Published online Feb 27, 2018. doi: 10.4254/wjh.v10.i2.287
Peer-review started: September 20, 2017
First decision: October 31, 2017
Revised: October 31, 2017
Accepted: February 5, 2018
Article in press: February 5, 2018
Published online: February 27, 2018
Processing time: 167 Days and 0.5 Hours
To investigate the influence of the umbilical cord-derived multipotent stromal cells (MSCs) on recovery of the liver after the subtotal resection, that is, removal of 80% of the organ mass, a renowned model of the small-for-size liver remnant syndrome.
The MSCs were obtained from the intervascular tissue of umbilical cords, dissected from rat fetuses, by the explant culture technique. The vital labeling of MSCs with РКН26 was carried out on the 3rd passage. The subtotal resection was performed on male Sprague-Dawley rats. The experimental group animals received a transplant 106 MSCs infused into the spleen. Hepatocyte proliferation was assessed by counting of either mitotic figures or Ki67-positive cells in microscopic images. MSC differentiation was assessed with antibodies to hepatocyte-specific marker cytokeratin 18 (CK18), cholangiocyte-specific protein CK19, smooth muscle cell-specific protein α-SMA, the endothelial cell marker CD31, or the active fibroblast marker FAPα. Total macrophages of the liver were selectively stained in cryosections incubated with anti-CD68 antibodies (1:100, Abcam), while the M2a and M2c macrophage populations were selectively stained with anti-CD206 antibodies. Expression of interleukin and growth factor genes was evaluated with PCR-RT.
Intrasplenic allogeneic transplantation of the umbilical cord-derived multipotent stromal cells stimulates reparative processes within the residual liver tissue after subtotal resection (removal of 80% of the organ mass), as indicated by increased rates of hepatocyte proliferation and accelerated organ mass recovery. These effects may result from paracrine influence of the transplanted cells on the resident macrophage population of the liver. The transplantation favors polarization of macrophages to M2 phenotype (the M2-polarized macrophages specifically express CD206; they are known to suppress inflammation and support tissue repair). No differentiation of the transplanted cells into any of the liver cell types have been observed in the study.
We found no direct evidence for the paracrine effect of MSCs on liver regeneration after the subtotal liver resection in rats. However, the paracrine mechanism of the therapeutic activity of transplanted MSC is indirectly indicated by a decrease in the total number of CD68 + macrophages and an increase in the proportion of M2 pro-repair macrophages in the regenerating liver as compared to animals in which the transplantation was only mimicked.
Core tip: Umbilical cord-derived multipotent stromal cells stimulate reparative processes within the liver after subtotal resection (removal of 80% of the organ mass). Multipotent stromal cells stimulate hepatocyte proliferation in rats after subtotal resection and favor polarization of macrophages to M2 phenotype. The transplanted multipotent stromal cells do not differentiate into any of the liver cell types under these conditions.
- Citation: Elchaninov A, Fatkhudinov T, Usman N, Arutyunyan I, Makarov A, Lokhonina A, Eremina I, Surovtsev V, Goldshtein D, Bolshakova G, Glinkina V, Sukhikh G. Multipotent stromal cells stimulate liver regeneration by influencing the macrophage polarization in rat. World J Hepatol 2018; 10(2): 287-296
- URL: https://www.wjgnet.com/1948-5182/full/v10/i2/287.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i2.287
Multipotent stromal cells (MSCs) are found to be a helpful supplement in various repair processes in mammals, particularly in solid organs[1]. These cells are considered as a promising tool of regenerative medicine[2]. Their ability to stimulate reparative regeneration of damaged liver have been confirmed[3], but the mechanisms remain uncertain.
