Published online Oct 27, 2020. doi: 10.4254/wjh.v12.i10.841
Peer-review started: May 3, 2020
First decision: May 24, 2020
Revised: May 25, 2020
Accepted: September 15, 2020
Article in press: September 15, 2020
Published online: October 27, 2020
Processing time: 174 Days and 1.3 Hours
Hepatitis C virus (HCV) is a disease with a significant global impact, affecting approximately 2%-2.5% of the world’s population. New direct-acting antivirals (DAAs) have been introduced over the past few years with great success in viral eradication. The association of chronic HCV infection with a wide spectrum of cutaneous manifestations has been widely reported in the literature.
To assess the effect of treating HCV with DAAs on the extrahepatic cutaneous manifestations of HCV.
This prospective observational study included 1039 HCV positive Egyptian patients who were eligible to receive DAAs. A total of 30 patients were diagnosed with extrahepatic cutaneous manifestations and fulfilled the inclusion criteria of the study. Of these patients, 6 had classic lichen planus, 8 were diagnosed with psoriasis vulgaris and 16 had pruritus. All patients received DAAs from October 2018 to July 2019 in the form of a three-month course of sofosbuvir/daclatasvir combination. Patients with lichen planus or psoriasis were dermoscopically evaluated before treatment and 6 mo after treatment, while patients with hepatic pruritus were assessed using the 12-Item Pruritus Severity Scale over the same period.
All patients with psoriasis showed significant improvement in all psoriatic plaques, and all patients with hepatic pruritus scored 0 on the 12-Item Pruritus Severity Scale indicating total improvement of pruritus. In addition, four of six patients with lichen planus showed complete improvement.
Treatment of HCV with DAAs was significantly effective in improving virus-related extrahepatic cutaneous manifestations.
Core Tip: In this study, we investigated the impact of hepatitis C virus (HCV) clearance using direct-acting antivirals (DAAs) on the dermatological extrahepatic manifestations of HCV. To our knowledge, this is the largest cohort of patients with cutaneous manifestations of HCV to be treated in the literature (30 patients). In addition, we used dermoscopy for the first time in this study to better evaluate the response of cutaneous diseases to DAAs.
- Citation: El Kassas M, Hegazy OM, Salah EM. Effect of treating chronic hepatitis C with direct-acting antivirals on extrahepatic cutaneous manifestations. World J Hepatol 2020; 12(10): 841-849
- URL: https://www.wjgnet.com/1948-5182/full/v12/i10/841.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i10.841
Hepatitis C virus (HCV) is one of the human hepatotropic viruses that infects around 71 million individuals globally[1]. Spontaneous clearance of the virus fails in nearly half of cases and acute infection progresses to chronic hepatitis C, and further progression of the disease could lead to cirrhosis, and hepatocellular carcinoma (HCC)[2]. Egypt was considered a country with a high prevalence of HCV. After recognizing the magnitude of this problem, the Egyptian National Committee for Control of Viral Hepatitis (NCCVH), established specialized treatment centers for the evaluation and management of viral hepatitis[3-7]. Treatment of chronic HCV showed a significant shift after the introduction of direct-acting antivirals (DAAs), which proved a great success with an acceptable safety profile compared to the previous standard of care treatment (pegylated interferon and ribavirin)[8-10]. Many treatment regimens have been considered for the management of HCV, which is usually a combination of two or more DAAs classes[11-13].
Being a hepatotropic and lymphotropic virus, HCV does not only cause hepatic manifestations, it also leads to a significant number of extra-hepatic manifestations. Around 74% of patients with HCV will show HCV-related extrahepatic manifestations in their lifetime[14]. The development of HCV-related extrahepatic manifestations most likely involves autoimmune mechanisms. This theory is supported by the appearance of autoimmune features, such as palpable purpura, complex lymphoproliferative disorders (e.g., lymphomas), and immune complex deposit diseases that cause local and/or systemic complications[14,15]. Among the extrahepatic manifestations, dermatologic manifestations significantly add to the morbidity of the disease[16]. The association of chronic HCV infection with a broad spectrum of cutaneous manifestations has been widely reported in the literature, with varying strengths of epidemiological association. In registry-based studies, approximately 17% of HCV patients have at least one skin manifestation, which can be induced directly or indirectly by chronic HCV infection[17]. Dermatosis disorders are known to be linked to HCV infection including mixed cryoglobulinemia, lichen planus, porphyria cutanea tarda, and necrolytic acral erythema[18]. Besides these dermatoses, HCV can also be associated with autoimmune cutaneous diseases. Vitiligo is one of the autoimmune cutaneous diseases reported in studies to have a similar prevalence in patients with HCV infection and in the healthy population with vitiligo[19]. Psoriasis can also be found in association with HCV infection. This was confirmed by studies that found anti-HCV antibodies in psoriatic patients as well as HCV-RNA in the lesions of patients with psoriasis and HCV infection[20]. Pruritus is also a common dermatologic manifestation that is recognized as an early sign of chronic HCV infection[21,22]. This study aimed to assess the effect of treating HCV with DAAs on the extrahepatic cutaneous manifestations of HCV.
