Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1004
Peer-review started: June 30, 2020
First decision: August 8, 2020
Revised: August 24, 2020
Accepted: October 12, 2020
Article in press: October 12, 2020
Published online: November 27, 2020
Processing time: 146 Days and 19 Hours
Obesity is a global health problem that is continuing to increase in the young population. In Brazil, the frequency of obesity in 2018 was 19.8%. Several comorbidities are directly associated with obesity, such as non-alcoholic fatty liver disease (NAFLD), which is considered the most common liver disorder in Western countries and affects up to 46% of adults. Bariatric surgery is effective in treating obesity and can improve NAFLD; however, the effect of bariatric surgery on body composition, phase angle (PA), and improving NAFLD needs to be further studied.
To analyze the PA in the postoperative period of bariatric surgery and to correlate it with changes in body composition and liver disease.
This study is a retrospective cohort study of the analysis of the medical records of patients undergoing bariatric surgery in a reference center of a teaching hospital in Porto Alegre over a 2-year period. Patients older than 18 years whose record contained all information relevant to the study were included. The data analyzed were body composition and PA through electrical bioimpedance and NAFLD through liver biopsy in the pre- and postoperative period. The level of significance adopted for the statistical analyses was 5%.
We evaluated 379 patients with preoperative data. Regarding PA, 169 patients were analyzed, and 33 patients had liver biopsy pre- and postoperatively with NAFLD information. In total, 79.4% were female, with a mean age of 39.1 ± 10.6 years. The average body mass index (BMI) was 45.9 ± 7.5 kg/m². The PA showed a mean of 5.8 ± 0.62° in the preoperative period and a significant reduction in the postoperative period. A postoperative reduction in body composition data (skeletal muscle mass, fat percentage, fat mass, body cell mass, BMI and visceral fat area) was shown as well. Regarding liver disease, all patients presented a reduction in the degrees and stages of liver disease in the postoperative period, and some had no degree of liver disease at all.
PA decreased after bariatric surgery, with a direct correlation with weight loss and changes in body composition. The decrease in PA was not correlated with the improvement in NAFLD.
Core Tip: We retrospectively evaluated 379 patients who underwent bariatric surgery, with non-alcoholic fatty liver disease in the preoperative period; we compared body composition, phase angle (PA) behavior and change in non-alcoholic fatty liver disease (NAFLD) in the pre- and postoperative period. There was an important improvement in body composition/body fat percentage and an improvement in NAFLD after bariatric surgery. Worsening PA was directly correlated with weight loss and skeletal muscle mass.
- Citation: Teixeira J, Marroni CA, Zubiaurre PR, Henz A, Faina L, Pinheiro LK, Mottin CC, Fernandes SA. Phase angle and non-alcoholic fatty liver disease before and after bariatric surgery. World J Hepatol 2020; 12(11): 1004-1019
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1004.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1004
Obesity is a complex chronic inflammatory disease characterized by excessive accumulation of body fat. It has multiple etiologies, such as biochemical, genetic, behavioral, social, environmental, and nutritional factors and imbalance between food intake and energy expenditure[1-4]. This disease presents its own pathophysiology as well as associated comorbidities[3,5].
According to VIGITEL Brazil 2018[6], the frequency of obese adults was 19.8% in a society that is aging and more ill. Obesity is closely associated with cardiovascular diseases, dyslipidemia, fatty liver, diabetes mellitus (DM) and other endocrine and metabolic disorders[1]. In obese people, a common comorbidity is non-alcoholic fatty liver disease (NAFLD).
NAFLD is defined as accumulation of fat in more than 5% of hepatocytes[7] without secondary cause, such as alcohol consumption (> 20 g for women and > 30 g for men, daily), use of steatogenic medications, or hereditary disorders[8].
The gold standard for the diagnosis of NAFLD is liver biopsy[9], and its classification encompasses a wide spectrum of histopathological changes, from simple hepatic steatosis, which can evolve from non-alcoholic steatohepatitis (NASH), to cirrhosis and/or hepatocellular carcinoma[10]. Therefore, NAFLD, due to its different staging, may or may not present fibrosis in hepatocytes[11,12]. NAFLD is considered the most common liver disease in Western countries, affecting 90% of morbidly obese patients eligible for bariatric surgery[11].
Because of the difficulty found in the clinical treatment of obesity, bariatric surgery is more efficient as a treatment option for individuals with severe obesity, when compared to non-surgical interventions[13]. Furthermore, surgical treatment shows improvement or remission in NAFLD[14].
According to the Brazilian Society of Bariatric and Metabolic Surgery[15], bariatric surgery combines techniques aimed to treat morbid obesity, severe obesity, and diseases associated with excess body fat or exacerbated by it. Gastric bypass surgery in Y by Roux (BPGYR) or Fobi-Capella surgery is the most commonly performed technique in Brazil and worldwide.
Electrical bioimpedance (BIA) is a method for analyzing body composition and is based on the principle of resistance and reactance that cells impose on the electrical current emitted by the device[16]. The human body is constituted of conductors like water and non-conductors, like body fat[17,18].
Several parameters are measured using BIA, including body water, lean mass, fat mass, and phase angle (PA). BIA assesses nutritional status and can be a good method for prognostic evaluation, as it is practical, fast, non-invasive, and low cost[19]. However, body composition values in patients with dysmorphia (edema, ascites, and morbid obesity), as measured by BIA, may suffer interference. This is why PA has been widely used, since it is not associated with interference[20,21].
Currently, there are segmented, multifrequency BIA devices with greater precision for assessing body composition in morbidly obese patients, as validated by Faria et al[22].
PA was originally described by Baumgartner et al[23] for the diagnosis of metabolic disorders. It is a parameter applicable in clinical practice because it reliably helps to describe cell vitality and integrality. High values (up to 8°) may indicate body homeostasis, whereas values below 6°, depending on the disease, reflect a poor clinical prognosis, indicating changes in the selective permeability of the cell membrane[23,24].
As already mentioned, PA reflects cellular integrity and functionality by measuring, through an electrical current, the values of resistance and reactance of the membrane of these cells, with skeletal muscle being a conductor of electrical current and the opposite occurring with fat mass[23-25]. In view of this fact, we believe that the body change resulting from bariatric surgery will reflect an improvement in NAFLD and can be measured by PA.
To date, there are not enough studies evaluating morbid obesity, body composition (described by the BIA), and associated comorbidities, such as NAFLD.
The present study aims to analyze the behavior of PA in the postoperative period of bariatric surgery, correlating it with changes in body composition and improvement of liver disease.
This is a retrospective cohort study that analyzed the medical records of patients undergoing bariatric surgery in a referral center of a teaching hospital in Porto Alegre. Patients over 18-years-old whose record contained all the information relevant to the study in the electronic or physical medical record were included. Patients who did not contain complete data were excluded. For convenience, the sample was carried out from July 2015 to July 2017. The data obtained were related to the protocol for routine pre- and postoperative care at the service's outpatient clinic.