The experimentally proven enhancement of liver regeneration by MSCs must be essentially paracrine, because their transplantation to residual livers causes an increase in concentrations of HGF and several other growth factors within the regenerating tissues[4]. Therapeutic activity of MSCs is apparently related to their anti-inflammatory properties, which also represent a sort of paracrine regulation, as manifested by a local increase in IL-4, IL-13, and TSG-6 production paralleled by relative shortage of TNFα and IL-6[5]. Modulation of inflammatory reactions may be implemented via influence of these, or similar, paracrine factors on immune cells, especially on macrophages[6,7]. Several studies, however, confirm the ability of MSCs to differentiate into hepatocytes, which may support quite a different explanation for the positive influence of MSCs on liver repair[3,8].
In a number of clinical cases, a residual portion of the liver left after an extended resection, e.g., due to a tumor or metastasis, or even a transplanted portion of donor liver tissue supposed to replace the bulk of liver tissue in the host, is too small to effectively support the body homeostasis[9]. The resulting post-hepatectomy liver failure is a major manifestation of the small-for-size liver remnant syndrome, when the critically reduced liver mass is not only insufficient to maintain normal liver function, but also incapable of compensatory growth. The problem could be solved by some specific controllable stimulation of this growth combined with intensive liver care and management for acute hepatic failure.
This study deals with influence of the umbilical cord-derived MSCs on the reparative regeneration of the liver after the subtotal resection, that is, removal of the 80% organ mass, a renowned model of the small-for-size liver remnant syndrome.
Outbred male Sprague-Dawley rats, body weight 250-270 g, were obtained from the Institute for Bioorganic Chemistry branch animal facilities (Pushchino, Moscow region, Russia). All experimental work involving animals was carried out according to the standards of laboratory practice (National Guidelines No. 267 by Ministry of Healthcare of the Russia, June 1, 2003), and all efforts were made to minimize suffering. The study was approved by the Ethical Review Board at the Scientific Research Institute of Human Morphology (Protocol No. 16, November 19, 2015).
The MSCs were obtained from the intervascular tissue of umbilical cords, dissected from rat fetuses, by the explant culture technique. Their identity as MSCs was verified by their capacity of clonogenic growth on the untreated plastic, expression of specific set of surface antigens, and their ability to differentiate into mesodermal derivatives (Arutyunyan et al[2], 2016). The vital labeling of MSCs with РКН26 (Sigma-Aldrich Co LLC, United States) was carried out on the 3rd passage. The labeled cells were washed twice with saline (PanEco, Russia) and transferred to culture dishes for the labeling quality assessment or into syringes for transplantation to experimental animals.
The animals (n = 83) were operated as described earlier[15]. Hepatic tissue from sham-operated animals (n = 43) served as additional control in the assessment of hepatocyte proliferation, immunostaining and genes expression.
Animal survival was calculated as (the total number of operated animals minus the number of spontaneous deaths divided by the total number of operated animals. The animals were drawn from the experiment in CO2-chamber at 3 h, 6 h, 24 h, 48 h, 3 d, 7 d, or 10 d after the surgery (5-6 animals for each term) between 9 am and 11 am. The regenerating livers were promptly dissected, weighed (along with the whole animal, to determine the liver-to-total mass ratio, with intact animals of similar age (n = 10) being used for comparison), and preserved for analysis. A part of the material was fixed in 10% buffered formalin for a routine histological procedure (dehydration, paraffin sectioning, HE staining, mounting, and microscopy). Another part of the material was frozen in liquid nitrogen for in vivo cell tracing and/or immunochemistry (see below).
Hepatocyte proliferation was assessed by counting of either mitotic figures or Ki67-positive cells in microscopic images.
Mitotic index of hepatocytes was calculated for each animal individually as a number of mitoses per 6 × 103 hepatocytes, expressed in promille (‰).
Ki67-positive cells were counted on cryosections immunostained with corresponding primary antibodies followed by FITC-conjugated secondary antibodies, both supplied by Abcam, United Kingdom, and used in 1:100 and 1:200 dilutions, respectively; cell nuclei were counterstained with DAPI (Sigma-Aldrich Co LLC). The Ki67 proliferation index was calculated for each animal individually as a number of Ki67-positive hepatocytes per 3 × 103 hepatocytes.