This prospective observational study included 1039 HCV patients, who were recruited from New Cairo Viral Hepatitis Treatment Center, one of the specialized treatment centers for HCV management, affiliated to the Egyptian NCCVH[7], from October 2018 to July 2019. These patients received HCV antiviral therapy with sofosbuvir/ daclatasvir (SOF/DAC) combination regimen for 12 wk and were followed-up until assessment of virological response 12 wk after treatment cessation. Patients were treated with DAAs according to the standardized protocol for HCV treatment issued by the NCCVH. The main exclusion criteria were: Child-Turcotte-Pugh (CTP) class C, hemoglobin level below 10 g/dL, platelet count less than 50000/mm3, diagnosed with HCC 6 mo following a successful intervention, extrahepatic malignancy after 2 years of cure, co-infection with hepatitis B or HIV, pregnancy or inability to use effective contraception, and hypersensitivity to any of the treatment medications[5]. In addition to the previous contraindication, we excluded patients who received dermatological treatment for their cutaneous manifestations, patients with renal failure, patients with autoimmune diseases, and those who received simeprevir as it can induce skin lesions as a complication[9].
A total of 30 patients were diagnosed with extrahepatic cutaneous manifestations and fulfilled the study inclusion criteria: Six patients were diagnosed with classic lichen planus, eight patients with psoriasis vulgaris, and sixteen patients had hepatic pruritus. A full medical history was obtained for these patients, followed by a clinical examination, and a complete liver biochemical profile. All subjects were tested for HCV viremia by polymerase chain reaction, and hepatitis B surface antigen was determined to exclude chronic HBV infection. Kidney function tests were also performed to exclude patients with renal failure. In addition an antinuclear antibody test was carried out to exclude patients with autoimmune hepatitis.
A dermatological assessment was performed by photographing skin lesions (in cases with lichen planus lesions and psoriatic plaques), before and after antiviral treatment. Dermoscopic photography of the lichen planus lesions and psoriatic plaques was performed before receiving and after completing treatment using a Dermlite DL4 3Gen. dermoscope. Patients with hepatic pruritus were evaluated before and after treatment using the 12-Item Pruritus, Severity Scale developed and validated by Reich and colleagues[23].
Data were collected, revised, coded, and entered into the SPSS version 26 software. Qualitative data are presented as numbers and percentages, while quantitative data are presented as mean, standard deviations and ranges. Comparisons between two groups with qualitative data were carried out using the Chi-square test was used instead of the Chi-square test when the expected count in any cell was found less than 5. Comparisons between two independent groups with quantitative data and parametric distribution was performed using the independent t-test. Comparisons between more than two groups with parametric distribution was performed using One Way Analysis of Variance. Pearson correlation coefficients were used to assess the relationship between two studied parameters in the same group. The receiver operating characteristic curve was used to assess the cut-off point with the best sensitivity, specificity, positive predictive value, and negative predictive value. The confidence interval was set to 95% and the margin of error accepted was set to 5%. Thus, the significance of the following P values was considered as follows: P > 0.05: Non significant, P < 0.05: Significant, and P < 0.01: Highly significant.
Thirty patients were included in the study. The mean age was 45.67 years and ranged from 24 to 60 years. Sixteen of the 30 recruited subjects were female. All patients received the SOF/DAC regimen, and all reached SVR12.
Cutaneous manifestations associated with chronic HCV infection were observed in all 30 recruited subjects and consisted of hepatic pruritus in 16 patients, lichen planus in 6 patients and psoriasis vulgaris in 8 patients. Age and sex distribution of the studied patients are shown in Table 1.
