Copyright
©The Author(s) 2025.
World J Hepatol. Sep 27, 2025; 17(9): 109035
Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.109035
Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.109035
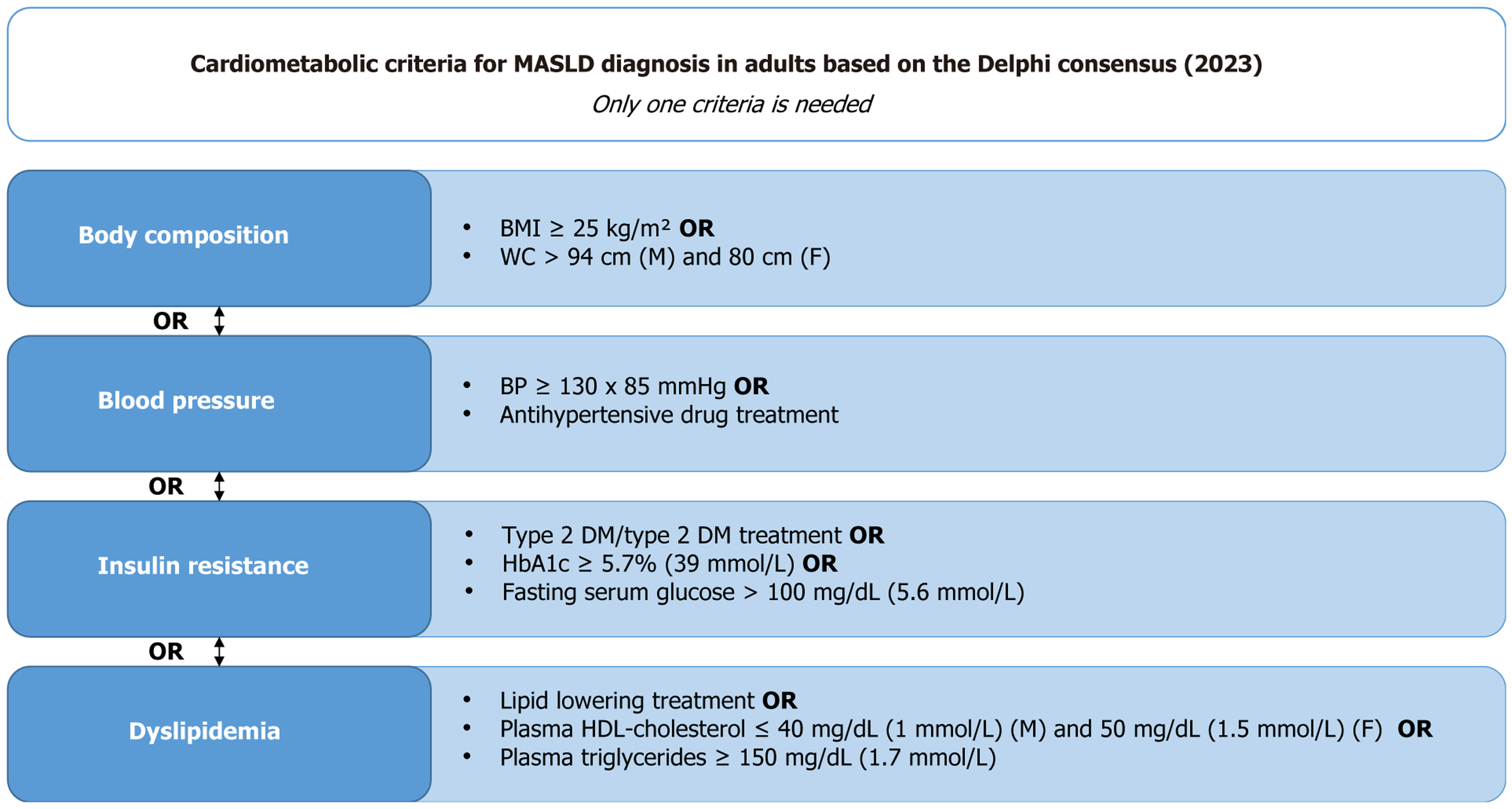
Figure 1 Cardiometabolic criteria for the diagnosis of metabolic dysfunction-associated steatotic liver disease in adults.