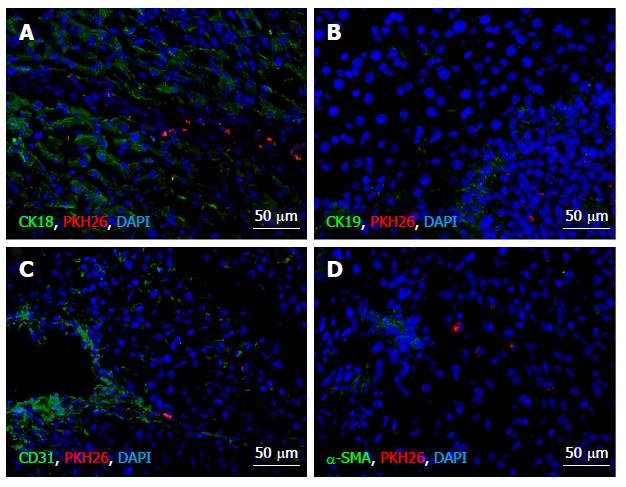
The cryosections were stained with antibodies to hepatocyte-specific marker cytokeratin 18 (CK18), cholangiocyte-specific protein CK19, smooth muscle cell-specific protein α-SMA, the endothelial cell marker CD31, or the active fibroblast marker FAPα. All of the primary antibodies were obtained from Abcam and used in 1:100 dilutions as recommended by the manufacturer and followed by FITC-conjugated secondary antibodies (1:200, Abcam); cell nuclei were counterstained with DAPI (Sigma-Aldrich Co LLC). Expression of the cell type-specific proteins was meticulously sought out in MSCs identified within regenerating liver tissue by PKH26 label. The observations were done using Leica DM 4000 B fluorescent microscope with LAS AF v.3.1.0 build 8587 software (Leica Microsystems CMS GmbH, Germany).
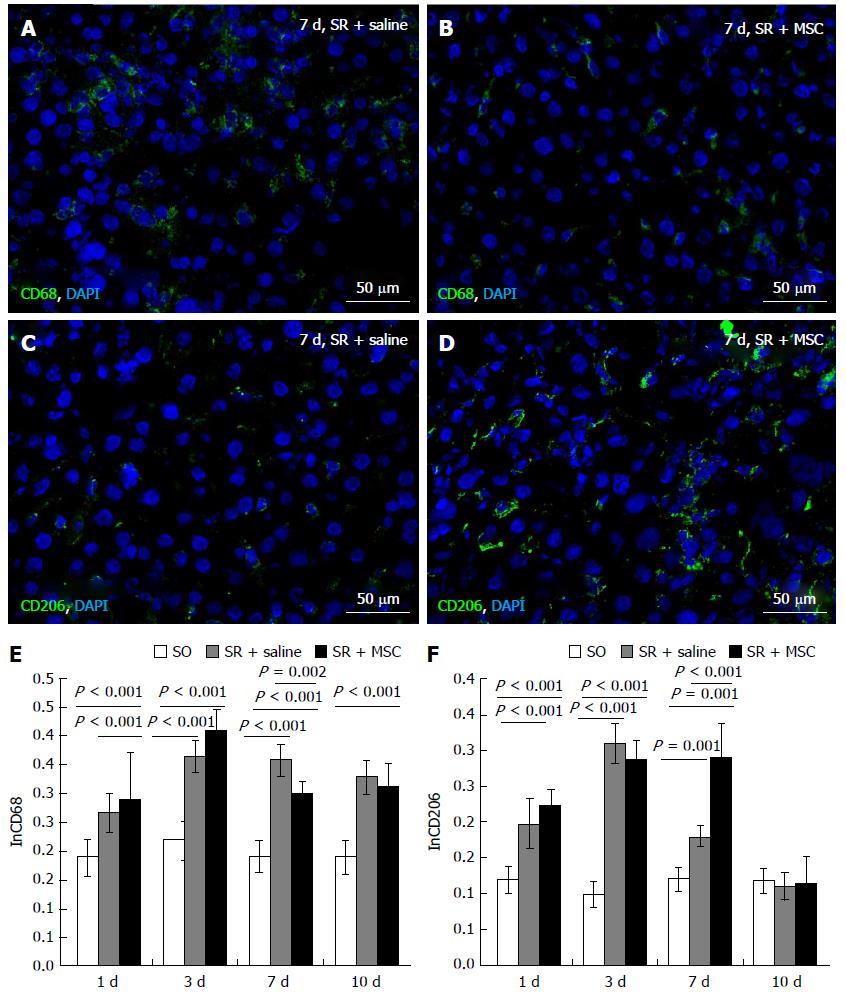
Total macrophages of the liver were selectively stained in cryosections incubated with anti-CD68 antibodies (1:100, Abcam), while the M2a and M2c macrophage populations were selectively stained with anti-CD206 antibodies (Santa-Cruz, United States) used in 1:100 dilution as recommended by the manufacturer. The signal was visualized by using FITC-conjugated secondary antibodies (1:200, Abcam) combined with DAPI-counterstaining of the nuclei. The CD68+ and CD206+ cells were counted in microscopic images and related to the total cell counts to obtain corresponding indexes of macrophage content.
