Published online Jan 27, 2018. doi: 10.4254/wjh.v10.i1.82
Peer-review started: March 28, 2017
First decision: June 30, 2017
Revised: November 10, 2017
Accepted: December 4, 2017
Article in press: December 7, 2017
Published online: January 27, 2018
Processing time: 305 Days and 23.2 Hours
To provide a simple surrogate marker predictive of liver cirrhosis (LC).
Specimens from 302 patients who underwent resection for hepatocellular carcinoma between January 2006 and December 2012 were retrospectively analyzed. Based on pathologic findings, patients were divided into groups based on whether or not they had LC. Parameters associated with hepatic functional reserve were compared in these two groups using Mann-Whitney U-test for univariate analysis. Factors differing significantly in univariate analyses were entered into multivariate logistic regression analysis.
There were significant differences between the LC group (n = 100) and non-LC group (n = 202) in prothrombin activity, concentrations of alanine aminotransferase, aspartate aminotransferase, total bilirubin, albumin, cholinesterase, type IV collagen, hyaluronic acid, indocyanine green retention rate at 15 min, maximal removal rate of technitium-99m diethylene triamine penta-acetic acid-galactosyl human serum albumin and ratio of mean platelet volume to platelet count (MPV/PLT). Multivariate analysis showed that prothrombin activity, concentrations of alanine aminotransferase, aspartate aminotransferase, total bilirubin and hyaluronic acid, and MPV/PLT ratio were factors independently predictive of LC. The area under the curve value for MPV/PLT was 0.78, with a 0.8 cutoff value having a sensitivity of 65% and a specificity of 78%.
The MPV/PLT ratio, which can be determined simply from the complete blood count, may be a simple surrogate marker predicting LC.
Core tip: Although liver biopsy is considered the gold standard in the diagnosis of liver fibrosis and cirrhosis, liver biopsy is an invasive procedure, with attendant morbidity. Less invasive procedures are needed in the diagnosis of liver cirrhosis. Multivariate analysis showed that the mean platelet volume to platelet count ratio was independently predictive of liver cirrhosis. This ratio, which can be determined from a routine complete blood count, may be a simple surrogate marker predicting liver cirrhosis.
- Citation: Iida H, Kaibori M, Matsui K, Ishizaki M, Kon M. Ratio of mean platelet volume to platelet count is a potential surrogate marker predicting liver cirrhosis. World J Hepatol 2018; 10(1): 82-87
- URL: https://www.wjgnet.com/1948-5182/full/v10/i1/82.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i1.82
Mean platelet volume (MPV) is a machine-calculated measurement of average platelet size, usually included in complete blood count testing. Normal MPV ranges from 7.5 fL to 11.5 fL. Because average platelet size is directly proportional to the numbers of platelets produced, MPV is indicative of platelet production in bone marrow. Moreover, MPV is higher when there is destruction of platelets, as observed in patients with inflammatory bowel disease, immune thrombocytopenic purpura, myeloproliferative diseases and Bernard-Soulier syndrome[1]. MPV may also be higher in patients with pre-eclampsia and those recovering from transient bone marrow hypoplasia[2]. In contrast, abnormally low MPV values are indicative of thrombocytopenia because of impaired platelet production, as observed in patients with aplastic anemia.
Several studies have reported that liver cirrhosis (LC) and fibrosis are related to MPV[3-6]. Increased MPV, as well as decreased platelet count (PLT), were found to reflect a greater degree of fibrosis. These findings suggested that the ratio of MPV to PLT may correlate strongly with the degree of liver fibrosis. This study was, therefore, designed to determine whether liver fibrosis and LC are associated with the MPV/PLT ratio or not.
This retrospective study assessed samples obtained from 302 patients who underwent liver resection for hepatocellular carcinoma (HCC) between January 2006 and December 2012. All patients were assessed pathologically by stage of fibrosis in nontumor liver tissue using the new Inuyama classification[7]. F0 was defined as no fibrosis (n = 22), F1 as chronic hepatitis with fibrous portal expansion (n = 67), F2 as chronic hepatitis with bridging fibrosis (n = 62), F3 as chronic hepatitis with bridging fibrosis and architectural distortion (n = 51), and F4 as LC with tendency toward nodular formation throughout the whole area. Patients classified as F0-F3 were assigned to the non-LC group (n = 202), and those classified as F4 to the LC group (n = 100).
