) | 72 (16.0) | |
| Metastasis | 128 (25.8) | 1 (2.2) | 127 (28.2) | < 0.01 |
| Tumor size < 50 mm | 177 (35.6) | 34 (91.9) | 143 (56.5) | < 0.01 |
| Treatment | ||||
| LRT | 24 (4.8) | 2 (4.3) | 22 (4.9) | 1.00 |
| Resection | 119 (23.9) | 21 (45.7) | 98 (21.8) | < 0.01 |
| Transplant | 50 (10.6) | 20 (43.5) | 30 (6.7) | < 0.01 |
| Median survival months (IQR) | 6 (1-22) | 96.5 (83.25-129.5) | 5 (1-16) | < 0.01 |
We compared racial differences in 12-mo, 36-mo, and 60-mo survival among white, black, Hispanics, API and others by χ2 test and there were no statistically significant differences. Among same groups, gender, age > 60 years-old, size > 50 mm, LRT, resection, or transplant did not show significant differences. We then compared API with non-Asians. API had higher resection rate 35.7% compared to 21.1% in non-Asians (P < 0.01) and higher rate of 60 mo survival 15.4% compared to 8.0% (P = 0.05). Gender, age > 60 years-old, rate of stage I or II disease, LRT, transplant, and size > 50 mm did not show significant difference.
After excluding 8 patients with other or unknown race (7) and surgery status (1), we conducted logistic regression model. Univariate analysis (Table 2) showed age > 60, stage I/II vs unknown, I/II vs III/IV, metastasis, tumor size < 50 mm, resection, and transplant were significant predictors (all P < 0.01). Sex, race, stage III/IV vs unknown, and LRT were not significant factors. After multivariate logistic regression (Table 2), tumor size < 50 mm [Odds ratio (OR): 2.415, P = 0.05], resection (OR: 12.849, P < 0.01), and transplant (OR: 27.129, P < 0.01) were statistically significant for 5-year survival. Age > 60, sex, race, AJCC stage, metastasis, and LRT were not significant. However, API vs white showed significant OR of 2.793 (CI: 1.120-6.967). It is important to note that 12 API and 18 Hispanic patients had untraced survival status.
| Univariate analysis | Multivariate analysis | |||
| OR [95%CI] | P value | OR [95%CI] | P value | |
| Age > 60 yr old | 0.372 [0.195-0.708]1 | < 0.01 | 0.502 [0.231-1.088] | 0.08 |
| Sex (Male) | 1.604 [0.774-3.323] | 0.20 | 1.264 [0.537-2.975] | 0.59 |
| Race | 0.13 | 0.07 | ||
| Black | 0.432 [0.099-1.888] | 0.483 [0.095-2.444] | ||
| Hispanic | 1.275 [0.547-2.976] | 2.043 [0.744-5.613] | ||
| API | 2.014 [0.971-4.176] | 2.793 [1.120-6.967]1 | ||
| AJCC stages | 0.41 | |||
| Stage I/II vs unknown | 8.143 [1.913-34.660]1 | < 0.01 | 1.048 [0.185-5.935] | |
| Stage III/IV vs unknown | 1.024 [0.202-5.186] | 0.06 | 0.498 [0.074-3.328] | |
| Stage I /II vs III/IV | 7.954 [3.284-19.264]1 | < 0.01 | ||
| Metastasis | 0.057 [0.008-0.416]1 | < 0.01 | 0.602 [0.059-6.165] | 0.67 |
| Tumor size < 50 mm | 6.098 [3.067-12.200]1 | < 0.01 | 2.415 [1.010-5.780]1 | 0.05 |
| LRT | 0.884 [0.201-3.886] | 0.87 | 4.622 [0.671-31.856] | 0.12 |
| Resection | 3.017 [1.620-5.620]1 | < 0.01 | 12.849 [3.359-49.142]1 | < 0.01 |
| Transplant | 10.769 [5.398-21.485]1 | < 0.01 | 28.129 [6.639-119.187]1 | < 0.01 |
Cox proportional hazard regression showed AJCC stage, tumor size < 50 mm, LRT, resection, and transplant showed significance (Table 3). Age > 60, sex, race, and metastasis did not show significant HR. Although overall race did not show significance, API showed HR of 0.654 (CI: 0.452-0.948) over black, and HR of 0.727 (CI: 0.555-0.952) over white. There was no difference for Hispanic over API, black, or white, and black over white.
| Hazard ratio | 95%CI | P value | |
| Age > 60 years old | 0.862 | 0.708-1.050 | 0.14 |
| Sex (Male) | 1.071 | 0.863-1.328 | 0.54 |
| Race | 0.07 | ||
| API vs black | 0.654 | 0.452-0.9481 | |
| API vs Hispanic | 0.838 | 0.595-1.180 | |
| API vs white | 0.727 | 0.555-0.9521 | |
| Black vs Hispanic | 1.280 | 0.884-1.852 | |
| Black vs white | 1.111 | 0.819-1.506 | |
| Hispanic vs white | 0.868 | 0.660-1.140 | |
| AJCC stages | < 0.01 | ||
| Stage I/II vs unknown | 0.547 | 0.390-0.7681 | |
| Stage III/IV vs unknown | 0.709 | 0.509-0.9881 | |
| Stage I/II vs III/IV | 0.772 | 0.571-1.042 | |
| Metastasis | 1.229 | 0.918-1.645 | 0.17 |
| Tumor size < 50 mm | 0.704 | 0.545-0.9081 | < 0.01 |
| LRT | 1.782 | 1.134-2.8011 | 0.01 |
| Resection | 2.770 | 2.137-3.5901 | < 0.01 |
| Transplant | 4.247 | 2.809-6.5421 | < 0.01 |
HCC/CC is a rare, aggressive variant with features of both HCC and CC and few centers have enough cases to understand how to effectively treat this. It is not clear if the treatments typically used for HCC will be effective. Before we offer specific therapies, we need to better understand the natural history of this disease so we can target our efforts appropriately. This study showed that of the clinical factors, tumor size < 50 mm, resection and transplant were predictors of 5-year survival. However, Age > 60, sex, race, AJCC stages, metastasis, and LRT were not associated with significant odds of 5-year survival. Treatment with liver transplantation or liver resection were associated with 5-year survival but transplantation had a higher odds-ratio. In addition, although overall race was not significant, API showed significant CI for OR over white, and HR over white and black.
Our study highlighted that API patients had a higher chance of 5-year survival compared to white and black. The reasons for this were not completely clear and may be related to the underlying chronic liver disease or access to care. The high prevalence of hepatitis B in Asia may account for some of these differences. A Chinese study suggested that hepatitis B was a strong risk factor for developing HCC/CC and while this is similar in both HCC and CC alone, there was no association between underlying hepatitis C and HCC/CC[22]. A previous SEER study on HCC showed that Asians had a higher proportion of localized HCC compared to advanced HCC[23]. This may suggest that the high prevalence of hepatitis B in Asians may have prompted HCC surveillance and earlier detection. Furthermore, hepatitis B infections may lead to primary liver cancers in the absence of cirrhosis which may have allowed for more aggressive attempts at surgical resection in Asians. Unfortunately, the SEER data does not have information on underlying disease or whether HCC/ICC was found with surveillance.
Our study also demonstrated that API had a survival advantage over whites and blacks but did not have a difference in survival compared to Hispanics. However, a slightly higher proportion of API and Hispanics had no survival information. Previous studies have shown a higher incidence of HCC in Hispanics compared to Asians and Hispanics were more likely to have underlying non-alcoholic fatty liver disease and chronic hepatitis C virus infections[24]. Ha et al[25] suggested that blacks and Hispanics were less likely to receive curative therapy for HCC due to the advanced stage at presentation of HCC. Similar observations in racial and socioeconomic disparities were found in Hispanic patients with CC[26,27]. While all of these observations suggest a worse outcome for Hispanics with HCC or CC, ours is the first to describe a non-inferior prognosis for Hispanics with the combined HCC/CC variant.
The burden of tumor likely affects overall prognosis and our study showed that tumors less than 5 cm were associated with better 5-year survival. Several other small studies have also suggested that tumor size > 5 cm was associated with a poor overall survival[15,28]. Multiple tumors and microvascular invasion were other factors associated with worse outcome after surgery[15]. However, there were several additional studies that did not support specific tumor characteristics as being prognostic in survival (Table 4). While our study demonstrated that tumor size affected long term survival, this was likely because patients with smaller tumors were more suitable candidates for surgery. Transplantation in the U.S. requires meeting Milan criteria or undergoing downstaging with LRT to meet Milan criteria and these presumably affected candidacy in our cohort.
| Ref. | Country | Number of HCC/CC patients | 1-year survival (%) | 3-year survival (%) | Factors predictive of survival |
| Park et al[32], 2013 | South Korea | Hepatic resection (n = 10) | 20 | 20 | Age, sex, TACE and T stage by univariate analysis, but none multivariate analysis |
| Antwi et al[33], 2018 | United States | Liver transplant (n = 19) | 84 | 74 | Response to neoadjuvant LRT |
| Groeschl et al[31], 2013 | United States | Hepatic resection (n = 35); Liver transplant (n = 19) | Resection: 71; Transplant: 89 | Resection: 46; Transplant: 48 | NA |
| Itoh et al[29], 2015 | Japan | Living donor transplant (n = 8) | 87.5 | 72.9 | NA |
| Li et al[30], 2018 | Meta-analysis | Hepatic resection (n = 1390); Liver transplant (n = 301) | Resection: 79; Transplant: 85 | Resection: 63; Transplant: 63 | Vascular invasion, lymph node involvement, tumor size > 5 cm and advanced stage |
The prognosis for HCC/CC is generally poor and our study showed that the median survival for HCC/CC was only 6 mo. Treatment with resection or transplant were associated with 5-year survival, however previous studies are divisive on which treatment is superior or how these treatments compare to patients with HCC. Itoh et al[29] compared long-term outcomes after living donor transplantation between 8 HCC/CC and 170 HCC patients and did not demonstrate a difference between overall and disease free survival. A meta-analysis of 1691 patients (42 studies) with HCC/CC suggested that there was no significant difference for 5-year overall survival after liver resection or transplantation[30]. However, Groeschl et al[31] compared the outcome between 3378 HCC and 54 HCC/CC patients and showed that both transplant and resection demonstrated a survival benefit in HCC/CC, but this benefit was inferior to transplant for HCC. They questioned the use of liver transplant in the HCC/CC variant.
Unfortunately, only a limited number of patients qualify for surgical treatment, and this is likely contributing to a poor median and 5-year survival rate. While surgical treatment can improve survival, the use of locoregional therapy or systemic therapies for this variant is not clear. While a small study did show improvement in survival from HCC/CC with transarterial chemoembolization[32], our study did not demonstrate a 5-year survival benefit in patients who received locoregional therapy. HCC/CC patients who respond to pre transplant locoregional therapy may have better post-liver transplantation 3-year overall survival[33]. However, unlike HCC, there are no large studies with established pre-transplant therapies for patients with HCC/ICC. The A.L.A.N. score, which is calculated with baseline actual neutrophil count, lymphocytes-monocytes ratio, albumin, and neutrophil-lymphocytes ratio developed by a group in the U.K., may provide prognostic information for patients with advanced biliary cancer who received the first-line chemotherapy[34].
The role of lymph node involvement in HCC/ICC may be contributing to the outcome. A previous report noted that up to 70% of HCC/CC cases demonstrate lymph node metastasis, similar to the frequency in CC cases[35,36]. Lymph node dissection is not generally done for HCC but is recommended for moderate and high-risk CC. Unfortunately, the SEER data did not have information as to whether a node dissection was performed so we cannot draw definite conclusions on the role for this in patients with the HCC/CC variant.
This study is limited in that it was based on a large database from multiple institutions, and may be subject to reporting bias and coding errors. Data from the SEER did not report the underlying chronic liver disease, laboratory studies to assess hepatic function, and calculate CHILD Pugh score or detailed information on tumor characteristics, which would be important in determining resectability and transplant candidacy and thus impact on 5-year survival. Due to the nature of the SEER database, it is not possible to know if patients received adjuvant chemotherapy after surgical treatment, which may improve recurrence-free survival if combined HCC/CC has a higher CC component[37].
It would have also been helpful to know if patients had their tumor found with surveillance or whether they were symptomatic as this would help identify any disparity in access to care, but this information is also unknown. In spite of these limitations, the strength of this study is that it included a large number of patients with a very rare variant. Individual institutions would never have enough cases of combined HCC/CC to have the statistical power to show differences in the factors analyzed.
Management of HCC/CC variant is difficult. The trend toward radiologic diagnosis of HCC may be facilitating misdiagnosis of this variant and delaying recognition until after the resected liver specimen has been examined. It is unclear if we should be treating HCC/ICC using similar protocols as HCC or if we should be adding adjuvant therapies to address nodal involvement of the CC component or perhaps some different approach altogether. We demonstrated that selection of tumors smaller than 5 cm and treatment with liver resection and transplant seem to be best associated with long term survival. While this study can help identify prognostic factors, further studies will be necessary to explain racial/ethnic differences, the effect of underlying chronic liver disease and the role of locoregional and systemic therapies in this rare variant.
Combined hepatocellular and cholangiocarcinoma (HCC/CC) is a rare primary hepatic malignancy which carries a poor prognosis due to its aggressive nature. Few centers have enough cases to draw definitive conclusions and there is limited understanding of prognosis.
As there has not been a randomized clinical trial done on this topic to elucidate the best treatment modality on HCC/CC, there is a need to better characterize the prognosis of this disease.
In this retrospective study, we attempted to identify associated factors for 5-year survival.
We conducted a retrospective study of The Surveillance, Epidemiology, and End Results database to identify HCC/CC cases from 2004 to 2015. We collected demographics, American Joint Committee on Cancer (AJCC) stage, treatment, tumor size, and survival data. Mann-Whitney U test was used to compare continuous variables without normal distribution, and t-test was used to compare continuous variables with a normal distribution. The Kaplan-Meier survival curve analyzed Five-year survival. These data were converted to categorical variables. Univariate and multivariate logistic regression model was used to analyze factors associated with 5-year survival. Multivariate Cox proportional hazard regression was done on 5-year survival.
We identified 497 patients with the following characteristics: Mean age 62.4 years, 149 (30.0%) were female, racial distributions were 276 (55.5%) white, 53 (10.7%) black, 84 (16.9%) Asian and Pacific Islander (API), 77 (15.5%) Hispanic, and 7 (1.4%) others or unknown. Stage I/II disease occurred in 41.5% and tumor size < 50 mm was seen in 35.6% of patients. The overall median survival was 6 mo. After multivariate logistic regression, tumor size < 50 mm [odds ratio (OR): 2.415, P = 0.05], resection (OR: 12.849, P < 0.01), and transplant (OR: 27.129, P < 0.01) showed significance for 5-year survival. Age > 60, sex, race, AJCC stages, metastasis, and LRT were not significant. However, API vs white showed significant OR of 2.793 (CI: 1.120-6.967). Cox proportional hazard regression showed AJCC stages, tumor size < 50 mm, LRT, resection, and transplant showed significant hazard ratio.
HCC/CC patients with tumor size < 50 mm, resection, and transplant were associated with an increase in 5-year survival. API showed advantageous OR and hazard ratios over white, black.
Prognosis and possible treatment modality for HCC/CC is different from hepatocellular carcinoma or cholangiocarcinoma alone. As we depend heavily on imaging diagnosis of hepatocellular carcinoma, this study may suggest the importance of role of biopsy to confirm correct diagnosis.
| 1. | Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 2. | Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 3. | Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] |
| 4. | Wachtel MS, Zhang Y, Xu T, Chiriva-Internati M, Frezza EE. Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin Med Pathol. 2008;1:43-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, Zhang BX. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Lee CH, Hsieh SY, Chang CJ, Lin YJ. Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J Gastroenterol Hepatol. 2013;28:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, Joh JW. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006;36:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, Bluteau O, Blanché H, Franco D, Monges G, Belghiti J, Sa Cunha A, Laurent-Puig P, Degott C, Zucman-Rossi J. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol. 2004;41:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Jung DH, Hwang S, Song GW, Ahn CS, Moon DB, Kim KH, Ha TY, Park GC, Hong SM, Kim WJ, Kang WH, Kim SH, Yu ES, Lee SG. Longterm prognosis of combined hepatocellular carcinoma-cholangiocarcinoma following liver transplantation and resection. Liver Transpl. 2017;23:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 12. | Maganty K, Levi D, Moon J, Bejarano PA, Arosemena L, Tzakis A, Martin P. Combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma: outcome after liver transplantation. Dig Dis Sci. 2010;55:3597-3601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289:816-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 869] [Article Influence: 108.6] [Reference Citation Analysis (1)] |
| 14. | Choi SH, Lee SS, Park SH, Kim KM, Yu E, Park Y, Shin YM, Lee MG. LI-RADS Classification and Prognosis of Primary Liver Cancers at Gadoxetic Acid-enhanced MRI. Radiology. 2019;290:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | Zhang H, Yu X, Xu J, Li J, Zhou Y. Combined hepatocellular-cholangiocarcinoma: An analysis of clinicopathological characteristics after surgery. Medicine (Baltimore). 2019;98:e17102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Song P, Midorikawa Y, Nakayama H, Higaki T, Moriguchi M, Aramaki O, Yamazaki S, Aoki M, Teramoto K, Takayama T. Patients' prognosis of intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma after resection. Cancer Med. 2019;8:5862-5871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 18. | Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database. Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000-2016) - Linke. Available from: https://www.seer.cancer.gov. |
| 19. | National Cancer Institute. About the SEER Registries. Surveillance, Epidemiol, End Results Program. Available from: https://seer.cancer.gov/registries/. |
| 20. | Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag, 2002. |
| 21. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 14533] [Article Influence: 1117.9] [Reference Citation Analysis (0)] |
| 22. | Zhou YM, Zhang XF, Wu LP, Sui CJ, Yang JM. Risk factors for combined hepatocellular-cholangiocarcinoma: a hospital-based case-control study. World J Gastroenterol. 2014;20:12615-12620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ha J, Yan M, Aguilar M, Bhuket T, Tana MM, Liu B, Gish RG, Wong RJ. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer. 2016;122:2512-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019; 156: 477-491. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1284] [Article Influence: 183.4] [Reference Citation Analysis (3)] |
| 25. | Ha J, Yan M, Aguilar M, Tana M, Liu B, Frenette CT, Bhuket T, Wong RJ. Race/Ethnicity-specific Disparities in Hepatocellular Carcinoma Stage at Diagnosis and its Impact on Receipt of Curative Therapies. J Clin Gastroenterol. 2016;50:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Lee RM, Liu Y, Gamboa AC, Zaidi MY, Kooby DA, Shah MM, Cardona K, Russell MC, Maithel SK. Race, ethnicity, and socioeconomic factors in cholangiocarcinoma: What is driving disparities in receipt of treatment? J Surg Oncol. 2019;120:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Ruiz JN, Kröner PT, Wijarnpreecha K, Corral JE, Harnois DM, Lukens FJ. Increased odds of cholangiocarcinoma in Hispanics: results of a nationwide analysis. Eur J Gastroenterol Hepatol. 2020;32:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Wakizaka K, Yokoo H, Kamiyama T, Ohira M, Kato K, Fujii Y, Sugiyama K, Okada N, Ohata T, Nagatsu A, Shimada S, Orimo T, Kamachi H, Taketomi A. Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol. 2019;34:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Itoh S, Ikegami T, Yoshizumi T, Wang H, Takeishi K, Harimoto N, Yamashita Y, Kawanaka H, Aishima S, Shirabe K, Maehara Y. Long-term outcome of living-donor liver transplantation for combined hepatocellular-cholangiocarcinoma. Anticancer Res. 2015;35:2475-2476. [PubMed] |
| 30. | Li DB, Si XY, Wang SJ, Zhou YM. Long-term outcomes of combined hepatocellular-cholangiocarcinoma after hepatectomy or liver transplantation: A systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2019;18:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Groeschl RT, Turaga KK, Gamblin TC. Transplantation versus resection for patients with combined hepatocellular carcinoma-cholangiocarcinoma. J Surg Oncol. 2013;107:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Park SE, Lee SH, Yang JD, Hwang HP, Hwang SE, Yu HC, Moon WS, Cho BH. Clinicopathological characteristics and prognostic factors in combined hepatocellular carcinoma and cholangiocarcinoma. Korean J Hepatobiliary Pancreat Surg. 2013;17:152-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Antwi SO, Habboush YY, Chase LA, Lee DD, Patel T. Response to Loco-Regional Therapy Predicts Outcomes After Liver Transplantation for Combined Hepatocellular-Cholangiocarcinoma. Ann Hepatol. 2018;17:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Salati M, Caputo F, Cunningham D, Marcheselli L, Spallanzani A, Rimini M, Gelsomino F, Reggiani-Bonetti L, Andrikou K, Rovinelli F, Smyth E, Baratelli C, Kouvelakis K, Kalaitzaki R, Gillbanks A, Michalarea V, Cascinu S, Braconi C. The A.L.A.N. score identifies prognostic classes in advanced biliary cancer patients receiving first-line chemotherapy. Eur J Cancer. 2019;117:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | O'Connor K, Walsh JC, Schaeffer DF. Combined hepatocellular-cholangiocarcinoma (cHCC-CC): a distinct entity. Ann Hepatol. 2014;13:317-322. [PubMed] |
| 36. | Navarro JG, Lee JH, Kang I, Rho SY, Choi GH, Han DH, Kim KS, Choi JS. Prognostic significance of and risk prediction model for lymph node metastasis in resectable intrahepatic cholangiocarcinoma: do all require lymph node dissection? HPB (Oxford). 2020;22:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Messina C, Merz V, Frisinghelli M, Trentin C, Grego E, Veccia A, Salati M, Messina M, Carnaghi C, Caffo O. Adjuvant chemotherapy in resected bile duct cancer: A systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol. 2019;143:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujino Y, Messina C S-Editor: Wang JL L-Editor: A P-Editor: Li X