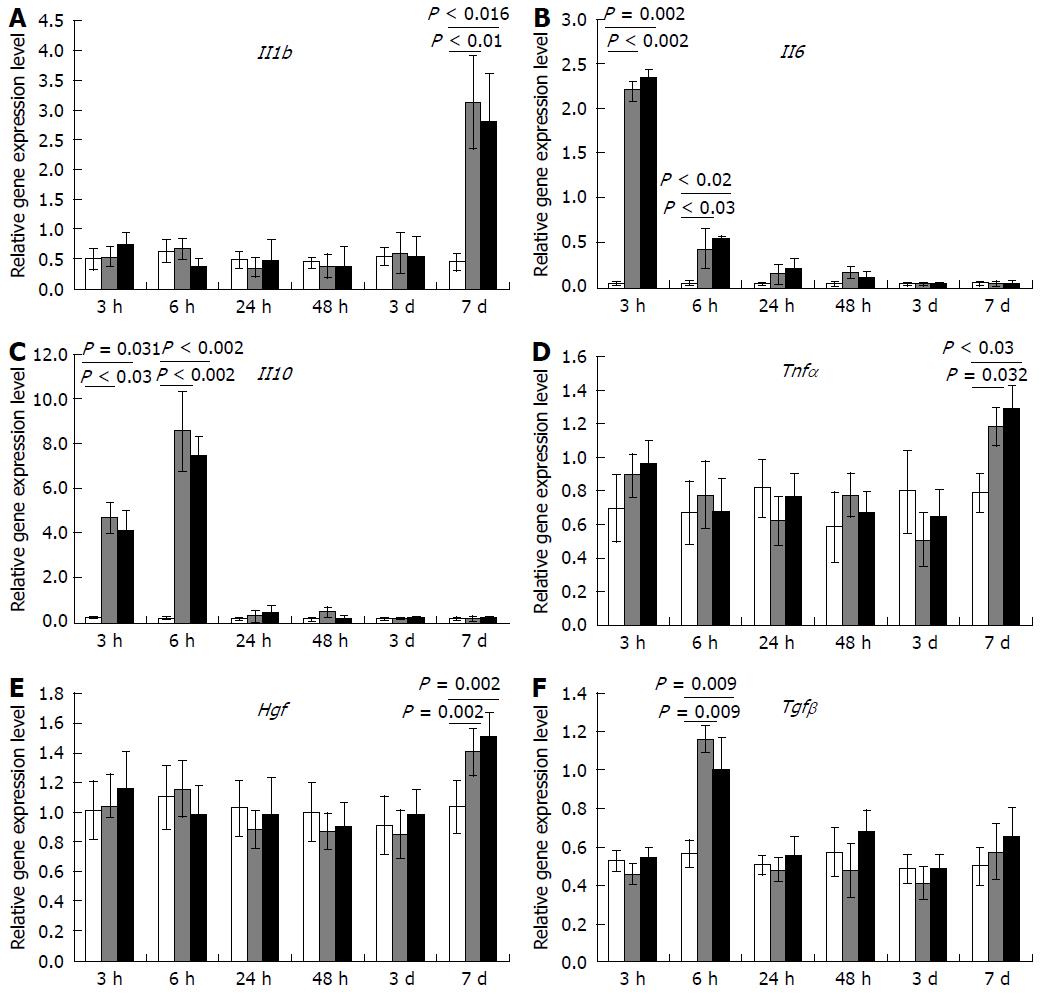
Total RNA was isolated with RNeasy Plus Mini Kit (QIAGEN). Estimated concentration of RNA in the eluate was 0.1 g/L; the quality was controlled by electrophoresis. To remove traces of genomic DNA, the samples were treated with RNase-free DNase I (Thermo Scientific, Waltham, MA, United States; 1 U per μg of RNA); the efficacy of DNA elimination was confirmed by PCR with nontranscribed genomic region-specific primers. Reverse transcription reactions were set up using MMLV RT Kit (Evrogen CJSC, Moscow, Russia) and held at 39 °C for 1 h.
