Published online Jul 27, 2018. doi: 10.4254/wjh.v10.i7.523
Peer-review started: February 19, 2018
First decision: March 2, 2018
Revised: March 27, 2018
Accepted: May 30, 2018
Article in press: May 30, 2018
Published online: July 27, 2018
Processing time: 158 Days and 7 Hours
Right-sided ligamentum teres (RSLT) is a congenital anomaly in which the right umbilical ligament becomes dominant and anomalous ramifications of the hepatic vessels and biliary system are present. A male patient in his 70s was diagnosed with advanced gallbladder cancer directly infiltrating the right hepatic duct (RHD), together with RSLT. Preoperative three-dimensional simulation of the liver based on multiple detector computed tomography images after cholangiography revealed ramifications of all segmental portal veins from the portal trunk and discordance of the arterial and biliary branching patterns of segment 8. Fusion analysis of the biliary architecture and segmental volumetry showed that the RHD drained segments 1r, 5, 6, and 7. We successfully performed a modified right-sided hepatectomy sparing segment 8 (i.e., resection of the RHD drainage territory), with negative surgical margins. This report is the first to describe major hepatectomy for advanced gallbladder cancer with RSLT.
Core tip: Right-sided ligamentum teres (RSLT) is a congenital anomaly that involves anomalous ramifications of the hepatic vessels and biliary system. A male in his 70s with RSLT was diagnosed with advanced gallbladder cancer directly infiltrating the right hepatic duct (RHD). Based on preoperative liver simulation, we successfully performed a modified right-sided hepatectomy sparing segment 8, which corresponded to resection of the RHD drainage territory (segments 1r, S5, S6, and S7), with negative surgical margins. This report is the first to describe major hepatectomy for advanced gallbladder cancer with RSLT.
- Citation: Goto T, Terajima H, Yamamoto T, Uchida Y. Hepatectomy for gallbladder-cancer with unclassified anomaly of right-sided ligamentum teres: A case report and review of the literature. World J Hepatol 2018; 10(7): 523-529
- URL: https://www.wjgnet.com/1948-5182/full/v10/i7/523.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i7.523
Right-sided ligamentum teres (RSLT) is a rare congenital anomaly in which the umbilical ligament on the left side atrophies and the right ligament becomes dominant at the 7-mm embryo stage[1]. The incidence of RSLT has been reported to be 0.2%-1.2% in adults[1,2]. RSLT is associated with anomalous ramifications in the arterial, portal vein, and biliary systems; therefore, precise evaluation of the branching patterns of the vascular and biliary architecture is required to avoid injuring the hepatic vasculature