Data on body composition and weight were measured using BIA in all patients in the week preceding bariatric surgery without prior preparation. For BIA, the patient stood upright on the InBody 770 device from Ottoboni, with an electric current intensity of 80 µA and 50/60 kHz frequency. The PA was obtained through the values of resistance and reactance through the formula: PA = tangent arc (Xc/R) × 180/3.1416, described in the result sheet. The BIA was performed in a second step, and for comparative postoperative analysis, we analyzed those patients who used the same BIA device 6 mo to 12 mo after bariatric surgery for routine postoperative evaluation.
Height was measured using a wall Tonelli stadiometer, model E150 A, with the patient standing upright and barefoot, with their feet together, and with their backs positioned against the wall. Body mass index (BMI) was calculated using the equation weight in kilograms, divided by height in meters squared and classified according to the World Health Organization[4]: BMI ≥ 30 kg/m² to 34.9 kg/m² Obesity Grade I, BMI between 35 kg/m² to 39.9 kg/m² Obesity Grade II, and BMI ≥ 40 kg/m² Obesity Grade III. The group of patients with a BMI greater than 50 kg/m² was analyzed separately.
The bariatric surgery used was the BPGYR with intestinal derivation after monitoring and preparation with a multidisciplinary service team. All patients met the criteria for bariatric surgery. The surgeries were performed by four specialist surgeons, from the same team, trained and with much experience.
Liver biopsies were routinely performed during the bariatric surgery trans operation by the surgeon under direct vision using a Tru-Cut needle at the beginning of the surgical procedure before liver withdrawal. The biopsies were analyzed by the same pathologist at the Hospital's Pathology Laboratory. The classification was made using the criteria of Kleiner et al[26], as follows: Absence or presence of NAFLD and/or cirrhosis; steatosis activity (absent, discreet, moderate, accented, and massive– according to grade and location of the injury); ballooning and lobular inflammation; degrees of NASH (1, 2, 3, and 4), and fibrosis stages (1, 2, 3 and 4). The liver biopsies performed in the postoperative period were obtained by the same surgical team in patients who underwent a second intervention (cholecystectomy or appendectomy) in the period from 6 to 12 mo after bariatric surgery and analyzed in the same way as those of the first biopsy.
Quantitative variables are described as mean and standard deviation or median and interquartile range. Categorical variables are described by absolute and relative frequencies.
To compare means before and after bariatric surgery, the t-student test for paired samples was applied. When comparing nominal categorical variables, the McNemar test was used and, for ordinals, the Wilcoxon test. To compare means between genders, the t-student test for independent samples was applied.
In the association between quantitative and ordinal variables, Pearson or Spearman correlation tests were used.
The level of significance adopted was 5% (P < 0.05), and the analyses were performed using the SPSS version 21.0 program (Armonk, NY, United States).
The project was elaborated in accordance with resolution 466 of 2012, which regulates the conduct of research in human beings, and submitted to and approved by the Research Ethics Committee under number 2.423.466. Patients who accepted to participate in the study signed the Informed Consent Term.
This study was reviewed by our specialist Biostatistics, Mestre, Ceres Andréia Vieira de Oliveira.
Of the 727 patients operated on in the period, 379 who had complete preoperative information were allocated. For the analysis of data related to PA, 169 of these patients who underwent pre- and post-evaluation on the same device were allocated. Regarding NAFLD, we analyzed 33 patients who underwent postoperative liver biopsy.
Of the 379 patients, 79.4% were female, with a mean age of 39.1 ± 10.6 years and a BMI of 45.9 ± 7.5 kg/m², classified as Obesity Grade III[4]. It is noteworthy that more than 22% of patients had a BMI greater than 50 kg/m². Full-body PA showed an average of 5.89° ± 0.62°, with a minimum of 4.3° and a maximum of 7.9°. The other characteristics of the sample are shown in Table 1.
| Variables | n = 379 |
| Gender, n (%) | |
| Male | 78 (20.6) |
| Female | 301 (79.4) |
| Age in yr, mean ± SD | 39.1 ± 10.6 |
| Weight in kg, mean ± SD | 123.7 ± 24.8 |
| Minimum | 77 |
| Maximum | 235 |
| BMI in kg/m2, mean ± SD | 45.9 ± 7.5 |
| BMI classification, n (%) | |
| 30-34.99 kg/m² | 3 (0.8) |
| 35-39.99 kg/m² | 82 (21.6) |
| 40-49.99 kg/m² | 210 (55.4) |
| 50 kg/m² or more | 84 (22.2) |
| SMM in kg, mean ± SD | 34.1 ± 7.5 |
| BCM in kg, mean ± SD | 39.4 ± 8.3 |
| Fat mass in kg, mean ± SD | 62.6 ± 15.1 |
| % Fat-mean ± SD | 50.7 ± 4.6 |
| Visceral fat area in cm², mean ± SD | 243.7 ± 31.2 |
| Phase angle °, mean ± SD | |
| Full-body | 5.89 ± 0.62 |
| RA | 5.58 ± 0.62 |
| LA | 5.42 ± 0.65 |
| Tr | 7.97 ± 1.18 |
| RL | 6.14 ± 0.81 |
| LL | 6.07 ± 0.84 |
All patients (n = 379) were diagnosed with NAFLD by liver biopsy, and the histological characteristics are shown in Table 2 Regarding the degrees of the disease, 78.1% had NASH and 43% fibrosis, ranging from F1 to F3. No patient had cirrhosis.
| Variables | n (%) |
| NAFLD | |
| Yes | 379 (100.0) |
| No | 0 (0.0) |
| Hepatic steatosis | |
| Absent | 0 (0.0) |
| Discreet | 159 (42.0) |
| Moderate | 104 (27.4) |
| Accented | 84 (22.2) |
| Massive | 32 (8.4) |
| Ballooning | |
| Absent | 91 (24.0) |
| Discreet | 221 (58.3) |
| Moderate | 18 (4.7) |
| Accented | 49 12.9) |
| Massive | 0 (0.0) |
| Lobular inflammation | |
| Absent | 243 (64.1) |
| Discreet | 100 (26.4) |
| Moderate | 28 (7.4) |
| Accented | 8 (2.1) |
| Massive | 0 (0.0) |
| NASH | |
| Absent | 83 (21.9) |
| Grade 1 | 201 (53.0) |
| Grade 2 | 67 (17.7) |
| Grade 3 | 28 (7.4) |
| Grade 4 | 0 (0.0) |
| Absent | 216 (57.0) |
| Grade 1 | 86 (22.7) |
| Grade 2 | 44 (11.6) |
| Grade 3 | 33 (8.7) |
| Grade 4 | 0 (0.0) |
| Cirrhosis | |
| Yes | 0 (0.0) |
| No | 379 (100.0) |
The difference in pre- and postoperative body composition (n = 379) is found in Table 3, with all items statistically significant (P < 0.001).
| Variables | Pre | Post | P value |
| mean ± SD | mean ± SD | ||
| BMI in kg/m² | 45.9 ± 7.5 | 31.2 ± 5.0 | < 0.001 |
| SMM in kg | 34.1 ± 7.5 | 29.0 ± 6.4 | < 0.001 |
| BCM in kg | 39.4 ± 8.3 | 33.9 ± 6.9 | < 0.001 |
| Fat mass in kg | 62.6 ± 15.1 | 31.9 ± 11.1 | < 0.001 |
| % Fat | 50.7 ± 4.6 | 37.2 ± 8.1 | < 0.001 |
| Visceral fat area in cm² | 243.7 ± 31.2 | 152.0 ± 51.0 | < 0.001 |
The associations of body composition variables with PA, stratified by gender (n = 379), are shown in Table 4. In males, there was an inverse, statistically significant association between the percentage of fat, fat mass, and weight with PA in most body compartments, except the torso.
| Variables | Weight in kg | SMM in kg | BCM in kg | Fat mass in kg | % Fat | Visceral fat area in cm² |
| Male gender, n = 78 | ||||||
| PA full body | -0.418c | -0.118 | -0.101 | -0.469c | -0.401c | -0.075 |
| PA RA | -0.299b | -0.083 | -0.064 | -0.337b | -0.287a | -0.095 |
| PA LA | -0.286a | -0.031 | -0.008 | -0.349b | -0.341b | -0.136 |
| PA Tr | -0.074 | 0.074 | 0.094 | -0.134 | -0.185 | 0.311b |
| PA RL | -0.493c | -0.179 | -0.170 | -0.531c | -0.421c | -0.101 |
| PA LL | -0.509c | -0.211 | -0.209 | -0.534c | -0.403c | -0.066 |
| Female gender, n = 301 | ||||||
| PA full body | -0.106 | 0.125a | 0.122b | -0.152b | -0.393c | -0.098 |
| PA RA | 0.011 | 0.169b | 0.148a | -0.036 | -0.245c | -0.111 |