PCR mixtures based on qPCRmix-HS SYBR system (Evrogen CJSC) containing oligonucleotide primers (custom made by SYNTOL, Moscow, Russia) in 0.2-0.4 μmol/L final concentrations were set up in duplicates. Structures of the oligonucleotides with corresponding target symbols and descriptions are given in Table 1. Real-time PCR was carried out in DT-96 Real-Time PCR Cycler (DNA-Technology JSC, Moscow, Russia) at 95 °C 15 s, 62 °C for 10 s + reading, 72 °C for 20 s. The relative expression values were calculated by approach originally introduced (Pfaffl MW[10], 2001) with modifications (Vandesompele J et al[11], 2002) using actb, b2m, and gapdh (Table 1) as reference targets.
| Target designation | 5'-end primer | 3'-end primer |
| Il1β | CTGTCTGACCCATGTGAGCT | ACTCCACTTTGGTCTTGACTT |
| Il6 | TACATATGTTCTCAGGGA GAT | GGTAGAAACGGAACTCCAG |
| Il10 | GCCCAGAAATCAAGGAGCAT | TGAGTGTCACGTAGGCTT CTA |
| Tnfα | CCACCACGCTCTTCTGTCTA | GCTACGGGCTTGTCACTCG |
| Hgf | GGCCATGGTGCTACACTCTT | TTGTGGGGGTACTGCGAATC |
| Tgfβ1 | CCGCAACAACGCAATCTATG | AGCCCTGTATTCCGTCTCCTT |
| Actβ | GAGATTACTGCCCTGGCTCC | GCTCAGTAACAGTCCGCCTA |
| B2m | CTCGCTCGGTGACCGTGAT | GGACAGATCTGACATCTCGA |
| Gapdh | GCGAGATCCCGCTAACATCA | CCCTTCCACGATGCCAAAGT |
The data were analyzed using SigmaStat 3.5 (Systat Software Inc., Chicago, IL, United States). Proportion values were compared by 2-sample z-test; relative gene expression values were compared by the Mann-Whitney U test; more-than-two-groups comparisons were done using ANOVA on ranks; P-values < 0.05 were considered significant.
Some of the animals died within 3 d after the surgery, but none of them died after the 3 d timepoint, animal survival in the comparison group (72.1% ± 6.8%, n = 43) was significantly higher than in the MSC transplantation group (47.5% ± 7.8%, n = 40).
MSC transplantation stimulated compensatory growth of residual hepatic tissues, which was expressed in more rapid liver mass recovery (Table 2). In the MSC transplantation group, the liver-to-body ratio returned to normal values by day 10 after the surgery, while in the comparison group it still did not reach the initial level (as measured for the intact control animals).
Mitotic index of hepatocytes was significantly higher in the MSC transplantation group than in the comparison group on day 3 as well as on day 10 after the surgery (Figure 1A, C, D, F and G). On day 7 after the surgery, however, this index was significantly lower in the MSC transplantation group than in the comparison group (Figure 1B, E and G).