of the remnant liver[3-5]. Recent papers have demonstrated the significance of three-dimensional (3D) liver simulation before hepatectomy in RSLT patients to determine surgical strategies, such as the hepatectomy procedure and the cutting plane[6-8]. For gallbladder cancer, macroscopically complete surgical resection with negative microscopic margins remains the only potentially curative treatment, and major hepatectomy, such as extended right hepatectomy, is usually required for locally advanced gallbladder cancer[9]. This case report is the first to describe major hepatectomy for advanced gallbladder cancer concomitant with RSLT.
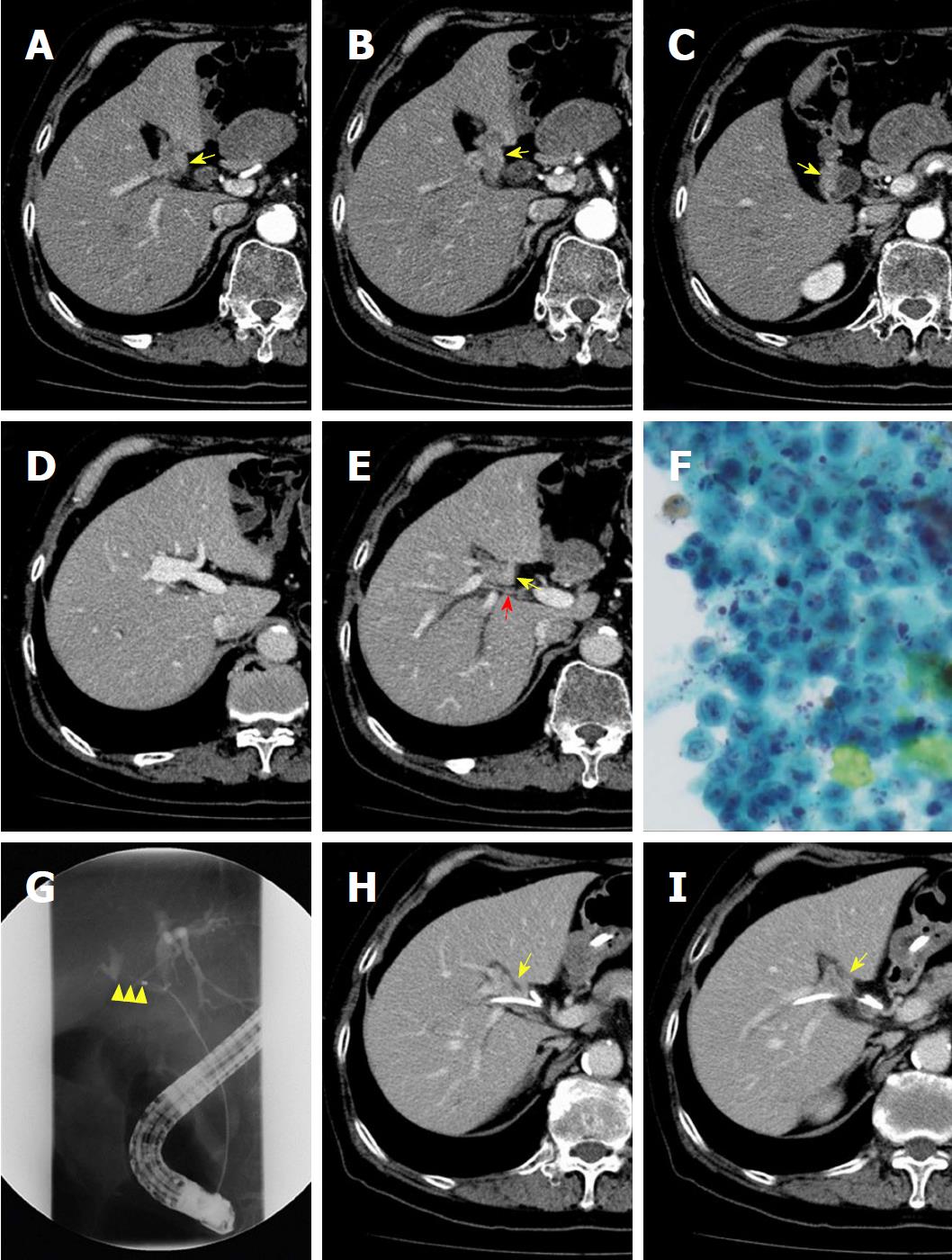
A male patient in his 70s was incidentally diagnosed with a gallbladder tumor by computed tomography (CT) during a preoperative examination of an inguinal hernia. He had no appreciable disease and had a good exercise tolerance. Laboratory tests showed normal levels of carcinoembryonic antigen and CA19-9. Hyperbilirubinemia was not observed. The indocyanine green (ICG) retention rate at 15 min was 3.6%; the normal range is less than 10%[10]. The patient’s Child-Turcotte-Pugh score was 5 points, Grade A[11]. An abdominal contrast-enhanced CT scan showed an atrophied gallbladder with tumor-like localized wall thickness enhanced by contrast medium, strongly suggesting the possibility of gallbladder cancer. The gallbladder was attached to the left side of the liver and complicated variations of the intrahepatic vascular architecture were detected, suggesting RSLT (Figure 1A-E). Endoscopic retrograde gallbladder drainage (ERGBD) was performed for cytological examination of bile in the gallbladder and adenocarcinoma was detected two consecutive times (Figure 1F). To prevent obstructive jaundice and to evaluate horizontal extension of the tumor along the bile duct, ERGBD was switched to endoscopic nasobiliary drainage (ENBD) one week later. Two ENBD tubes were placed both in the right hepatic duct (RHD) and the left hepatic duct (LHD). ENBD tube cholangiography demonstrated a severe stenotic change of the RHD, and the confluence of the LHD and the common bile duct was intact (Figure 1G-I). No metastases of lymph nodes and distant organs were detected by CT scanning. Based on these findings, the patient was diagnosed with advanced gallbladder cancer with direct infiltration of the RHD rather than hilar cholangiocarcinoma. The gallbladder carcinoma was classified as cStage IIIA (T3; right bile duct, N0, M0) according to the Union for International Cancer Control system[12]. Considering the patient’s eligibility for radical surgery, we finally determined that right-sided major hepatectomy with resection of the RHD drainage territory was required.