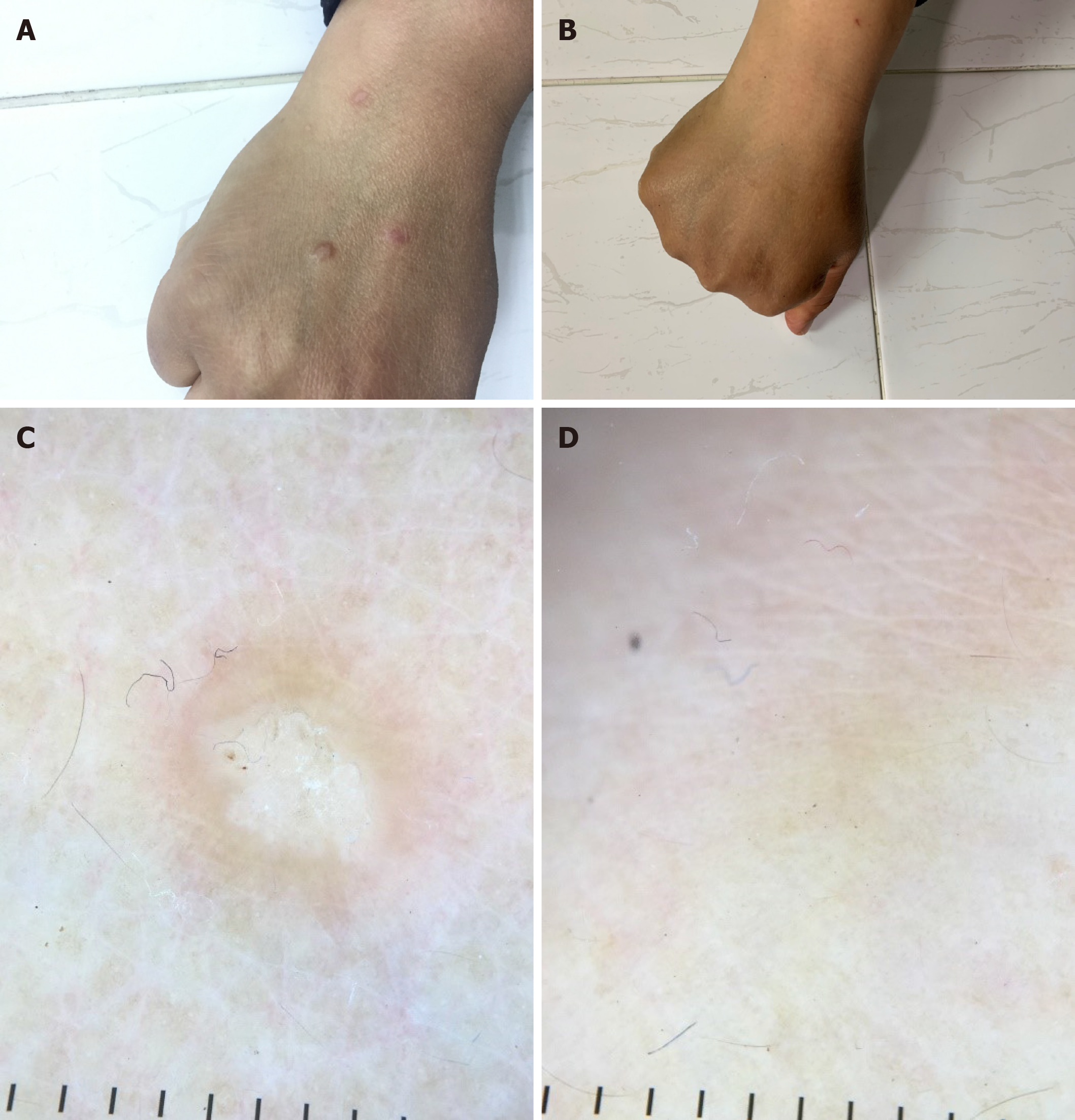
Four of six patients with lichen planus showed complete improvement of lesions following treatment, while no improvement was seen in the remaining two patients. The impact of HCV treatment on lichen planus is shown in Table 2. Figure 1 shows lichen planus papules on the hand of a patient before and after receiving DAAs, with dermoscopic images of lichen planus papules before and after treatment for HCV.