Parameters associated with hepatic functional reserve were assessed in all patients; these included: MPV, PLT, and the MPV/PLT ratio; prothrombin activity (PT); concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, cholinesterase, type IV collagen, and hyaluronic acid; indocyanine green retention rate at 15 min (ICGR15); and, the maximal removal rate of technitium-99m diethylene triamine penta-acetic acid (99mTc-DTPA)-galactosyl human serum albumin (GSA-Rmax), a marker of hepatic functional reserve, as determined by scintigraphy[8,9]. These factors were compared between the LC and non-LC groups. Multivariate regression analysis was performed to identify factors independently predictive of LC, and cutoff values were calculated. In addition, patients were divided by fibrosis stage (F0-F4), and these parameters were compared among the five subgroups.
Comorbidities that could be associated with an increase or decrease in the MPV/PLT ratio, such as inflammatory bowel disease, immune thrombocytopenic purpura, myeloproliferative disease or Bernard-Soulier syndrome, were not observed in any of the patients.
Parameters predictive of hepatic functional reserve in the LC and non-LC groups were compared using the Mann-Whitney U-test. Factors differing significantly in univariate analyses were entered into multivariate logistic regression analysis. Receiver operating characteristic (ROC) curves were used to calculate areas under the curve (AUC) and cutoff values. All analyses were performed using JMP 9 statistical analysis software (SAS Institute Inc., Cary, NC, United States), with a P value of < 0.05 defined as statistically significant.
There were 161 patients with hepatitis C and 53 patients with hepatitis B. The remaining 88 patients were negative for hepatitis B and C. The average age was 69.6 ± 9.7 years in the non-LC group and 68.2 ± 7.6 years in the LC group (P = 0.21). The ratio of males to females was larger in the non-LC group, with 164 (81.2%) male and 38 (18.8%) female patients; there were 69 (69.0%) male and 31 (31.0%) female patients in the LC group (P = 0.02).
Table 1 compares parameters (univariate analysis) between the LC and non-LC groups. The rate of hepatitis C was greater in the LC group than in the non-LC group (P < 0.001). The Edmondson-Steiner grade[10] for HCC grade I was a little smaller and for grade II a little larger in the LC group; however, the difference was not significant (P = 0.07). The average PLT was 11.6 ± 4.6 × 104/μL and 18.9 ± 8.1 × 104/μL, respectively, and the average MPV was 10.8 ± 0.9 fL and 10.2 ± 0.9 fL, respectively (P < 0.05 for each). The MPV/PLT ratio was significantly higher in the LC group than in the non-LC group (1.10 ± 0.51 vs 0.64 ± 0.30, P < 0.05). Other factors associated with hepatic functional reserve also differed significantly between the two groups, including PT, the concentrations of AST, ALT, total bilirubin, albumin, cholinesterase, type IV collagen and hyaluronic acid, ICGR15 and GSA-Rmax (P < 0.05 for each).
| Non-LC group, n = 202 | LC group, n = 100 | P-value | |
| Etiology | |||
| HBV | 43 (21.2%) | 10 (10.0%) | |
| HCV | 89 (44.1%) | 72 (72.0%) | |
| NBNC | 70 (34.7%) | 18 (18.0%) | < 0.001 |
| Edmonson-Steiner grade | |||
| I | 30 (30.0%) | 36 (17.8%) | 0.07 |
| II | 64 (64.0%) | 151 (74.8%) | |
| III | 4 (4.0%) | 13 (6.4%) | |
| IV | 2 (2.0%) | 2 (1.0%) | |
| PLT, × 104/μL | 18.9 ± 8.1 | 11.6 ± 4.6 | < 0.0001 |
| MPV, fL | 10.2 ± 0.9 | 10.8 ± 0.9 | < 0.0001 |
| MPV/PLT ratio | 0.64 ± 0.30 | 1.10 ± 0.51 | < 0.0001 |
| PT, % | 92.9 ± 11.7 | 82.5 ± 11.1 | < 0.0001 |
| AST, IU/L | 42 ± 26 | 51 ± 23 | < 0.0001 |
| ALT, IU/L | 40 ± 30 | 47 ± 33 | 0.02 |
| Total-bilirubin, mg/dL | 0.67 ± 0.23 | 0.90 ± 0.34 | < 0.0001 |
| Albumin, g/dL | 3.8 ± 0.5 | 3.6 ± 0.4 | 0.01 |
| Cholinesterase, IU/L | 235 ± 80 | 193 ± 60 | < 0.0001 |
| Type 4 collagen, ng/mL | 6.3 ± 2.4 | 9.1 ± 4.4 | 0.04 |
| Hyaluronic acid, ng/mL | 147 ± 179 | 312 ± 318 | < 0.0001 |
| ICGR15, % | 14.1 ± 8.3 | 22.1 ± 12.1 | < 0.0001 |
| GSA Rmax, mg/min | 0.62 ± 0.21 | 0.44 ± 0.17 | < 0.0001 |
Table 2 shows multivariate analysis of factors predictive of LC in these patients. MPV/PLT ratio, PT, and concentrations of AST, ALT, total bilirubin and hyaluronic acid were independent predictors of LC. The highest odds ratio was 3.71 for the MPV/PLT ratio. Although albumin, cholinesterase and type IV collagen concentrations, as well as ICGR15 and GSA-Rmax, were also predictors of LC on univariate analysis, they were not independently predictive on multivariate analysis.