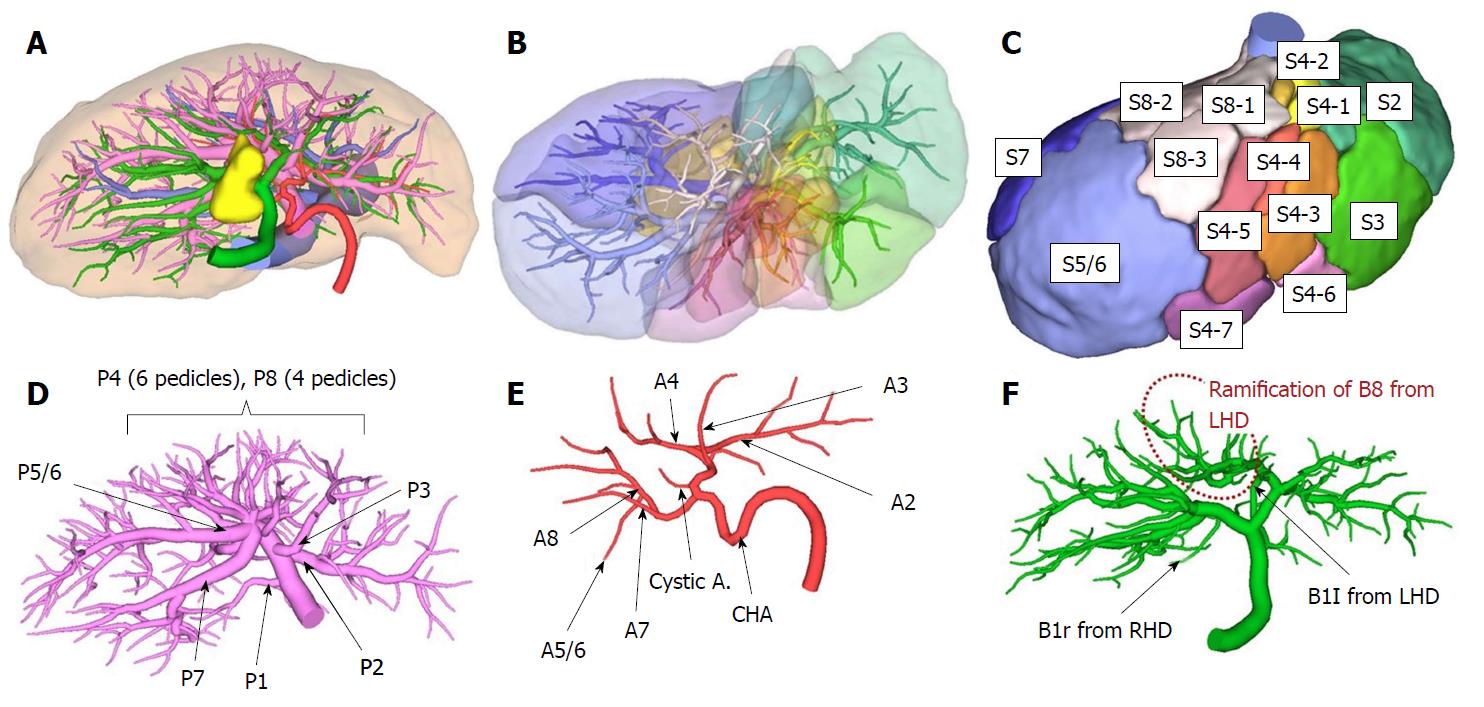
Preoperative simulation was performed to plan the hepatectomy using multiple detector CT (MDCT) with a slice thickness of 1 mm after contrast cholangiography through an ENBD tube. After a quarto-phase (non-contrast, arterial, portal venous, and hepatic venous phases) contrast study was performed, two-dimensional CT images were transferred to a workstation (SYNAPSE VINCENT: FUJIFILM Medical Co., Ltd., Tokyo, Japan), and 3D images were automatically constructed for some areas and manually constructed for other areas. The intrahepatic vasculature was reconstructed at the 4th order division level (Figure 2A) and was verified by 4 liver surgeons independently. We labeled each sub-segment following the Couinaud classification[13] and the Brisbane 2000 terminology of Liver Anatomy and Resections[14] to define the anatomical divisions of the liver (Figure 2B and C). All segmental portal branches were separately ramified from the portal trunk (Figure 2D). No arterial anomalies were detected (Figure 2E). Bile duct analysis demonstrated that the bile duct of segment 8 (B8) was ramified from the LHD, and the RHD drained segments 5, 6, and 7. The bile duct of the right side of segment 1 (B1r) was ramified from the RHD, and the bile duct of the left side (B1l) was ramified from the LHD (Figure 2F).
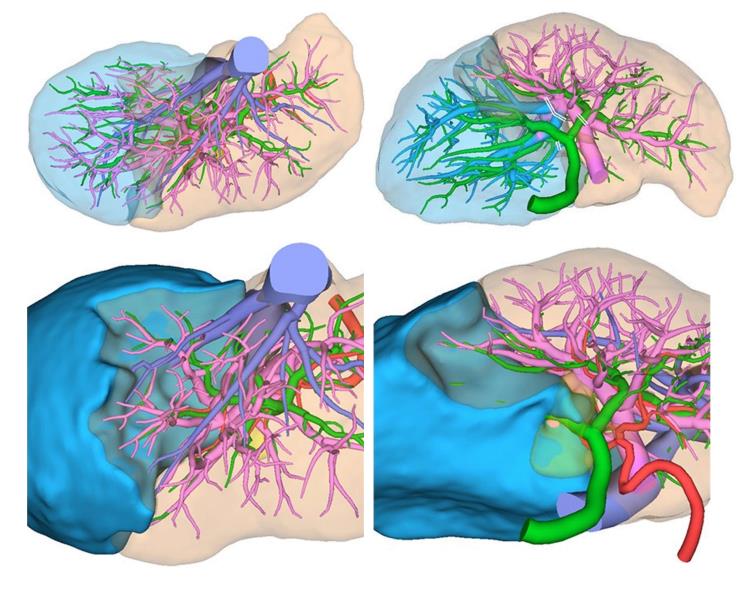
Based on this simulation, resection of the gallbladder bed and the RHD drainage territory (segments 1r, 5, 6, and 7) was planned (Figure 3). The future liver remnant (FLR) volume was 605 mL. The plasma clearance rate of ICG in the FLR (calculated as the plasma clearance rate of ICG × the proportion of the FLR) was 0.131; 0.05 is the cut-off value for predicting mortality and morbidity[15]. The functional count rate of the FLR according to technetium-99m diethylenetriamine pentaacetic acid galactosyl human serum albumin scintigraphy was 50.2%[16]. The hepatic artery of segment 8 (A8) branched from the right hepatic artery, while B8 branched from the LHD.