| PA LA | 0.043 | 0.193b | 0.155b | -0.009 | -0.226c | -0.135a |
| PA Tr | 0.024 | 0.120a | 0.133a | -0.030 | -0.172b | -0.058 |
| PA RL | -0.154b | 0.083 | 0.068 | -0.182b | -0.406c | -0.043 |
| PA LL | -0.151b | 0.069 | 0.052 | -0.187b | -0.388c | -0.086 |
In females, there was a negative, statistically significant association between the percentage of fat and PA in all compartments. There was a positive, statistically significant association between skeletal muscle mass (SMM) and body cell mass (BCM) with PA in most body compartments, except the legs.
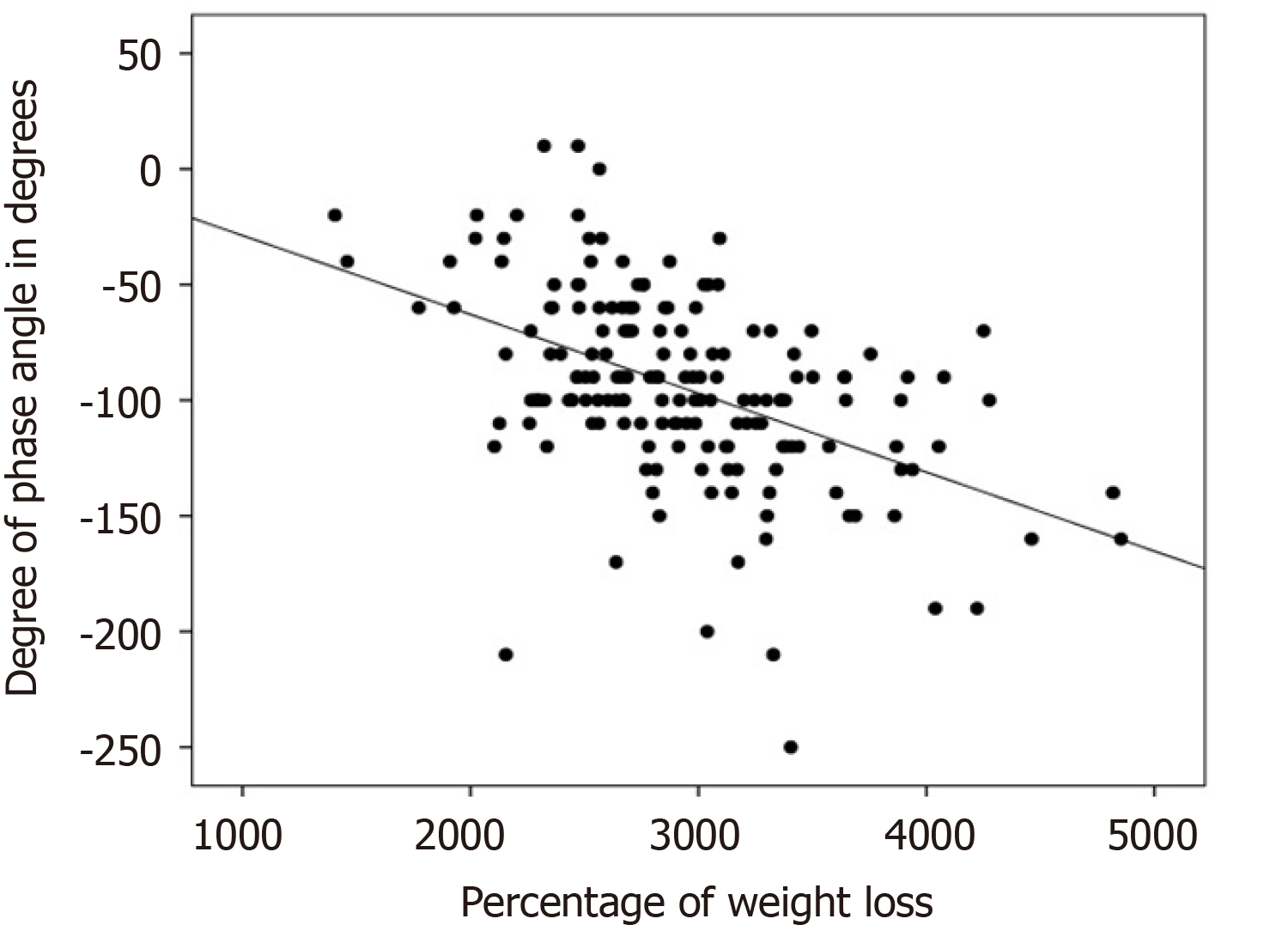
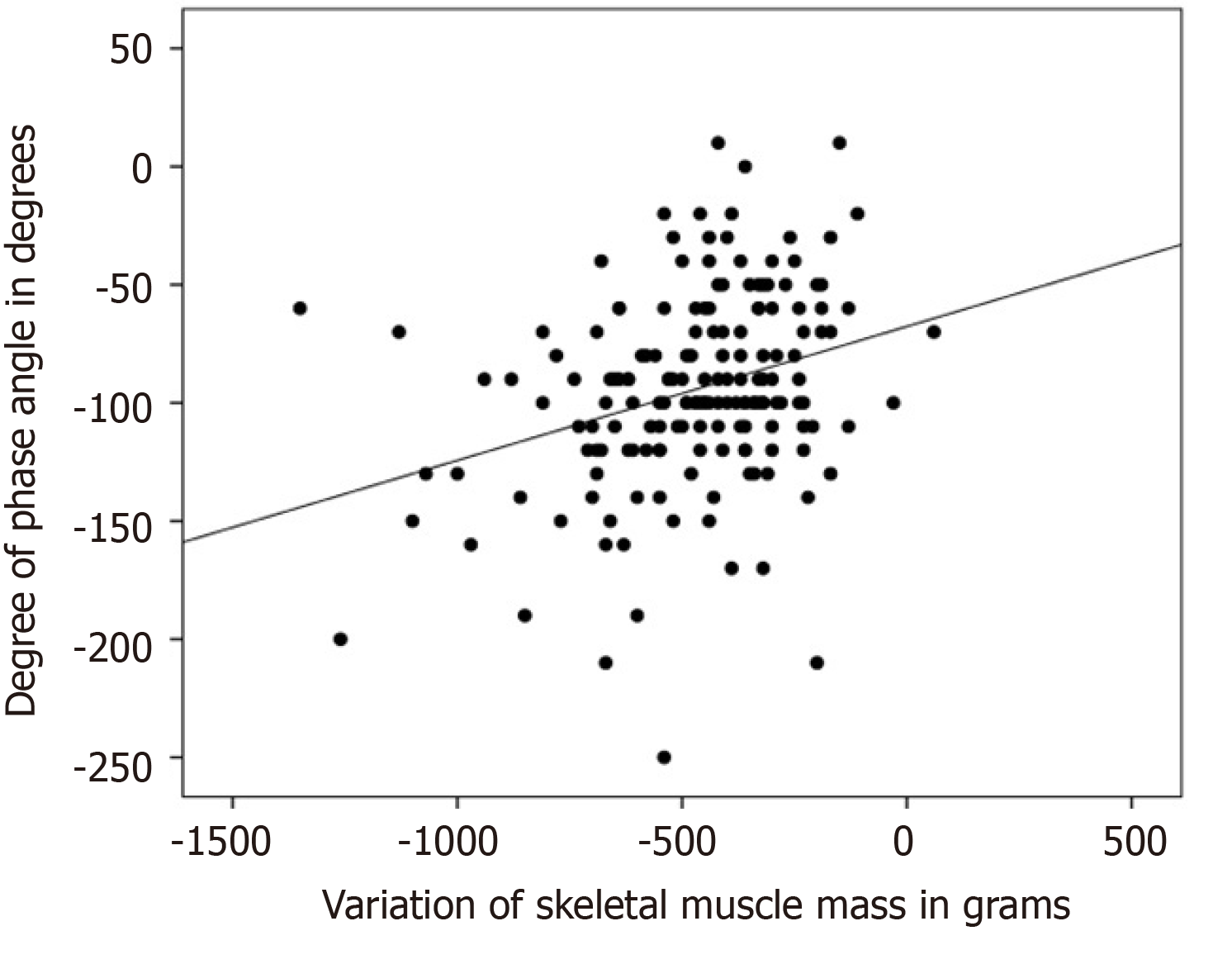
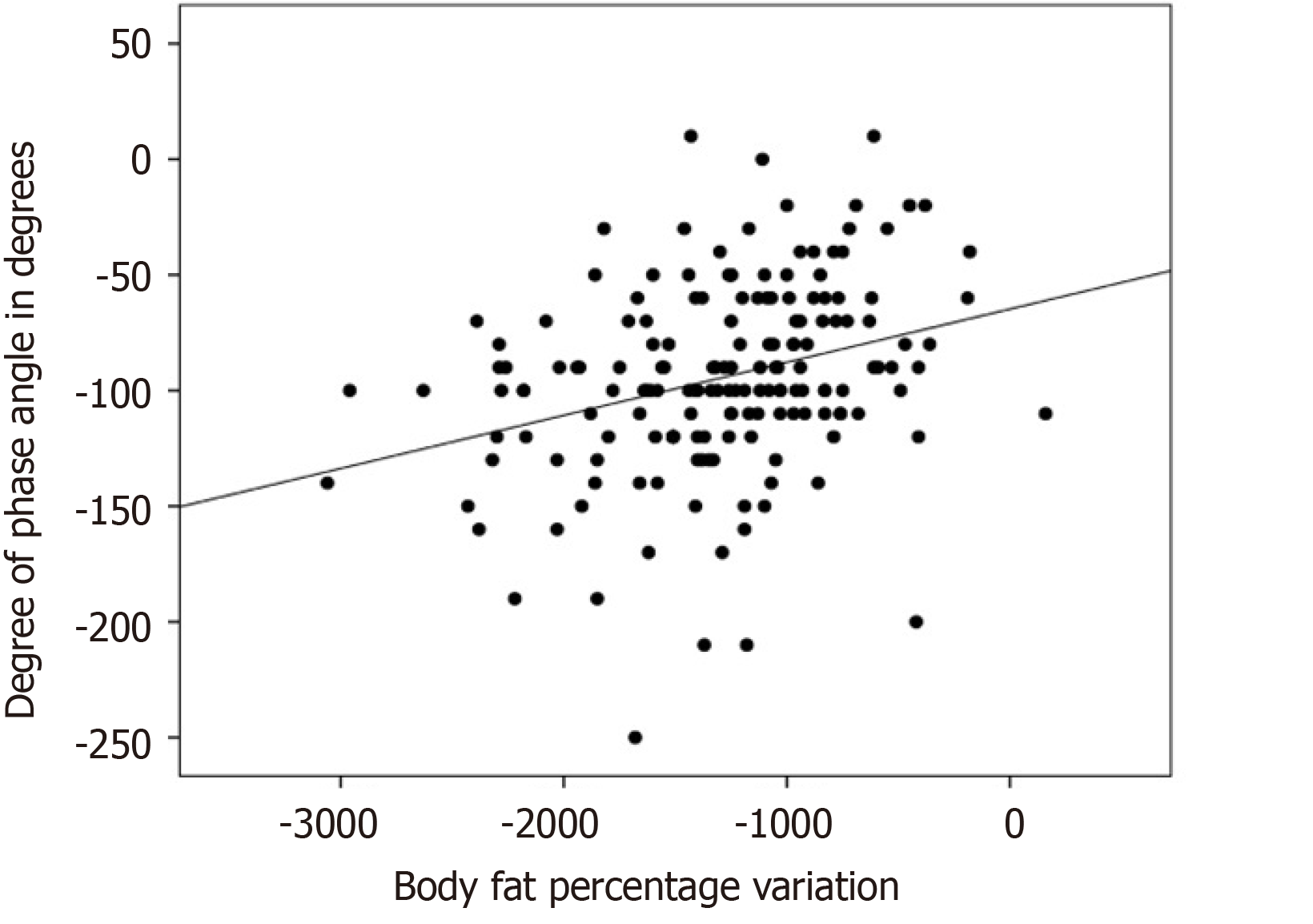
There was a negative, statistically significant association (r = -0.483; P < 0.001) between the reduction of full-body PA and the percentage of weight loss, as shown in Figure 1. There was a statistically significant positive association between the reduction of full-body PA with loss of SMM (r = 0.307; P < 0.001), as shown in Figure 2. The association of the reduction in PA of the whole body was also positive and significant with the loss of fat mass, (r = 0.280; P < 0.001), MCC (r = 0.287; P < 0.001), visceral fat area (r = 0.275; P < 0.001), BMI (r = 0.413; P < 0.001), and variation in the fat percentage (r = 0.304; P < 0.001), as shown in Figure 3.
Men showed a more marked and significant reduction in the percentage of fat (-16 ± 4.9 vs -12.3 ± 5.3; P < 0.001) and visceral fat area (-110.7 ± 58.9 vs -90 ± 42.8; P = 0.024) when compared to women.
After bariatric surgery, a significant reduction in PA values (n = 169) was observed in all body compartments (P < 0.001), as described in Table 5.
| Variables | Pre | Post | P value |
| mean ± SD | mean ± SD | ||
| PA full body | 5.92 ± 0.55 | 4.98 ± 0.55 | < 0.001 |
| PA RA | 5.58 ± 0.60 | 4.73 ± 0.59 | < 0.001 |
| PA LA | 5.42 ± 0.63 | 4.54 ± 0.57 | < 0.001 |
| PA Tr | 7.87 ± 1.15 | 6.76 ± 1.29 | < 0.001 |
| PA RL | 6.25 ± 0.66 | 5.19 ± 0.65 | < 0.001 |
| PA LL | 6.18 ± 0.70 | 5.14 ± 0.66 | < 0.001 |
There was a statistically significant inverse association between BMI and full-body PA (P < 0.018), right leg and left leg (P < 0.001), as shown in Table 6. Regarding PA and NAFLD, there was no significant association (P > 0.05).
| Variables | BMI | |
| r | P value | |
| PA full body | -0.121 | 0.018 |
| PA RA | 0.031 | 0.551 |
| PA LA | 0.057 | 0.265 |
| PA Tr | 0.054 | 0.298 |
| PA RL | -0.262 | < 0.001 |
| PA LL | -0.262 | < 0.001 |
The analysis of the postoperative liver biopsy, compared with the preoperative biopsy of these patients (n = 33), showed that all of them had a reduction in the degrees and stages of liver disease, and 18.2% had no degree of liver disease (P < 0.05).
The other histological changes, which decreased from 75 to 90%, are described in Table 7. The body composition of this group (n = 33) showed that all parameters significantly decreased (P < 0.001) and that there was a reduction in PA in all compartments (P < 0.001), as described in Table 8.
| Variables | Pre | Post | P value |
| n (%) | n (%) | ||
| NAFLD | 0.031 | ||
| Yes | 33 (100) | 27 (81.8) | |
| No | 0 (0.0) | 6 (18.2) | |
| Hepatic steatosis | < 0.001 | ||
| Absent | 0 (0.0) | 6 (18.2) | |
| Discreet | 14 (42.4) | 24 (72.7) | |
| Moderate | 8 (24.2) | 3 (9.1) | |
| Accented | 8 (24.2) | 0 (0.0) | |
| Massive | 3 (9.1) | 0 (0.0) | |
| Ballooning | < 0.001 | ||
| Absent | 5 (15.2) | 29 (87.9) | |
| Discreet | 23 (69.7) | 4 (12.1) | |
| Moderate | 3 (9.1) | 0 (0.0) | |
| Accented | 2 (6.1) | 0 (0.0) | |
| Lobular inflammation | 0.003 | ||
| Absent | 21 (63.6) | 30 (90.9) | |
| Discreet | 7 (21.2) | 3 (9.1) | |
| Moderate | 3 (9.1) | 0 (0.0) | |
| Accented | 2 (6.1) | 0 (0.0) | |
| NASH | < 0.001 | ||
| Absent | 6 (18.2) | 25 (75.8) | |
| Grade 1 | 20 (60.6) | 5 (15.2) | |
| Grade 2 | 4 (12.1) | 3 (9.1) | |
| Grade 3 | 3 (9.1) | 0 (0.0) | |
| Fibrosis | 0.033 | ||
| Absent | 26 (78.8) | 27 (81.8) | |
| Grade 1 | 3 (9.1) | 4 (12.1) | |
| Grade 2 | 0 (0.0) | 0 (0.0) | |
| Grade 3 | 4 (12.1) | 2 (6.1) |
| Variables | Pre | Post | P value |
| mean ± SD | mean ± SD | ||
| BMI in kg/m² | 45.2 ± 6.9 | 31.8 ± 5.4 | < 0.001 |
| SMM in kg | 31.9 ± 6.8 | 27.1 ± 5.3 | < 0.001 |
| BCM in kg | 37.0 ± 7.8 | 32.1 ± 5.9 | < 0.001 |
| Fat mass in kg | 60.7 ± 13.3 | 33.6 ± 11.1 | < 0.001 |
| % Fat | 51.6 ± 3.5 | 39.8 ± 8.2 | < 0.001 |
| Visceral fat area in cm² | 249.3 ± 23.1 | 162.5 ± 52.3 | < 0.001 |
| PA full body ° | 5.93 ± 0.65 | 4.91 ± 0.60 | < 0.001 |
| PA RA ° | 5.55 ± 0.77 | 4.61 ± 0.64 | < 0.001 |
| PA LA ° | 5.44 ± 0.86 | 4.44 ± 0.58 | < 0.001 |
| PA Tr ° | 7.84 ± 1.24 | 6.73 ± 1.03 | < 0.001 |
| PA RL ° | 6.42 ± 0.72 | 5.18 ± 0.68 | < 0.001 |
| PA LL ° | 6.38 ± 0.74 | 5.19 ± 0.66 | < 0.001 |
There was a positive, statistically significant association between BMI (kg/m²) and lobular inflammation (P < 0.05), NASH (P < 0.01), and fibrosis (P < 0.05). The other body composition variables did not correlate with the different histological characteristics of NAFLD.
There was an association between the variations of PA before and after bariatric surgery regarding the degree of lobular inflammation (rs = -0.593; P = < 0.001) but not steatosis (rs = 0.305; P = 0.095), ballooning (rs = 0.057; P = 0.760), NASH (rs = -0.197; P = 0.288), and fibrosis (rs = -0.183; P = 0.324).