A similar tendency was revealed by using the Ki67 immunostaining (Figure 2). Although no significant differences in the Ki67 proliferation index between the groups were observed on day 3 after the surgery (Figure 2A, D and G), this index for the MSC transplantation group on day 7 was significantly lower (Figure 2B, E and G), and on day 10 significantly higher, than for the comparison group (Figure 2C, F and G).
The transplanted cells expressed neither CK18 or CK19 (Figure 3A and B), nor α-SMA (Figure 3D). Solitary CD31-expressing PKH26-labeled cells were observed at the site of injection on days 3 and 10 after the surgery (Figure 3С).
It turned out, that on day 7 after the surgery the animals of the MSC transplantation group had significantly decreased numbers of total liver macrophages defined as CD68+ cells (Figure 4A, B and E). At the same time, calculated numbers of M2 macrophages (defined as CD206+ cells) in regenerating livers on day 7 after the surgery were higher in the MSC transplantation group than in the comparison group (Figure 4C, D and F).
No transplantation-dependent changes in gene expression at the site of transplantation have been revealed in this study, despite that such changes were extensively sought for. We analyzed expression dynamics for a set of genes, which reportedly participate in regulation of mammalian liver recovery, and found no changes that could be related to the MSC transplantation (Figure 5).
In this study we observed a stimulating effect of the umbilical cord-derived MSCs on liver regeneration after subtotal resection in rats, manifested as increased survival of the operated animals, increased rates of liver mass recovery, and increased proliferation activity of hepatocytes.
The obtained evidence of the effect of MSCs on hepatocyte proliferation is consistent with the results of other studies[4]. It should be noted that the stimulating effect MSCs on hepatocyte proliferation is, apparently, due to accelerated passage of the cell cycle phases. This is indicated by the fact that on day 3 after the subtotal liver resection combined with MSC administration (mimicked by sham injection of saline in the comparison group), the Ki67 index did not differ between the groups indicating similar extent of hepatocyte engagement in cell cycle for both groups. At the same time, the proportion of hepatocytes undergoing mitosis per second was significantly higher in animals which received the MSCs, that is, their hepatocytes passed G1, S, and G2 phases more rapidly, to be able to divide by day 3 after the surgery. Such acceleration of cell cycles apparently led to their synchronization resulting in waves of hepatocyte proliferation with a temporary decrease in the mitotic activity of in between[12]. It is possible, that the increased rates of cell cycling in the early postoperative period were the cause of significantly better survival of animals in the MSC transplantation group.
Despite the clearly demonstrated regeneration-stimulating effect of the umbilical cord-derived MSC transplantation, exact mechanisms of this stimulation remained obscure.
However, the obtained data indicate that the replacement mechanism of stimulation of regeneration in this case is not the leading one.
The transplanted MSCs did not differentiate into hepatocytes, cholangiocytes, smooth muscle cells, active fibroblasts, or myofibroblasts. Solitary РКН26-labeled MSCs at the site of transplantation were positive for the endothelial cell marker CD31. It is known that endothelial differentiation of MSCs is stimulated by VEGF and supported the endothelium-derived extracellular matrix; in combination they make MSCs to express the endothelium-specific markers[13]. It is also known, that the umbilical cord-derived MSCs give a stronger response to endothelial induction than the bone marrow-derived MSCs[14]. Despite that, only a minor fraction of transplanted cells could possibly differentiate into endothelial cells upon transplantation. This is consistent with our previously reported data on rapid elimination of transplanted MSCs by host macrophages. In particular, about 90% of transplanted cells in spleen and regenerating liver were destroyed by association with CD68+ cells[15].
We found no direct evidence for the paracrine effect of MSCs on liver regeneration after the subtotal liver resection in rats. Although MSCs show the ability to synthesize a rich set of biologically active molecules[2], the paracrine effect provided by MSCs is dose-dependent and typically short-term, and apparently difficult to study in vivo. Besides, several other studies demonstrating the positive effect of MSCs on liver regeneration show that repeated administration of MSCs, as well as an “in advance″ transplantation of the cells before damage, gives a stronger effect, which may have a stronger paracrine component[16,17].
However, the paracrine mechanism of the therapeutic activity of transplanted MSC is indirectly indicated by a decrease in the total number of CD68 + macrophages and an increase in the proportion of M2 pro-repair macrophages in the regenerating liver as compared to animals in which the transplantation was only mimicked. It is known that the effect of MSC on inflammation is mediated by a local increase in IL-4, IL-13, TSG-6 and a decrease in TNFα, IL-6[5] influencing the immune system cells, especially macrophages[6,7]. It has been shown in many studies on various models that after MSC transplantation the number of activated macrophages of the alternative M2 phenotype (which have pro-repair and anti-inflammatory properties) increases, and the number of pro-inflammatory M1 macrophages decreases[18,19]. This is consistent with the data obtained by us on the model of liver regeneration after its subtotal resection in rats.
Exact molecular mechanisms of the paracrine effect of MSC transplantation on hepatocyte proliferation and liver macrophage behavior in the aftermath of subtotal liver resection remain unclear. Further studies in this direction are very desirable, since any possibility of controlling hepatocyte proliferation and/or liver macrophage polarization could be of great therapeutic importance, especially in severe liver injuries.
The resulting post-hepatectomy liver failure is a major manifestation of the small-for-size liver remnant syndrome, when the critically reduced liver mass is insufficient to maintain normal liver function. The problem can be solved by some specific controllable stimulation of compensatory growth combined with intensive liver care and management for acute hepatic failure. Multipotent stromal cells may therefore represent a reasonable choice not only in cases of extensive hepatectomy but also for other types of severe liver damage (e.g. cirrhosis). Multipotent stromal cells are found to be a helpful supplement in various repair processes in mammals, particularly in solid organs. These cells are considered as a promising tool of regenerative medicine. Their ability to stimulate reparative regeneration of damaged liver has been confirmed, but the mechanisms remain uncertain.
The experimentally proven enhancement of liver regeneration by multipotent stromal cells (MSCs) must be essentially paracrine, because their transplantation to residual livers causes an increase in concentrations of HGF and several other growth factors within the regenerating tissues. Therapeutic activity of MSCs is apparently related to their anti-inflammatory properties, which also represent a sort of paracrine regulation, as manifested by a local increase in IL-4, IL-13, and TSG-6 production paralleled by relative shortage of TNFα and IL-6. Modulation of inflammatory reactions may be implemented via influence of these, or similar, paracrine factors on immune cells, especially on macrophages. Several studies, however, confirm the ability of MSCs to differentiate into hepatocytes, which leads to quite a different explanation for the positive influence of MSCs on liver repair.
In our experiments we found that intrasplenic allogeneic transplantation of the umbilical cord-derived multipotent stromal cells stimulated hepatocyte proliferation and organ mass recovery after subtotal resection. These effects may result from positive paracrine influence of the transplanted cells on polarization of the liver resident macrophages to M2 phenotype.
The MSCs were obtained from the intervascular tissue of umbilical cords, dissected from rat fetuses, by the explant culture technique. The vital labeling of MSCs with РКН26 was carried out on the 3rd passage. The subtotal resection was performed on male Sprague-Dawley rats. The experimental group animals received a transplant 106 MSCs infused into the spleen. Hepatocyte proliferation was assessed by counting of either mitotic figures or Ki67-positive cells in microscopic images. MSC differentiation was assessed with antibodies to hepatocyte-specific marker cytokeratin 18 (CK18), cholangiocyte-specific protein CK19, smooth muscle cell-specific protein α-SMA, the endothelial cell marker CD31, or the active fibroblast marker FAPα. Total macrophages of the liver were selectively stained in cryosections incubated with anti-CD68 antibodies (1:100, Abcam), while the M2a and M2c macrophage populations were selectively stained with anti-CD206 antibodies. Expression of interleukin and growth factor genes was evaluated with PCR-RT.
Intrasplenic allogeneic transplantation of the umbilical cord-derived multipotent stromal cells stimulates reparative processes within the residual liver tissue after subtotal resection (removal of 80% of the organ mass), as indicated by increased rates of hepatocyte proliferation and accelerated organ mass recovery. These effects may result from paracrine influence of the transplanted cells on the resident macrophage population of the liver. The transplantation favors polarization of macrophages to M2 phenotype (the M2-polarized macrophages specifically express CD206; they are known to suppress inflammation and support tissue repair). No differentiation of the transplanted cells into any of the liver cell types have been observed in the study.
In this study we observed a stimulating effect of the umbilical cord-derived MSCs on liver regeneration after subtotal resection in rats, manifested as increased survival of the operated animals, increased rates of liver mass recovery, and increased proliferation activity of hepatocytes. We found no direct evidence for the paracrine effect of MSCs on liver regeneration after the subtotal liver resection in rats. However, the paracrine mechanism of the therapeutic activity of transplanted MSC is indirectly indicated by a decrease in the total number of CD68 + macrophages and an increase in the proportion of M2 pro-repair macrophages in the regenerating liver as compared to animals in which the transplantation was only mimicked.
Exact molecular mechanisms of the paracrine effect of MSC transplantation on hepatocyte proliferation and liver macrophage behavior in the aftermath of subtotal liver resection remain unclear. Further studies in this direction are very desirable, since any possibility of controlling hepatocyte proliferation and/or liver macrophage polarization could be of great therapeutic importance, especially in severe liver injuries.
| 1. | Chistiakov DA. Liver regenerative medicine: advances and challenges. Cells Tissues Organs. 2012;196:291-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016;2016:6901286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 3. | Wu XB, Tao R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int. 2012;11:360-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, Han Y. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One. 2013;8:e62363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 446] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 6. | Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Tögel F, Westenfelder C. The role of multipotent marrow stromal cells (MSCs) in tissue regeneration. Organogenesis. 2011;7:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Liu ZC, Chang TM. Intrasplenic transplantation of bioencapsulated mesenchymal stem cells improves the recovery rates of 90% partial hepatectomized rats. Stem Cells Int. 2012;2012:697094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 482] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [PubMed] |
| 11. | Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. [PubMed] |
| 12. | Lambotte L, Saliez A, Triest S, Tagliaferri EM, Barker AP, Baranski AG. Control of rate and extent of the proliferative response after partial hepatectomy. Am J Physiol. 1997;273:G905-G912. [PubMed] |
| 13. | Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 959] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 14. | Chen MY, Lie PC, Li ZL, Wei X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 15. | Arutyunyan I, Elchaninov A, Fatkhudinov T, Makarov A, Kananykhina E, Usman N, Bolshakova G, Glinkina V, Goldshtein D, Sukhikh G. Elimination of allogeneic multipotent stromal cells by host macrophages in different models of regeneration. Int J Clin Exp Pathol. 2015;8:4469-4480. [PubMed] |
| 16. | Lundup AV, Onishchenko NA, Shagidulin M.Yu. Stem / progenitor cells of the liver and bone marrow as regulators of regenerative regeneration of damaged liver. Bulletin of transplantology and artificial organs. Russia. 2011;100-107. |
| 17. | Onischenko NA, Lundup AV, Gazizov IM. Two-phase dynamics of the effect of mesenchymal multipotent stromal cells (MMSC) of the bone marrow on the liver in the modeling of fibrosing hepatitis. Bulletin of transplantology and artificial organs. Russia. 2011;51-58. |
| 18. | Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31:2042-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Fatkhudinov TKh, Bol’shakova GB, Goldshtein DV, Sukhikh GT. Mechanisms of therapeutic activity of multipotent cells in heart diseases. Bull Exp Biol Med. 2014;156:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Clemens DLL, Chen YK S- Editor: Cui LJ L- Editor: A E- Editor: Li RF