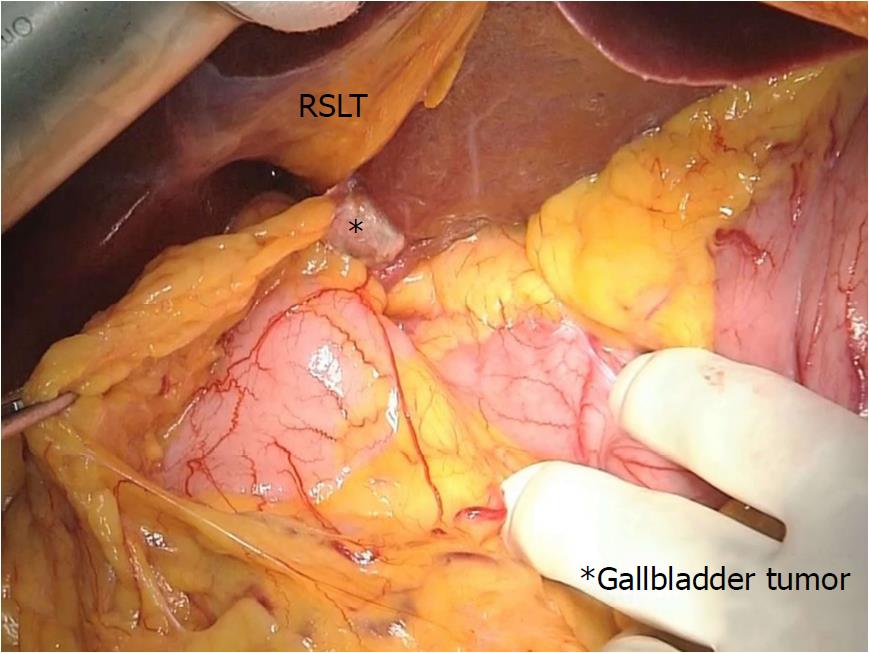
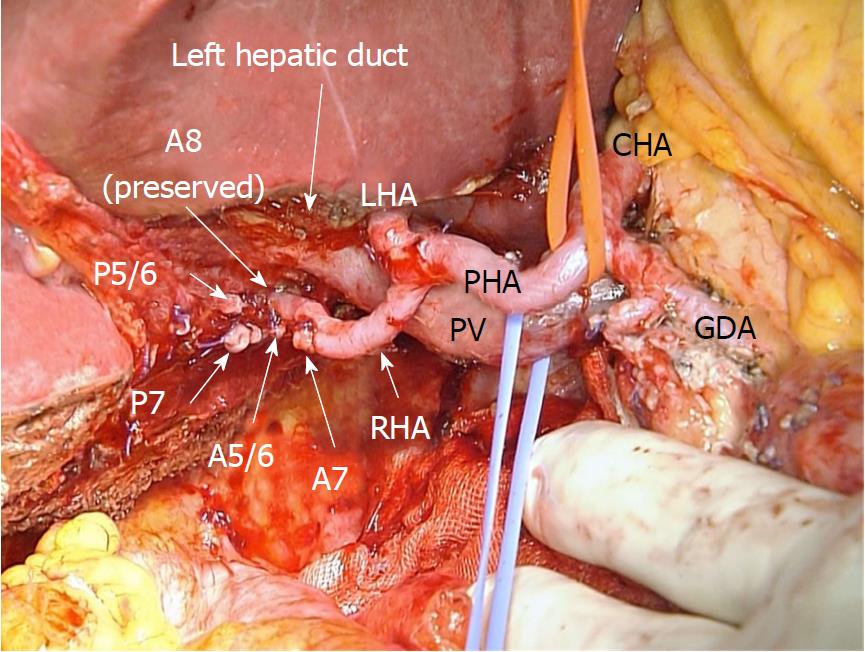
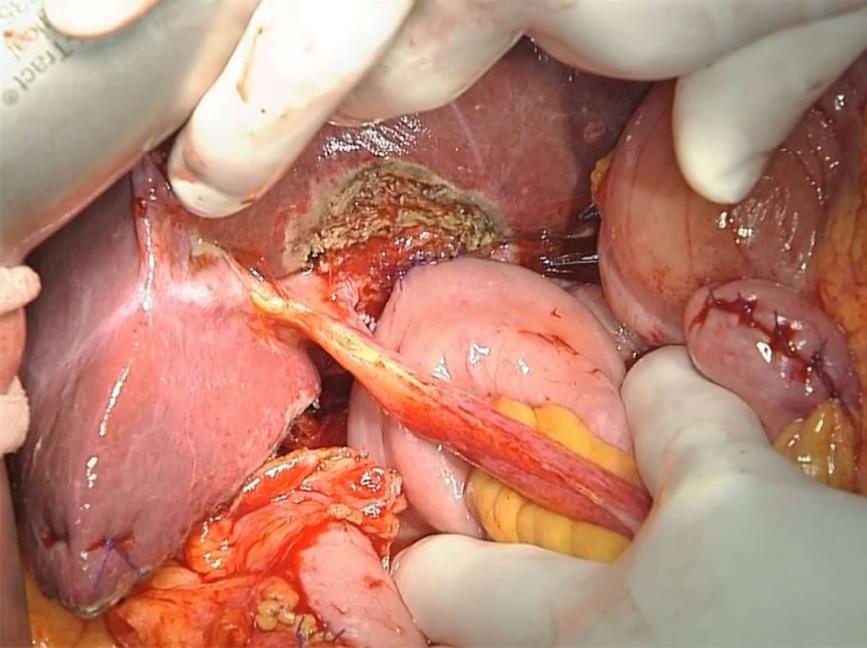
During laparotomy, the gallbladder was located on the left side of the RSLT (Figure 4). After mobilization of the right lobe, lymphadenectomy of the hepatoduodenal ligament was performed. The common bile duct and LHD were divided, resulting in pathologically negative surgical margins, followed by parenchymal dissection of the gallbladder bed. All portal and arterial branches of segments 5 to 8 were extrahepatically separated and taped. Each pedicle was temporarily clamped, and the intrahepatic blood flow was simultaneously monitored via ultrasonography. After dividing all inflow vessels of segments 5 to 7, the demarcation line was detected as preoperatively simulated (Figure 5). The liver parenchyma was transected using a Cavitron ultrasonic surgical aspirator and saline-coupled bipolar electrocautery[17], with an intermittent Pringle maneuver. After dividing the right hepatic vein, the specimen was excised (Figure 6). Hepaticojejunostomy to the LHD and jejunojejunostomy were conducted, and the operation was successfully completed as planned (Figure 7). The surgical time was 682 min, and the estimated blood loss was 430 g.
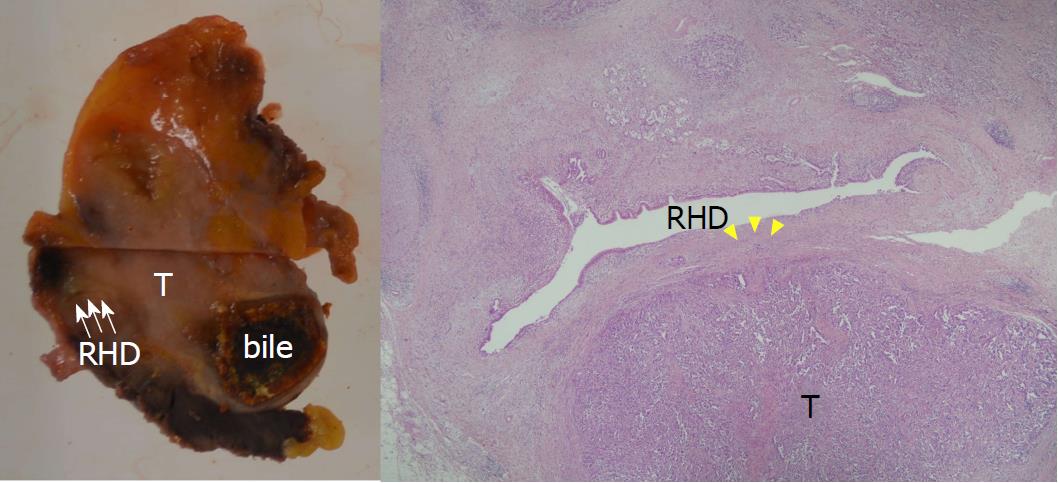