| Lichen planus | First visit, n (%) | Second visit, n (%) | Chi-square test | ||
| χ² | P value | Significance | |||
| Absent | 0 (0) | 4 (66.7) | 6.000 | 0.014 | S |
| Present | 6 (100) | 2 (33.3) | |||
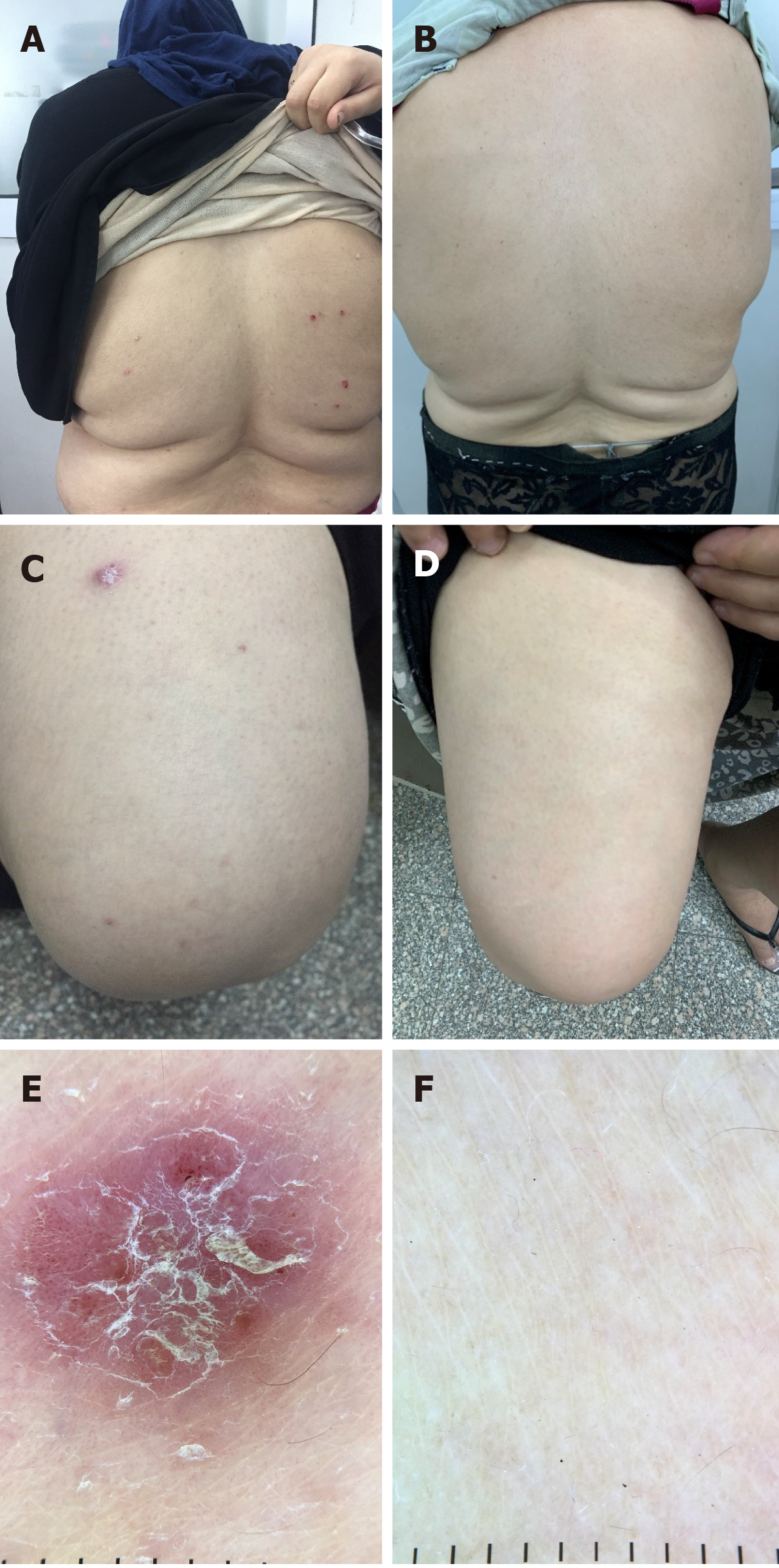
Eight patients in this study had psoriasis, and all patients showed complete improvement of psoriatic plaques 12 wk after finishing treatment. The impact of HCV treatment on psoriasis is shown in Table 3. Figure 2 shows psoriatic plaques on the back and thigh of a patient before and after receiving DAAs for HCV, in addition to dermoscopic images of psoriatic plaques before and after treatment.