| Odds ratio | P-value | 95%CI | ||
| MPV/PLT ratio | ≥ 0.71, n = 151 | 3.71 | < 0.0001 | 1.94-7.28 |
| < 0.71, n = 151 | ||||
| PT, % | ≥ 89.0, n = 151 | 2.68 | 0.0018 | 1.44-5.06 |
| < 89.0, n = 151 | ||||
| AST, IU/L | ≥ 39, n = 155 | 3.30 | 0.01 | 1.30-9.09 |
| < 39, n = 147 | ||||
| ALT, IU/L | ≥ 34, n = 153 | 2.57 | 0.04 | 1.02-7.09 |
| < 34, n = 149 | ||||
| Total-bilirubin, mg/dL | ≥ 0.7, n = 177 | 1.89 | 0.04 | 1.00-3.61 |
| < 0.7, n = 125 | ||||
| Albumin, g/dL | ≥ 3.8, n = 168 | 0.95 | 0.89 | 0.47-1.89 |
| < 3.8, n = 134 | ||||
| Cholinesterase, IU/L | ≥ 211, n = 152 | 0.98 | 0.96 | 0.48-1.98 |
| < 211, n = 150 | ||||
| Type 4 collagen, ng/mL | ≥ 6.5, n = 166 | 0.95 | 0.86 | 0.51-1.72 |
| < 6.5, n = 136 | ||||
| Hyaluronic acid, ng/mL | ≥ 124, n = 153 | 2.28 | 0.008 | 1.23-4.26 |
| < 124, n = 149 | ||||
| ICGR15, % | ≥ 14.3, n = 151 | 1.40 | 0.30 | 0.72-2.68 |
| < 14.3, n = 151 | ||||
| GSA Rmax, mg/min | ≥ 0.555, n = 151 | 1.51 | 0.24 | 0.75-3.04 |
| < 0.555, n = 151 |
The ROC curves of all six independently predictive factors (MPV/PLT ratio, PT, and concentrations of AST, ALT, total bilirubin and hyaluronic acid) are shown in Figure 1. Calculation of AUC for all six factors showed that the MPV/PLT ratio had the highest AUC (0.78). A cutoff value of 0.8 had a sensitivity of 65% and a specificity of 78% in predicting LC. This ratio was a better predictor of LC than other parameters of hepatic functional reserve.
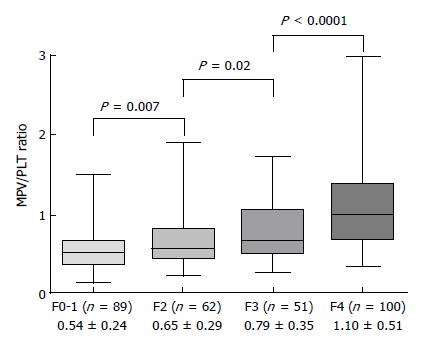
Patients were also divided by individual fibrosis stage and MPV/PLT ratio determined for each stage. The average MPV/PLT ratios for patients classified as F0-1, F2, F3 and F4 were 0.54 ± 0.24, 0.65 ± 0.29, 0.79 ± 0.35 and 1.10 ± 0.51, respectively, with each pairwise difference being statistically significant (Figure 2).