Pathological examinations demonstrated well-differentiated tubular adenocarcinoma of the gallbladder with direct invasion of the liver parenchyma and the RHD (Figure 8). The tumor cell invaded to the RHD wall, but not into the lumen. Surgical margins were pathologically negative, and the tumor was classified as pStage III A (T3; liver bed and right bile duct, N0, M0). Postoperative contrast-enhanced CT showed no ischemic regions in the remnant liver, including the spared segment 8. The patient was discharged 8 d after surgery without any complications more severe than a Grade II Clavien-Dindo classification[18]. Five months after surgery, solitary liver metastasis in segment 2, far from the surgical stump, was detected during adjuvant chemotherapy with gemcitabine. The liver metastasis disappeared on imaging analysis after 2 cycles of chemotherapy with gemcitabine plus cisplatin (GC). The patient underwent ileocecal resection for primary ascending colon cancer 7 months after surgery; thereafter, an additional 13 cycles of GC therapy were continued. Multiple mediastinal lymph node metastases were identified 18 mo after surgery, and second-line chemotherapy with S-1 was introduced. The patient has currently survived 26 months after hepatectomy.
This report is the first to describe major hepatectomy for curative resection of advanced gallbladder cancer with RSLT. Preoperative simulation enabled visualization of the complicated anatomical findings of the intrahepatic vascular and biliary architecture and provided valuable assistance in performing resection of the RHD drainage territory without injuring the hepatic vasculature of the remnant liver.