Diagnosis requires the presence of at least one of the following conditions: Altered body composition (body mass index ≥ 25 kg/m² or increased waist circumference adjusted for sex and ethnicity), elevated blood pressure, signs of insulin resistance (type 2 diabetes mellitus or markers), or dyslipidemia (use of lipid-lowering medication, reduced high-density lipoprotein-cholesterol, or elevated triglycerides). Adapted from the Delphi Consensus Statement (2023). BMI: Body mass index; BP: Blood pressure; DM: Diabetes mellitus; HbA1c: Glycated hemoglobin; HDL: High-density lipoprotein; MASLD: Metabolic dysfunction-associated steatotic liver disease; WC: Waist circumference.
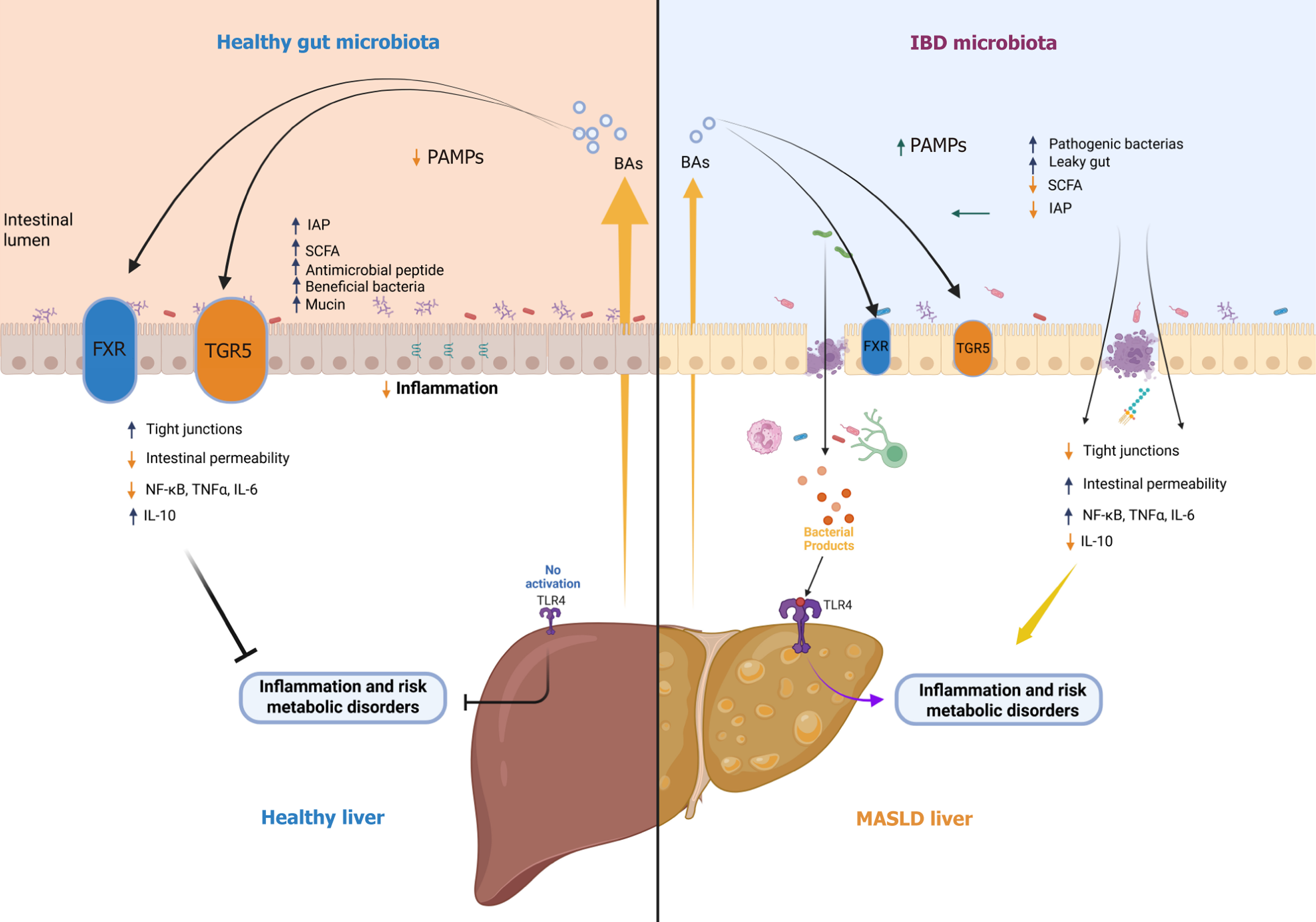
Figure 2 Gut–liver axis in health and disease: Reciprocal interactions between intestinal microbiota, bile acid signaling, barrier function, and hepatic inflammation linking inflammatory bowel disease and metabolic dysfunction-associated steatotic liver disease.
This schematic illustrates the bidirectional crosstalk of the gut–liver axis under physiological and pathological conditions. In an eubiotic state (left panel), a balanced gut microbiota produces short-chain fatty acids (SCFAs) such as butyrate, which reinforce intestinal barrier integrity by promoting tight junction expression and intestinal alkaline phosphatase (IAP) activity. This environment limits intestinal permeability and prevents the translocation of pathogen-associated molecular patterns into the portal circulation. Additionally, bile acids activate the farnesoid X receptor and Takeda G protein–coupled receptor 5, modulating anti-inflammatory pathways; reducing nuclear factor-kappa B, tumor necrosis factor-alpha, and interleukin (IL)-6 signaling; and enhancing IL-10 production, thereby preserving hepatic and intestinal