Additionally, we examined the correlation between the MPV/PLT ratio and the pathological inflammation level according to the new Inuyama classification. The average MPV/PLT ratios for patients classified as A0, A1, A2 and A3 were 0.68 ± 0.21, 0.70 ± 0.45, 0.82 ± 0.39 and 0.73 ± 0.10, respectively. There was no significant correlation between MPV/PLT and pathological inflammation level (P = 0.214).
LC is a result of advanced liver disease, in which normal liver tissue is replaced by fibrotic tissue. These changes lead to loss of liver function. LC is most frequently caused by alcoholism, infection with hepatitis B and hepatitis C viruses, and fatty liver disease, but it may have many other causes. LC arising from nonalcoholic steatohepatitis (NASH) was recently shown to be a worldwide problem.
The standard method of diagnosing LC is liver biopsy. However, liver biopsy is an invasive procedure and cannot be performed on patients with severe ascites or severe coagulation disorders. Several studies have, therefore, assessed surrogate markers in blood predictive of LC; these include FibroTest (FibroSure) and the AST platelet ratio index[11]. In addition, elastographic methods, such as FibroScan, have been reported to be noninvasive methods of predicting LC[12,13]. We could not compare these methods with the MPV/PLT ratio because we do not have these examination devices.
MPV may be another predictor of LC. Higher MPV has been reported in patients with hepatitis B[14], and LC, fibrosis level and MPV have been reported to correlate in patients with chronic hepatitis B[3-5]. MPV may also be predictive of LC in patients with chronic hepatitis C[6].
MPV has been associated not only with fibrosis stage but with degree of liver inflammation[15]. For example, higher MPV has been observed in patients with NASH[16], and MPV has been found to correlate with the presence of nonalcoholic fatty liver disease (NAFLD)[17], although another study reported no correlation[18]. In addition, MPV may or may not correlate with insulin resistance, which is closely related to NAFLD[19,20]. To date, however, the relationship between NAFLD and MPV has not been determined.
MPV has been reported to strongly correlate with the prognosis of patients with non-small cell lung cancer[21]. In addition, high MPV and MPV/PLT have been found to be associated with a high risk for HCC[22,23]. An examination of the correlation between MPV/PLT ratio and postoperative prognosis of patients undergoing hepatic resection for HCC found that the MPV/PLT ratio was unrelated to overall or recurrence-free survival rate after resection.
This study had several limitations, including its retrospective design, performance at a single center and small sample size. Moreover, all patients included had undergone resection for HCC. Therefore, the results should not be generalized to patients without HCC before verification. Prospective, multicenter studies with large numbers of patients are needed to confirm these findings.
In conclusion, we found that the MPV/PLT ratio was predictive of LC, suggesting that this ratio may be a simple surrogate marker predictive of LC.
Several noninvasive methods for predicting cirrhosis have been reported, but liver biopsy is the only method for obtaining a definitive diagnosis. However, liver biopsy is invasive, and a noninvasive diagnostic method is desirable. Mean platelet volume (MPV), the size of platelets, can be determined from routine complete blood count data of blood samples. Generally, if bone marrow hematopoietic function decreases, MPV decreases. In contrast, if spleen function increases, new platelets are made rapidly and MPV increases. In recent years, the relationship between MPV and liver disease has attracted attention.
There are reports that MPV correlates with liver function, and there are reports that MPV is related to the incidence of HCC. However, there is no report to evaluate the correlation between MPV/platelet count (PLT) and liver function, so we undertook this study.
The authors studied only patients who were diagnosed with cirrhosis histopathologically after liver resection. The MVP/PLT ratio could predict cirrhosis more sensitively than other general liver function tests.
The MPV/PLT ratio also correlated with the degree of hepatic fibrosis according to the Inuyama classification. The authors examined the relationship between prognosis after hepatic resection of hepatocellular carcinoma and the value of the MPV/PLT ration, but unfortunately no correlation was found.
MPV is the size of platelets and can be determined from routine complete blood count data of blood samples. Liver cirrhosis and fibrosis are related to MPV.