The anomalies associated with RSLT may lead to several surgical problems during hepatectomy. In the most common cases (e.g., an independent ramification of the right lateral portal pedicle), a surgeon’s misunderstanding and ligation of the left portal trunk result in a lack of portal flow in two-thirds of the whole liver during left hepatectomy[3]. Therefore, awareness of the type of anomaly is important before planning hepatectomy for patients with RSLT[1,3,4]. In 1997, Nagai et al[1] reported two types of portal vein classifications of RSLT, and later, Shindoh et al[3] and Nishitai et al[4] demonstrated the classification of arterial, portal, and biliary ramifications. Portal ramification patterns were classified into three types: Independent right lateral, bifurcation, and trifurcation. Our case may be defined as an unclassified type because four sectional portal branches did not exist, and all subsegmental branches were directly ramified from the portal trunk. In the bifurcation type, P4 runs from the right paramedian branch to the left paramedian area beyond the middle hepatic vein (MHV). However, our case showed that P4 was ramified from the main portal trunk and did not run beyond the MHV. Therefore, performing a conventional anatomical hepatectomy was difficult, and each subsegmental portal vein branch from the portal trunk had to be identified and divided. Hepatic arterial ramification patterns are classified into 3 types: Independent ramification of the left hepatic artery, common trunk formation between the left hepatic artery and the ventral branch of the right paramedian artery, and replaced left hepatic artery from the left gastric artery. Our case is classified as the first type. Intrahepatic bile duct confluence patterns are classified into 4 types: Symmetrical, independent right lateral, total left, and total right. Our case corresponds to the independent right lateral type, in which the LHD drains the right paramedian section.
No specific relationship has been reported among the patterns of the biliary, portal, and arterial ramifications in RSLT livers[4]. Embryologically, the portal ramifications induce restructuring of the ductal plate that surrounds them in the future intrahepatic ducts, which results in a periportal position of the intrahepatic bile ducts[19]. In principle, the formation of hepatic arteries follows the portal venous tributaries, and dissociations between the portal venous system and bile duct system have been reported[20]. Therefore, an uncorrelated pattern of portal vein and bile tree ramifications is possible. In our case, however, the arterial ramification pattern differed from the biliary confluence pattern; A8 was ramified from the right hepatic artery, and B8 entered into the LHD. This report is the first to describe an artery and bile duct that were not parallel. If the vascular and biliary anatomies of the expected remnant liver in major hepatectomy with hilar lymphadenectomy for hepatobiliary malignancies are extremely complicated, as in this case, hepatic resection with the Glissonian approach[6] is difficult to perform. Step-by-step extrahepatic division of all vasculature through hilar dissection based on precise preoperative simulation is the safest and most secure surgical strategy, as described in this paper.
Anomalies of the intrahepatic portal vein are not rare[2,21]. However, in our case, all subsegmental portal veins were ramified from the portal trunk extrahepatically. Therefore, we could intentionally encircle all portal branches in accordance with the detailed preoperative simulation. Although conventional right hepatectomy was also planned as an alternative, the infiltration of the tumor to the right hepatic artery was not evident from the intraoperative findings; therefore, modified right-sided hepatectomy was possible, as simulated.
Liver simulation, particularly 3D modeling and virtual planning, offers a new approach to liver resection, as reported by several groups[22-24]. Liver simulation has led to significantly shorter surgical times, and the predicted liver volume to be resected appears to correlate well with the actual resected liver weight[23]. Preoperative simulation has become a common practice in Japan because simulation software has been developed and has become commercially available, and the cost of preoperative liver simulation has been covered by national health care insurance since 2012[25]. The fusion image of the portal vein and bile duct is useful for hepatectomy with RSLT because both the symmetrical type and the total left type of bile duct confluence patterns are similar to the ramification pattern of the normal liver, and cholangiography alone may provide misleading information for the operation[4]. The fusion image from one examination modality is referred to as an “all-in-one” (bile ducts, arteries, and portal veins) 3D image, in which MDCT is performed after cholangiography using carbon dioxide or iodine[24]. We strongly recommend structuring “all-in-one” 3D simulation images when planning hepatectomy with RSLT to evaluate the horizontal and perpendicular spreading of a tumor and its influence on the surrounding intrahepatic vasculature.
A systematic review of preoperative liver simulation and navigation has demonstrated the characteristics of the ideal simulation and navigation tool[26]. Simulation can accurately predict the liver volume to be resected, even by complicated anatomical resection procedures, and permits stereotactic measurement of the length of the surgical margin, which could not be estimated previously[27]. In this case, the most useful contribution of liver simulation was the combination of volumetry with the 3D structure of the intrahepatic vasculature. Using volumetry, we could not only calculate the cubic content but also visualize the parenchymal shape that each subsegmental portal branch dominated. By comparing each subsegmental area with the 3D structure of the right-sided biliary trees, we could exactly determine the hepatic territory to be resected and divide the portal pedicles in the proper sequence, as simulated. Simulation also enabled us to perform a parenchyma-preserving hepatectomy, which may contribute to safe and curative resection in selected cases with liver neoplasm in the right anterior resection region[28]. Takamoto et al[29] demonstrated accurate, completed drawings and surgical strategies for malignant liver tumors to execute anatomic segmentectomy and subsegmentectomy using 3D simulation. We also successfully performed parenchyma-preserving anatomical resection at the 3rd order division level for oncologically curative resection based on a precise preoperative analysis. The current 3D simulation system cannot be used as a real-time navigation tool during surgery[29], and the development of new technology is ongoing.