homeostasis. In contrast, intestinal dysbiosis observed in inflammatory bowel disease (IBD) (right panel) is characterized by reduced microbial diversity, loss of beneficial SCFA-producing bacteria, decreased IAP, and increased pathogenic bacteria, leading to mucosal barrier disruption. This favors pathogen-associated molecular pattern translocation and toll-like receptor 4 activation in the liver, promoting hepatic inflammation and the progression to metabolic dysfunction-associated steatotic liver disease (MASLD). Furthermore, MASLD itself can impair bile acid homeostasis and alter gut microbiota composition, thereby exacerbating intestinal permeability, inflammation, and dysbiosis, which may, in turn, predispose or contribute to the development of IBD. This vicious cycle underscores the reciprocal, amplifying relationship between intestinal and hepatic disorders, positioning the gut–liver axis as a critical therapeutic target in the co-management of IBD and MASLD. FXR: Farnesoid X receptor; IAP: Intestinal alkaline phosphatase; IBD: Inflammatory bowel disease; IL: Interleukin; MASLD: Metabolic dysfunction-associated steatotic liver disease; NF-κB: Nuclear factor-kappa B; PAMPs: Pathogen-associated molecular patterns; SCFA: Short-chain fatty acid; TGR5: Takeda G protein–coupled receptor 5; TLR4: Toll-like receptor 4; TNF-α: Tumor necrosis factor-alpha.
- Citation: Lopes MA, Oliveira ECS, Quaglio AEV, Santos A, Imbrizi M, Mendes LER, Beraldo RF, Baima JP, Spiller AL, Magro DO, Sassaki LY. From gut to liver: Exploring the relationship between inflammatory bowel disease and metabolic dysfunction-associated steatotic liver disease. World J Hepatol 2025; 17(9): 109035
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/109035.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.109035