| 1. | Liu S, Ren J, Han G, Wang G, Gu G, Xia Q, Li J. Mean platelet volume: a controversial marker of disease activity in Crohn’s disease. Eur J Med Res. 2012;17:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Lippi G, Filippozzi L, Salvagno GL, Montagnana M, Franchini M, Guidi GC, Targher G. Increased mean platelet volume in patients with acute coronary syndromes. Arch Pathol Lab Med. 2009;133:1441-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Karagoz E, Ulcay A, Tanoglu A, Kara M, Turhan V, Erdem H, Oncul O, Gorenek L. Clinical usefulness of mean platelet volume and red blood cell distribution width to platelet ratio for predicting the severity of hepatic fibrosis in chronic hepatitis B virus patients. Eur J Gastroenterol Hepatol. 2014;26:1320-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Qi XT, Wan F, Lou Y, Ye B, Wu D. The mean platelet volume is a potential biomarker for cirrhosis in chronic hepatitis B virus infected patients. Hepatogastroenterology. 2014;61:456-459. [PubMed] |
| 5. | Ekiz F, Yüksel O, Koçak E, Yılmaz B, Altınbaş A, Çoban S, Yüksel I, Üsküdar O, Köklü S. Mean platelet volume as a fibrosis marker in patients with chronic hepatitis B. J Clin Lab Anal. 2011;25:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Purnak T, Olmez S, Torun S, Efe C, Sayilir A, Ozaslan E, Tenlik I, Kalkan IH, Beyazit Y, Yuksel O. Mean platelet volume is increased in chronic hepatitis C patients with advanced fibrosis. Clin Res Hepatol Gastroenterol. 2013;37:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Ichida F, Tsuji T, Omata M, Ichida T, Inoue K, Kamimura T, Yamada G, Hino K, Yokosuka O, Suzuki H, Suzuki H. New Inuyama classification: new criteria for histological assess- ment of chronic hepatitis. Int Hepatol Commun. 1996: 112. . |
| 8. | Ha-Kawa SK, Tanaka Y. A quantitative model of technetium-99m-DTPA-galactosyl-HSA for the assessment of hepatic blood flow and hepatic binding receptor. J Nucl Med. 1991;32:2233-2240. [PubMed] |
| 9. | Kwon AH, Matsui Y, Ha-Kawa SK, Kamiyama Y. Functional hepatic volume measured by technetium-99m-galactosyl-human serum albumin liver scintigraphy: comparison between hepatocyte volume and liver volume by computed tomography. Am J Gastroenterol. 2001;96:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [PubMed] |
| 11. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 12. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] |
| 13. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 966] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 14. | Turhan O, Coban E, Inan D, Yalcin AN. Increased mean platelet volume in chronic hepatitis B patients with inactive disease. Med Sci Monit. 2010;16:CR202-CR205. [PubMed] |
| 15. | Ceylan B, Fincanci M, Yardimci C, Eren G, Tözalgan Ü, Müderrisoğlu C, Paşaoğlu E. Can mean platelet volume determine the severity of liver fibrosis or inflammation in patients with chronic hepatitis B? Eur J Gastroenterol Hepatol. 2013;25:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Shin WY, Jung DH, Shim JY, Lee HR. The association between non-alcoholic hepatic steatosis and mean platelet volume in an obese Korean population. Platelets. 2011;22:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Ozhan H, Aydin M, Yazici M, Yazgan O, Basar C, Gungor A, Onder E. Mean platelet volume in patients with non-alcoholic fatty liver disease. Platelets. 2010;21:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Kilciler G, Genc H, Tapan S, Ors F, Kara M, Karadurmus N, Ercin CN, Karslioglu Y, Kilic S, Bagci S. Mean platelet volume and its relationship with carotid atherosclerosis in subjects with non-alcoholic fatty liver disease. Ups J Med Sci. 2010;115:253-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 19. | Arslan N, Makay B. Mean platelet volume in obese adolescents with nonalcoholic fatty liver disease. J Pediatr Endocrinol Metab. 2010;23:807-813. [PubMed] |
| 20. | Celikbilek M, Gürsoy S, Deniz K, Karaman A, Zararsiz G, Yurci A. Mean platelet volume in biopsy-proven non-alcoholic fatty liver disease. Platelets. 2013;24:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. 2014;83:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Cho SY, Yang JJ, You E, Kim BH, Shim J, Lee HJ, Lee WI, Suh JT, Park TS. Mean platelet volume/platelet count ratio in hepatocellular carcinoma. Platelets. 2013;24:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Kurt M, Onal IK, Sayilir AY, Beyazit Y, Oztas E, Kekilli M, Turhan N, Karaman K, Akdogan M. The role of mean platelet volume in the diagnosis of hepatocellular carcinoma in patients with chronic liver disease. Hepatogastroenterology. 2012;59:1580-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Atta H, Ji F, Preda CM S- Editor: Kong JX L- Editor: Filipodia E- Editor: Li D