In conclusion, 3D simulation based on precise intrahepatic vascular and biliary analysis enabled accurate and oncologically curative hepatic resection, even in a patient with rare anatomical anomalies.
A healthy and asymptomatic male in his 70s was incidentally diagnosed with a gallbladder tumor by preoperative computed tomography (CT) scanning for an inguinal hernia.
The patient, who had right-sided ligamentum teres (RSLT) with abnormal vascular anomaly, was diagnosed with advanced gallbladder cancer invading the right hepatic duct (RHD, cStage III A, T3; right bile duct, N0, M0).
Based on 2 important findings, including a solid tumor-like appearance of the highly atrophied gallbladder by CT scanning and cytological evidence of adenocarcinoma from bile obtained by endoscopic retrograde gallbladder drainage rather than endoscopic nasobiliary drainage, advanced gallbladder cancer with infiltration of the RHD was more convincing than hilar cholangiocarcinoma as a preoperative diagnosis.
Laboratory tests revealed that tumor markers and liver function were within the normal ranges.
Preoperative three-dimensional liver simulation based on multiple detector computed tomography scanning combined with endoscopic nasobiliary tube cholangiography revealed that all segmental portal veins were independently ramified from the portal trunk and that the RHD drained the right-sided liver except for segment 8 (segments 1r, 5, 6, and 7).
Pathological examination showed well-differentiated tubular adenocarcinoma of the gallbladder invading the hepatic parenchyma and the RHD.
The patient was treated with extended hepatectomy of the RHD drainage territory (segments 1r, 5, 6, and 7) without injuring the hepatic vasculature of the remnant liver based on preoperative liver simulation.
Shindoh et al demonstrated anomalous vascular architecture with right-sided ligamentum teres (RSLT) and Ome et al reported major hepatectomy for RSLT patients. Shindoh J, Akahane M, Satou S, Aoki T, Beck Y, Hasegawa K, Sugawara Y, Ohtomo K, Kokudo N. Vascular architecture in anomalous right-sided ligamentum teres: Three-dimensional analyses in 35 patients. HPB (Oxford) 2012; 14: 32-41 [PMID: 22151449 DOI: 10.1111/j.1477-2574.2011.00398.x]. Ome Y, Kawamoto K, Park TB, Ito T. Major hepatectomy using the glissonean approach in cases of right umbilical portion. World J Hepatol 2016; 8: 1535-1540 [PMID: 28008345 DOI: 10.4254/wjh.v8.i34.1535].
RSLT is a congenital anomaly in which the umbilical ligament on the left side atrophies, and it is associated with anomalous ramifications in the artery, portal vein, and biliary systems.
Preoperative 3D liver simulation based on precise intrahepatic vascular and biliary analysis enabled accurate and oncologically curative hepatic resection, even in a patient with rare anatomical anomalies.
We thank Naomi Matsuzaki MD, a specialist in pathology, for her pathological diagnosis.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Barreto SG, Kanazawa A, Kai K, Ramia JM, Shindoh J S- Editor: Ji FF L- Editor: A E- Editor: Yin SY
| 1. | Nagai M, Kubota K, Kawasaki S, Takayama T, BandaiY , Makuuchi M. Are left-sided gallbladders really located on the left side? Ann Surg. 1997;225:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 2. | Maetani Y, Itoh K, Kojima N, Tabuchi T, Shibata T, Asonuma K, Tanaka K, Konishi J. Portal vein anomaly associated with deviation of the ligamentum teres to the right and malposition of the gallbladder. Radiology. 1998;207:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Shindoh J, Akahane M, Satou S, Aoki T, Beck Y, Hasegawa K, Sugawara Y, Ohtomo K, Kokudo N. Vascular architecture in anomalous right-sided ligamentum teres: three-dimensional analyses in 35 patients. HPB (Oxford). 2012;14:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Nishitai R, Shindoh J, Yamaoka T, Akahane M, Kokudo N, Manaka D. Biliary architecture of livers exhibiting right-sided ligamentum teres: an indication for preoperative cholangiography prior to major hepatectomy. HPB (Oxford). 2016;18:929-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Kawai K, Koizumi M, Honma S, Tokiyoshi A, Kodama K. Right ligamentum teres joining to the right branch of the portal vein. Anat Sci Int. 2008;83:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Ome Y, Kawamoto K, Park TB, Ito T. Major hepatectomy using the glissonean approach in cases of right umbilical portion. World J Hepatol. 2016;8:1535-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Abe T, Kajiyama K, Harimoto N, Gion T, Shirabe K, Nagaie T. Resection of metastatic liver cancer in a patient with a left-sided gallbladder and intrahepatic portal vein and bile duct anomalies: A case report. Int J Surg Case Rep. 2012;3:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Sakaguchi T, Suzuki S, Morita Y, Oishi K, Suzuki A, Fukumoto K, Inaba K, Takehara Y, Baba S, Nakamura S. Hepatectomy for metastatic liver tumors complicated with right umbilical portion. Hepatogastroenterology. 2011;58:984-987. [PubMed] |