Obese patients, candidates for bariatric surgery diagnosed with NAFLD, have been extensively studied[8,27-31]; and, to date, changes in lifestyle are the only effective forms of treatment for NAFLD. It was established that a loss of 7%-10% of body weight is necessary to present any change[32]. However, in order to guarantee body homeostasis, it is essential that the weight loss of these patients is the highest possible percentage of fat mass and not of SMM, preserving muscle volume and functionality. Therefore, BIA and PA are essential tools for monitoring the body characteristics and health of these patients[19,33].
Most of our patients were women, with a mean age of 39.1 ± 10.6 years and a BMI of 45.9 ± 7.5 kg/m², findings similar to those of Losekann et al[9], who in 2013 analyzed 250 patients with liver biopsies performed in the bariatric surgery trans operation, which 80% were women, with a mean age of 36.8 ± 10.2 years and a BMI of 43.6 ± 5.2 kg/m².
BMI is an analytical, non-laboratory method that is easy to apply and reproducible, allowing an indirect assessment of body composition, and is a defining parameter of indication for bariatric surgery.
The mean BMI after surgery decreased to 31.2 ± 5.0 kg/m² (P < 0.001), changing from Grade III Obesity to Grade I. The reduction in BMI was significant in both sexes, with no significant difference between them. All body composition parameters had a significant decrease (P < 0.001), mainly on fat mass and visceral fat area. In the present study, men compared to women showed a more marked and significant reduction in the percentage of fat and in the area of visceral fat.
Perrone et al[34] reported that the decrease in BMI after bariatric surgery in men was greater than that in women, without influencing the improvement of comorbidities in the long term, because the BMI does not differentiate or qualify weight loss, which should be mostly fat and not SMM. A study by Hartwig et al[35], showed similar results in men and women regarding the decrease in the percentage of fat and fat mass in the postoperative period. De Paris et al[36] showed a reduction in body composition (weight, fat-free mass, SMM, fat mass, and fat percentage) in the postoperative period of bariatric surgery, results similar to ours.
All of our patients had NAFLD; accentuated or massive steatosis in 30.6%; ballooning in 76%; lobular inflammation in 35.9%; NASH in 78.1%; fibrosis in 43%; and no case of cirrhosis. NAFLD patients are obese[37], with a prevalence of 51% of cases[38]. In patients with obesity undergoing bariatric surgery, the percentage of steatosis ranged from 87.6% to 100%[8,27-31], ballooning from 58.9% to 88%[29,30], lobular inflammation from 23% to 88%[29,31], and fibrosis from 31% to 44.9%[28,29,31], findings similar to ours.
Some NAFLD patients present progression from simple steatosis to advanced stages, such as NASH and fibrosis, increasing the risk of cirrhosis and hepatocellular carcinoma. In addition, it is believed that NAFLD is implicated in the pathogenesis of type 2 DM and cardiovascular diseases[32]. These facts are fundamental in the search for the reduction of obesity that bariatric surgery provides.
We found a direct association between BMI and lobular inflammation, NASH, and fibrosis (P < 0.05), which is why it is important to monitor evolution of this group of patients.
The assessment of body composition is limited in several clinical conditions; and, therefore, the use of BIA data has gained increasing attention[39]. PA can be used as a biomarker for lean mass and/or for reducing muscle mass[39-41], besides being recognized as a marker of malnutrition[40,41] and predictor of morbidity and mortality in several diseases[18,42].
The PA of healthy individuals can vary between 6º and 7º, according to Bosy-Westphal et al[43] and 6.96º according to Barbosa-Silva et al[44]. There is no reference value that classifies PA for patients with obesity who have NAFLD. In the studied population, the PA was 5.89 ± 0.62°, lower than the values described above, probably due to obesity.
In Table 4, we evaluated the associations of PA with the variables of body composition, and we observed in men a significant negative relation with decreased weight, fat mass, and percentage of fat (P < 0.01). In women, this relation of PA was with the decrease in the percentage of fat (P < 0.01). There was a significant positive relation with SMM and BCM (P < 0.05). PA decreased in most variables related to weight loss (P < 0.05). Baumgartner et al[45], in 1988, observed a statistically significant negative correlation of PA with the percentage of body fat, corroborating the findings of our study. A study by Peres et al[46] analyzed 66 patients over 18 years of age with NAFLD, chronic hepatitis, cirrhosis, and hepatocellular carcinoma who had a mean PA of 5.1°, values similar to those found in the present study.
The post-surgical evaluation showed that PA decreased significantly in all evaluated segments (P < 0.001), as shown in Table 5, and its correlation with BMI showed a significant difference (P = 0.018), as shown in Table 6. These are apparently paradoxical findings, based on knowledge that the highest PA means improvement and the lowest means clinical worsening. We must consider the time of the second assessment, within the first year, as directly related to the decrease. A new evaluation of PA, at a longer time point, already programmed, may show an increase in PA.
Norman et al[47] described that BMI is one of the biological factors that influences PA. Furthermore, Llames et al[33] found a reduction in PA in individuals with a BMI greater than 35 kg/m². Bosy-Westphal et al[43] showed that PA increased with an increase in BMI up to 30 kg/m² and that such physiological behavior can be explained as a reflection of the increase in the number of cells (adipocytes and myocytes), since the reactivity of BIA is dependent on the amount of cell membranes. The same study showed that with BMI above 40 kg/m², there was an inverse relation between PA and BMI. The explanation is that severe obese individuals have a loss of functionality in the cell membrane, which may contribute to the decrease in PA.
In 2017, Vassilev et al[48] in Germany, with 173 patients undergoing bariatric surgery (BPGYR), showed a correlation between PA and weight loss, between 6 and 12 mo postoperatively, and a significant reduction in lean mass with a reduction in PA, results similar to ours. Koehler et al[49], with 20 patients undergoing bariatric surgery (BPGYR), showed significant results in reducing PA, at an earlier time, from 3 to 6 mo.
In the present study, there was a negative association between the reduction of full-body PA and the percentage of weight loss (P < 0.001), as shown in Figure 1. There was a positive association between the reduction of full-body PA with the variation of SMM (P < 0.001), as shown in Figure 2; and there was a positive association between the reduction of PA of the whole body and the percentage variation of fat (P < 0.001), as shown in Figure 3. These same associations were observed in the other parameters (loss of fat mass; BCM; visceral fat area, BMI), all with significant value (P < 0.001).
In the Vassilev et al study[48], the higher the percentage of body fat in the postoperative period, the lower the PA, and the reduction in BCM occurred with a reduction in PA after 9 mo of surgery. Thus, it is clear that the percentage of fat, even after weight loss, continues to have a negative influence on PA. The decrease in PA in the postoperative period is linked to weight loss, which can be a confusing factor when relating the reduction in the percentage of fat and PA, since the present study also associated a reduction in BMI with a decrease in PA.
There was an association between the variations of PA before and after bariatric surgery regarding the degree of lobular inflammation (rs = -0.593; P = < 0.001) but not with steatosis (rs = 0.305; P = 0.095), ballooning (rs = 0.057; P = 0.760), NASH (rs = -0.197; P = 0.288), and fibrosis (rs = -0.183; P = 0.324).
Regarding the role of PA and NAFLD staging, Peres et al[46] found no difference in PA values in patients with different degrees and stages of liver disease; our findings did not show significance associating the decrease in PA with the variation in the degree of steatosis, ballooning, NASH, and fibrosis in the pre- and postoperative period, demonstrating that the improvement of liver disease after bariatric surgery is not related to the worsening of PA.
The comparison of liver histology before and after surgery (n = 33) showed a significant improvement in NAFLD, where 18.2% had no degree of liver disease (P = 0.031); all parameters analyzed showed a reduction in histological changes, from 75% to 90% (P < 0.05), as described in Table 7.
Máttar et al[24] analyzed patients undergoing different bariatric surgery techniques and observed significant improvement in steatosis (from 88% to 8%), lobular inflammation (from 23% to 2%), and fibrosis (31% to 13%). In addition, 37% no longer had lobular inflammation, and 20% had no fibrosis, in line with our study. Similar data were found in Cazzo et al[50] review in 2017 with patients undergoing bariatric surgery using different surgical techniques[27,30,51-53].
A pioneering study by Silverman et al[54] in 1995 observed an improvement in liver disease in the postoperative period of bariatric surgery with the same surgical technique we used, with a 71% reduction in steatosis; 19% absence of steatosis; 76.9% absence of fibrosis, and 7.6% reduction in fibrosis, corroborating with our findings. There was a marked change in body composition in this group (n = 33), in all parameters (P < 0.001), as well as a reduction in PA (P < 0.001), as shown in Table 8. These data reinforce that weight loss is fundamental in the treatment of NAFLD.
The PA decreased after bariatric surgery, with a direct correlation with weight loss and changes in body composition. Grade III obesity became Grade I obesity. The decrease in the PA after bariatric surgery did not correlate with the improvement of NAFLD, even with the marked improvement of NAFLD. The decrease in the PA after performing bariatric surgery correlated with the decrease in the BMI, loss of SMM, and decreases in body fat (in percentages and kilograms), BCM, and visceral fat area.
We believe that PA should increase with more time after bariatric surgery, since the change in the body composition of the operated patient will reflect an improvement in body mass distribution and, consequently, less inflammatory process. An important point is what form of protein supplementation should be used with these patients after surgery in order to minimize the loss of muscle mass and thereby increase PA. With the data presented in this study, we suggest that PA may be a marker of the state of body composition linked to the functionality of SMM.
Obesity is a complex chronic inflammatory disease characterized by excessive accumulation of body fat. In obese people, a common comorbidity is non-alcoholic fatty liver disease (NAFLD). NAFLD is considered the most common liver disease in Western countries, affecting 90% of morbidly obese patients eligible for bariatric surgery. To evaluate this patients, bioimpedance (BIA) can be a good method for nutritional and prognostic evaluation, using phase angle (PA).
There are not enough studies to evaluate morbid obesity, its body composition (described by the BIA), and associated comorbidities, such as NAFLD. We believe that the body change resulting from bariatric surgery will reflect an improvement in NAFLD and can be measured by PA, since it reflects cellular integrity and functionality by measuring, through an electrical current, the values of resistance and reactance of the membrane of these cells, with skeletal muscle being a conductor of electrical current and the opposite occurring with fat mass.
The aim of this study was to analyze the behavior of PA in the postoperative period of bariatric surgery, correlating it with changes in body composition and improvement of liver disease.
This was a retrospective cohort study that analyzed the medical records of 727 patients undergoing bariatric surgery in a referral center of a teaching hospital in the south of Brazil. For convenience, the sample was carried out from July 2015 to July 2017. The data obtained were related to the protocol for routine pre- and postoperative care at the service's outpatient clinic. Quantitative and categorical variables analyses were performed to assess the association between PA, NAFLD, and body composition before and after bariatric surgery.
We analyzed 727 patients’ medical records, and 379 patients were selected for having all preoperative data. Regarding PA, 169 patients were analyzed, and 33 patients had liver biopsy pre-and postoperatively with NAFLD information. The PA showed a significant reduction in the postoperative period as well as body composition data. Regarding liver disease, all patients presented a reduction in the degrees and stages of liver disease in the postoperative period, and some had no degree of liver disease.
The PA decreased after bariatric surgery, with a direct correlation with weight loss and changes in body composition, and it did not correlate with the improvement of NAFLD. With the data presented in this study, we suggest that PA may be a marker of the state of body composition linked to the functionality of skeletal muscle mass.
Performing large scale prospective studies with long-term follow-up are needed to verify if PA increases with more time after bariatric surgery, since the change in the body composition of the operated patient will reflect an improvement in body mass distribution and, consequently, less inflammatory process.
| 1. | Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica (ABESO). Diretrizes brasileiras de obesidade, 2019. Available from: https://abeso.org.br/wp-content/uploads/2019/12/Diretrizes-Download-Diretrizes-Brasileiras-de-Obesidade-2016.pdf. |
| 2. | de Moraes TS. Nutritional intervention in the treatment of patients obesity. Revista Brasileira de Obesidade, Nutrição e Emagrecimento 2007; 1: 38-47. Available from: http://www.rbone.com.br/index.php/rbone/article/view/27/25. |
| 3. | Prado WL, Lofrano MC, Oyama LM, Dâmaso AR. Obesity and inflammatory adipokines: Practical implications for exercise prescription. Rev Bras Med Esporte. 2009;15:378-383. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Conway B, Rene A. Obesity as a disease: no lightweight matter. Obes Rev. 2004;5:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 210] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Brasil. Ministério da Saúde. VIGITEL Brasil 2018 [cited 2019 Nov 5]. Available from: https://portalarquivos2.saude.gov.br/images/pdf/2019/julho/25/vigitel-brasil-2018.pdf. |
| 7. | European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3285] [Article Influence: 328.5] [Reference Citation Analysis (7)] |
| 8. | Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology. 2016;63:2032-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. | Losekann A, Weston AC, Carli LA, Espindola MB, Pioner SR, Coral GP. Nonalcoholic fatty liver disease in severe obese patients, subjected to bariatric surgery. Arq Gastroenterol. 2013;50:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2651] [Article Influence: 189.4] [Reference Citation Analysis (3)] |
| 11. | Tovo CV, Fernandes SA, Buss C, de Mattos AA. Sarcopenia and non-alcoholic fatty liver disease: Is there a relationship? World J Hepatol. 2017;9:326-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Losekann A, Weston AC, de Mattos AA, Tovo CV, de Carli LA, Espindola MB, Pioner SR, Coral GP. Non-Alcoholic Steatohepatitis (NASH): Risk Factors in Morbidly Obese Patients. Int J Mol Sci. 2015;16:25552-25559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1-190, 215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 665] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 14. | Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Bordalo LA, Teixeira TF, Bressan J, Mourão DM. [Bariatric surgery: how and why to supplement]. Rev Assoc Med Bras (1992). 2011;57:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Rezende F, Rosado L, Franceschinni S, Rosado G, Ribeiro R, Marins JC. [Critical revision of the available methods for evaluate the body composition in population-based and clinical studies]. Arch Latinoam Nutr. 2007;57:327-334. [PubMed] |
| 17. | Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Coppini LZ, Waitzberg DL, Campos AC. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr Opin Clin Nutr Metab Care. 2005;8:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Marroni CA, Miranda D, Boemeke L, Fernandes SA. Phase Angle Bioelectrical Impedance Analysis (BIA) as a Biomarker Tool for Liver Disease. In: Patel V, Preedy V. (eds) Biomarkers in Liver Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. 2017: 735-751. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 20. | Fernandes SA, de Mattos AA, Tovo CV, Marroni CA. Nutritional evaluation in cirrhosis: Emphasis on the phase angle. World J Hepatol. 2016;8:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Boemeke L, Raimundo FV, Bopp M, Leonhardt LR, Fernandes SA, Marroni CA. The correlation of neck circumference and insulin resistance in NAFLD patients. Arq Gastroenterol. 2019;56:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Faria SL, Faria OP, Cardeal MD, Ito MK. Validation study of multi-frequency bioelectrical impedance with dual-energy X-ray absorptiometry among obese patients. Obes Surg. 2014;24:1476-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Baumgartner RN, Heymsfield SB, Lichtman S, Wang J, Pierson RN Jr. Body composition in elderly people: effect of criterion estimates on predictive equations. Am J Clin Nutr. 1991;53:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 160] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Máttar JA. Application of total body bioimpedance to the critically ill patient. Brazilian Group for Bioimpedance Study. New Horiz. 1996;4:493-503. [PubMed] |
| 25. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, Lilienthal Heitmann B, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, M W J Schols A, Pichard C; ESPEN. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1517] [Article Influence: 72.2] [Reference Citation Analysis (1)] |
| 26. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8577] [Article Influence: 408.4] [Reference Citation Analysis (9)] |
| 27. | Mottin CC, Moretto M, Padoin AV, Kupski C, Swarowsky AM, Glock L, Duval V, da Silva JB. Histological behavior of hepatic steatosis in morbidly obese patients after weight loss induced by bariatric surgery. Obes Surg. 2005;15:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Mattar SG, Velcu LM, Rabinovitz M, Demetris AJ, Krasinskas AM, Barinas-Mitchell E, Eid GM, Ramanathan R, Taylor DS, Schauer PR. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610-617; discussion 618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Liu X, Lazenby AJ, Clements RH, Jhala N, Abrams GA. Resolution of nonalcoholic steatohepatits after gastric bypass surgery. Obes Surg. 2007;17:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Clark JM, Alkhuraishi AR, Solga SF, Alli P, Diehl AM, Magnuson TH. Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes Res. 2005;13:1180-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Moretto M, Kupski C, da Silva VD, Padoin AV, Mottin CC. Effect of bariatric surgery on liver fibrosis. Obes Surg. 2012;22:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 615] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 33. | Llames L, Baldomero V, Iglesias ML, Rodota LP. [Values of the phase angle by bioelectrical impedance; nutritional status and prognostic value]. Nutr Hosp. 2013;28:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 34. | Perrone F, Bianciardi E, Benavoli D, Tognoni V, Niolu C, Siracusano A, Gaspari AL, Gentileschi P. Gender Influence on Long-Term Weight Loss and Comorbidities After Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: a Prospective Study With a 5-Year Follow-up. Obes Surg. 2016;26:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Hartwig TW, dos Santos FAI, González MC, Rombaldi AJ. Bariatric surgery and body composition of adults. Rev Bras Cineantropom Desempenho Hum 2013; 15: 686-694. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | de Paris FGC, Padoin AV, Mottin CC, de Paris MF. Assessment of Changes in Body Composition During the First Postoperative Year After Bariatric Surgery. Obes Surg. 2019;29:3054-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263-8276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 543] [Cited by in RCA: 540] [Article Influence: 60.0] [Reference Citation Analysis (15)] |
| 38. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7941] [Article Influence: 794.1] [Reference Citation Analysis (8)] |
| 39. | Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle. 2017;8:187-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 40. | Basile C, Della-Morte D, Cacciatore F, Gargiulo G, Galizia G, Roselli M, Curcio F, Bonaduce D, Abete P. Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Exp Gerontol. 2014;58:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Kilic MK, Kizilarslanoglu MC, Arik G, Bolayir B, Kara O, Dogan Varan H, Sumer F, Kuyumcu ME, Halil M, Ulger Z. Association of Bioelectrical Impedance Analysis-Derived Phase Angle and Sarcopenia in Older Adults. Nutr Clin Pract. 2017;32:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Stobäus N, Pirlich M, Valentini L, Schulzke JD, Norman K. Determinants of bioelectrical phase angle in disease. Br J Nutr. 2012;107:1217-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Bosy-Westphal A, Danielzik S, Dörhöfer RP, Later W, Wiese S, Müller MJ. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr. 2006;30:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 419] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 44. | Barbosa-Silva MC, Barros AJ. Bioelectric impedance and individual characteristics as prognostic factors for post-operative complications. Clin Nutr. 2005;24:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am J Clin Nutr. 1988;48:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 300] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Peres WA, Lento DF, Baluz K, Ramalho A. Phase angle as a nutritional evaluation tool in all stages of chronic liver disease. Nutr Hosp. 2012;27:2072-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 47. | Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 755] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 48. | Vassilev G, Hasenberg T, Krammer J, Kienle P, Ronellenfitsch U, Otto M. The Phase Angle of the Bioelectrical Impedance Analysis as Predictor of Post-Bariatric Weight Loss Outcome. Obes Surg. 2017;27:665-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Koehler KB, Moraes RAG, Rodrigues JB, Portela BSM, Miguel GPS, Pedrosa RG, Haraguchi FK. Bioimpedance phase angle is associated with serum transthyretin but not with prognostic inflammatory and nutritional index during follow-up of women submitted to bariatric surgery. Clin Nutr ESPEN. 2019;33:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Cazzo E, Pareja JC, Chaim EA. Nonalcoholic fatty liver disease and bariatric surgery: a comprehensive review. Sao Paulo Med J. 2017;135:277-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Barker KB, Palekar NA, Bowers SP, Goldberg JE, Pulcini JP, Harrison SA. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2006;101:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Csendes A, Smok G, Burgos AM. Histological findings in the liver before and after gastric bypass. Obes Surg. 2006;16:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | de Almeida SR, Rocha PR, Sanches MD, Leite VH, da Silva RA, Diniz MT, Diniz Mde F, Rocha AL. Roux-en-Y gastric bypass improves the nonalcoholic steatohepatitis (NASH) of morbid obesity. Obes Surg. 2006;16:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Silverman EM, Sapala JA, Appelman HD. Regression of hepatic steatosis in morbidly obese persons after gastric bypass. Am J Clin Pathol. 1995;104:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sasaki A S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xing YX