| Psoriasis | First visit, n (%) | Second visit, n (%) | Chi-square test | ||
| χ² | P value | Significance | |||
| Absent | 0 (0) | 8 (100) | 16.000 | 0.000 | HS |
| Present | 8 (100) | 0 (0) | |||
Sixteen patients in this study complained of pruritus. All patients with pruritus showed complete improvement and reported total relief of pruritus according to the 12-Item Pruritus Severity Scale. According to this scale, the mean score before treatment was 9.94 ± 1.61, with a range of 7-12. Complete disappearance of pruritus was reported with a score of zero in all patients at the follow-up visit.
The association of chronic HCV infection with a wide spectrum of cutaneous manifestations has been widely reported in the literature, with varying strengths of epidemiological association. In registry-based studies, approximately 17% of HCV patients showed at least one skin manifestation, which was induced directly or indirectly by chronic HCV infection[17]. This study was an observational prospective hospital-based study, which included 30 HCV positive patients with associated extrahepatic cutaneous manifestations. All cutaneous lesions were assessed clinically and dermoscopically before receiving treatment and during the follow-up visit after six months of treatment. To our knowledge, this is the first study to assesses and follow-up HCV patients with extrahepatic cutaneous manifestations using a dermatoscope. Out of 1039 HCV patients who were referred to receive antiviral therapy, only those with dermatological manifestations were included in the study (30 patients). Of these 30 patients, six had cutaneous lichen planus, eight had psoriasis vulgaris, and sixteen had hepatic pruritus. Some dermatological disorders which are more frequent and are closely related to HCV such as porphyria cutanea tarda and necrolytic acral erythema were not observed. This could be explained by the pathognomonic nature of disorders such as HCV, and hence most of the patients with these disorders were diagnosed with HCV infection and treated early in the HCV treatment project which started in Egypt in 2015.
All patients with hepatic pruritus in this study reported complete resolution after finishing antiviral therapy. Although pruritus is often associated with HCV infection, the exclusion of all other causes of pruritus either dermatological or systemic, and its disappearance after viral clearance could confirm this association, and raise the suspicion of pruritus as an extrahepatic manifestation of HCV infection.
All patients with psoriasis showed total resolution of all psoriatic plaques. The results obtained by Enomoto et al[24], were concordant with our results, as they reported a male patient with a nine-year history of refractory psoriasis, with a Psoriasis Area and Severity Index (PASI) score of 3.8. The patient's symptoms and signs gradually resolved after a 12-wk course of oral, fixed-dose ledipasvir–sofosbuvir. The same results were also confirmed in a report from Egypt, where the authors described an 18-year-old male with psoriasis who received sofosbuvir and ribavirin and showed a sustained virologic response and the disappearance of skin lesions without the use of topical or systemic treatments for psoriasis six months after the end of treatment[25]. This was also in agreement with a published report of a patient with refractory psoriasis. The patient with HCV infection was treated with daclatasvir and asunaprevir combination, and his PASI score decreased from 3.4 to 0[26].
Four of the six patients diagnosed with lichen planus in the present study had a complete cure, while the remaining two patients showed no improvement in their lesions. Ansari et al[27] in 2017, supported these study findings in a 55-year-old HCV positive male patient with associated lichen planus. The patient received HCV treatment with ledipasvir-sofosbuvir, which led to a marked improvement in cutaneous lesions.
The main limitation in our study was the small number of recruited patients (30 subjects), who were enrolled after screening 1039 HCV patients over a 7-mo period. This relatively small number could be explained by the fact that most cases with HCV-related dermatological manifestations were discovered and treated at earlier stages of the HCV treatment project in Egypt. As this was a pilot study, it was the first time that a dermoscope was used to evaluate the response of extrahepatic cutaneous manifestations to HCV treatment with DAAs. Further studies with a larger number of patients and more diverse dermatological lesions are warranted to confirm our findings.
In conclusion, treatment of HCV infection with DAAs was effective in all patients with hepatic pruritus and psoriasis, and in most patients with lichen planus. These findings confirm the association between HCV infection and the extrahepatic dermatological manifestations of this virus. Based on our results, treatment of HCV infection in patients with extrahepatic dermatological manifestations is highly recommended.
Hepatitis C virus (HCV) is a disease with a significant global impact, affecting approximately 2%-2.5% of the world’s population. New direct-acting antivirals (DAAs) have been introduced over the past few years leading to successful viral eradication.
The association of chronic HCV infection with a wide spectrum of cutaneous manifestations has been widely reported in the literature.
This study aimed to assess the effect of treating HCV with DAAs on the extrahepatic cutaneous manifestations of HCV.
A prospective observational study included HCV positive Egyptian patients who were eligible to receive DAAs. Patients with lichen planus or psoriasis were dermoscopically evaluated before treatment and 6 mo after treatment, while patients with hepatic pruritus were assessed using the 12-Item Pruritus Severity Scale over the same period. All patients received DAAs from October 2018 to July 2019 in the form of a three-month course of sofosbuvir/daclatasvir combination.
A total of 30 from 1039 patients eligible for antiviral treatment were diagnosed with extrahepatic cutaneous manifestations and fulfilled the inclusion criteria of this study. Of these 30 patients, 6 patients had classic lichen planus, 8 patients had psoriasis vulgaris and 16 had hepatic pruritus. All patients with psoriasis showed significant improvement of all psoriatic plaques, and all patients with hepatic pruritus scored 0 on the 12-Item Pruritus Severity Scale indicating total improvement of pruritus. In addition, four of six patients with lichen planus showed complete improvement.
Treatment of HCV with DAAs was effective in improving HCV-related extrahepatic cutaneous manifestations.
Further studies with a larger number of patients and more diverse dermatological lesions are warranted to confirm our findings.
| 1. | Lazarus JV, Roel E, Elsharkawy AM. Hepatitis C Virus Epidemiology and the Impact of Interferon-Free Hepatitis C Virus Therapy. Cold Spring Harb Perspect Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Bostan N, Mahmood T. An overview about hepatitis C: a devastating virus. Crit Rev Microbiol. 2010;36:91-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 3. | El Kassas M, Elbaz T, Elsharkawy A, Omar H, Esmat G. HCV in Egypt, prevention, treatment and key barriers to elimination. Expert Rev Anti Infect Ther. 2018;16:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Omran D, Alboraie M, Zayed RA, Wifi MN, Naguib M, Eltabbakh M, Abdellah M, Sherief AF, Maklad S, Eldemellawy HH, Saad OK, Khamiss DM, El Kassas M. Towards hepatitis C virus elimination: Egyptian experience, achievements and limitations. World J Gastroenterol. 2018;24:4330-4340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 5. | El-Akel W, El-Sayed MH, El Kassas M, El-Serafy M, Khairy M, Elsaeed K, Kabil K, Hassany M, Shawky A, Yosry A, Shaker MK, ElShazly Y, Waked I, Esmat G, Doss W. National treatment programme of hepatitis C in Egypt: Hepatitis C virus model of care. J Viral Hepat. 2017;24:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 6. | Gomaa A, Allam N, Elsharkawy A, El Kassas M, Waked I. Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med. 2017;9:17-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 7. | El Kassas M, Alboraie M, Omran D, Salaheldin M, Wifi MN, ElBadry M, El Tahan A, Ezzat S, Moaz E, Farid AM, Omar H, Abouelkhair M, Afify S, Elsaeed K, Shazly Y, Doss W, Esmat G. An account of the real-life hepatitis C management in a single specialized viral hepatitis treatment centre in Egypt: results of treating 7042 patients with 7 different direct acting antiviral regimens. Expert Rev Gastroenterol Hepatol. 2018;12:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Elbaz T, El-Kassas M, Esmat G. New era for management of chronic hepatitis C virus using direct antiviral agents: A review. J Adv Res. 2015;6:301-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | El Kassas M, Elbaz T, Hafez E, Esmat G. Safety of direct antiviral agents in the management of hepatitis C. Expert Opin Drug Saf. 2016;15:1643-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Esmat G, El Kassas M, Hassany M, Gamil M, El Raziky M. Optimizing treatment for HCV genotype 4: PEG-IFN alfa 2a vs. Liver Int. 2014;34 Suppl 1:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Omar H, El Akel W, Elbaz T, El Kassas M, Elsaeed K, El Shazly H, Said M, Yousif M, Gomaa AA, Nasr A, AbdAllah M, Korany M, Ismail SA, Shaker MK, Doss W, Esmat G, Waked I, El Shazly Y. Generic daclatasvir plus sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt. Aliment Pharmacol Ther. 2018;47:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Eletreby R, Elakel W, Said M, El Kassas M, Seif S, Elbaz T, El Raziky M, Abdel Rehim S, Zaky S, Fouad R, Gamal Eldeen H, Abdo M, Korany M, Yosry A, El Serafy M, El-Sayed MH, ElShazly Y, Waked I, Doss W, Esmat G. Real life Egyptian experience of efficacy and safety of Simeprevir/Sofosbuvir therapy in 6211 chronic HCV genotype IV infected patients. Liver Int. 2017;37:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | El Kassas M, Alboraie M, Omar H, El Latif YA, Algaber MA, El Tahan A, El Halwagy H, Afify S, Elserafy M, Elsaeed K, Doss W. High success rates for the use of ombitasvir/paritaprevir/ritonavir containing regimens in treatment of naïve and experienced chronic hepatitis C genotype 4: Real world results. J Med Virol. 2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB; Italian Association of the Study of Liver Commission on Extrahepatic Manifestations of HCV infection. Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Ramos-Casals M, Font J. Extrahepatic manifestations in patients with chronic hepatitis C virus infection. Curr Opin Rheumatol. 2005;17:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Younossi ZM, Stepanova M, Mishra A, Venkatesan C, Henry L, Hunt S. The impact of chronic hepatitis C on resource utilisation and in-patient mortality for Medicare beneficiaries between 2005 and 2010. Aliment Pharmacol Ther. 2013;38:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Garcovich S, Garcovich M, Capizzi R, Gasbarrini A, Zocco MA. Cutaneous manifestations of hepatitis C in the era of new antiviral agents. World J Hepatol. 2015;7:2740-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Wiznia LE, Laird ME, Franks AG Jr. Hepatitis C virus and its cutaneous manifestations: treatment in the direct-acting antiviral era. J Eur Acad Dermatol Venereol. 2017;31:1260-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Jadali Z, Eslami M, Sanati M, Mansouri P, Mahmoudi M, Maghsoudi N, Esfahanian F. Hepatitis C virus antibodies and vitiligo disease. Iran J Publ Health. 2005;34:23-26. |
| 20. | Jadali Z, Alavian SM. Autoimmune diseases co-existing with hepatitis C virus infection. Iran J Allergy Asthma Immunol. 2010;9:191-206. [PubMed] |
| 21. | Halawani M. Screening of hepatitis C virus genotypes in urticaria patients in Saudi Arabia. Genet Test Mol Biomarkers. 2012;16:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Cordel N, Chosidow O, Francès C. Cutaneous disorders associated with hepatitis C virus infection. Ann Med Interne (Paris). 2000;151:46-52. [PubMed] |
| 23. | Reich A, Bożek A, Janiszewska K, Szepietowski JC. 12-Item Pruritus Severity Scale: Development and Validation of New Itch Severity Questionnaire. Biomed Res Int. 2017;2017:3896423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Enomoto M, Tateishi C, Tsuruta D, Tamori A, Kawada N. Remission of Psoriasis After Treatment of Chronic Hepatitis C Virus Infection With Direct-Acting Antivirals. Ann Intern Med. 2018;168:678-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Elfert AA. Sustained improvement of psoriasis associated with HCV after virologic response to sofosbuvir/ribavirin. Arab J Gastroenterol. 2017;18:234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kikuchi S, Umezawa Y, Chihara M, Asahina A, Nakagawa H. Case of psoriatic patient who maintains long-term remission after anti-hepatitis C virus agents and ustekinumab treatment. J Dermatol. 2018;45:e59-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Ansari U, Henderson LI, Stott G, Parr K. Treatment with ledipasvir-sofosbuvir for hepatitis C resulting in improvement of lichen planus. JAAD Case Rep. 2017;3:67-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Egyptian Association for Research and Training in Hepatogastroenterology, No. 01.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu XY S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Wang LL