| 9. | Fairweather M, Balachandran VP, D’Angelica MI. Surgical management of biliary tract cancers. Chin Clin Oncol. 2016;5:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Hemming AW, Scudamore CH, Shackleton CR, Pudek M, Erb SR. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg. 1992;163:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 150] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5813] [Article Influence: 109.7] [Reference Citation Analysis (2)] |
| 12. | Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Hoboken, NJ: John Wiley Sons 2011; . |
| 13. | Couinaud C. Le foie: Études anatomiques et chirurgicales. Paris: Masson Cie 1957; . |
| 14. | Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford). 2002;4:99; author reply 99-99; author reply 100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 271] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Yokoyama Y, Nishio H, Ebata T, Igami T, Sugawara G, Nagino M. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br J Surg. 2010;97:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Nanashima A, Abo T, Kudo T, Sakamoto I, Hayashi H, Murakami G, Takeshita H, Hidaka S, Kido Y, Nagayasu T. Usefulness of examining hepatic functional volume using technetium-99m galactosyl serum albumin scintigraphy in hepatocellular carcinoma. Nucl Med Commun. 2013;34:478-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Yamamoto Y, Ikai I, Kume M, Sakai Y, Yamauchi A, Shinohara H, Morimoto T, Shimahara Y, Yamamoto M, Yamaoka Y. New simple technique for hepatic parenchymal resection using a Cavitron Ultrasonic Surgical Aspirator and bipolar cautery equipped with a channel for water dripping. World J Surg. 1999;23:1032-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 25984] [Article Influence: 1181.1] [Reference Citation Analysis (1)] |
| 19. | Cheynel N, Arnal E, Rat P, Benoit L, Trouilloud P, Favre JP. Absence of the portal bifurcation at the hilum of the liver due to intrahepatic origin of the left branch of the portal vein. Surg Radiol Anat. 2001;23:355-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Hsu SL, Chen TY, Huang TL, Sun CK, Concejero AM, Tsang LL, Cheng YF. Left-sided gallbladder: its clinical significance and imaging presentations. World J Gastroenterol. 2007;13:6404-6409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 21. | Sanli EC, Kurtoglu Z, Kara A, Uzmansel D. Left sided-right portal joined round ligament with an anomaly of the intrahepatic portal vein. Saudi Med J. 2006;27:1897-1900. [PubMed] |
| 22. | Miyamoto R, Oshiro Y, Hashimoto S, Kohno K, Fukunaga K, Oda T, Ohkohchi N. Three-dimensional imaging identified the accessory bile duct in a patient with cholangiocarcinoma. World J Gastroenterol. 2014;20:11451-11455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Nakayama K, Oshiro Y, Miyamoto R, Kohno K, Fukunaga K, Ohkohchi N. The Effect of Three-Dimensional Preoperative Simulation on Liver Surgery. World J Surg. 2017;41:1840-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 24. | Okuda Y, Taura K, Seo S, Yasuchika K, Nitta T, Ogawa K, Hatano E, Uemoto S. Usefulness of operative planning based on 3-dimensional CT cholangiography for biliary malignancies. Surgery. 2015;158:1261-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Mise Y, Tani K, Aoki T, Sakamoto Y, Hasegawa K, Sugawara Y, Kokudo N. Virtual liver resection: computer-assisted operation planning using a three-dimensional liver representation. J Hepatobiliary Pancreat Sci. 2013;20:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Hallet J, Gayet B, Tsung A, Wakabayashi G, Pessaux P; 2nd International Consensus Conference on Laparoscopic Liver Resection Group. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: current status and future perspectives. J Hepatobiliary Pancreat Sci. 2015;22:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 27. | Yamanaka J, Okada T, Saito S, Kondo Y, Yoshida Y, Suzumura K, Hirano T, Iimuro Y, Fujimoto J. Minimally invasive laparoscopic liver resection: 3D MDCT simulation for preoperative planning. J Hepatobiliary Pancreat Surg. 2009;16:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kurimoto A, Yamanaka J, Hai S, Kondo Y, Sueoka H, Ohashi K, Asano Y, Hirano T, Fujimoto J. Parenchyma-preserving hepatectomy based on portal ramification and perfusion of the right anterior section: preserving the ventral or dorsal area. J Hepatobiliary Pancreat Sci. 2016;23:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Takamoto T, Hashimoto T, Ogata S, Inoue K, Maruyama Y, Miyazaki A, Makuuchi M. Planning of anatomical liver segmentectomy and subsegmentectomy with 3-dimensional simulation software. Am J Surg. 2013;206:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |