Copyright
©The Author(s) 2025.
World J Hepatol. Sep 27, 2025; 17(9): 107671
Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.107671
Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.107671
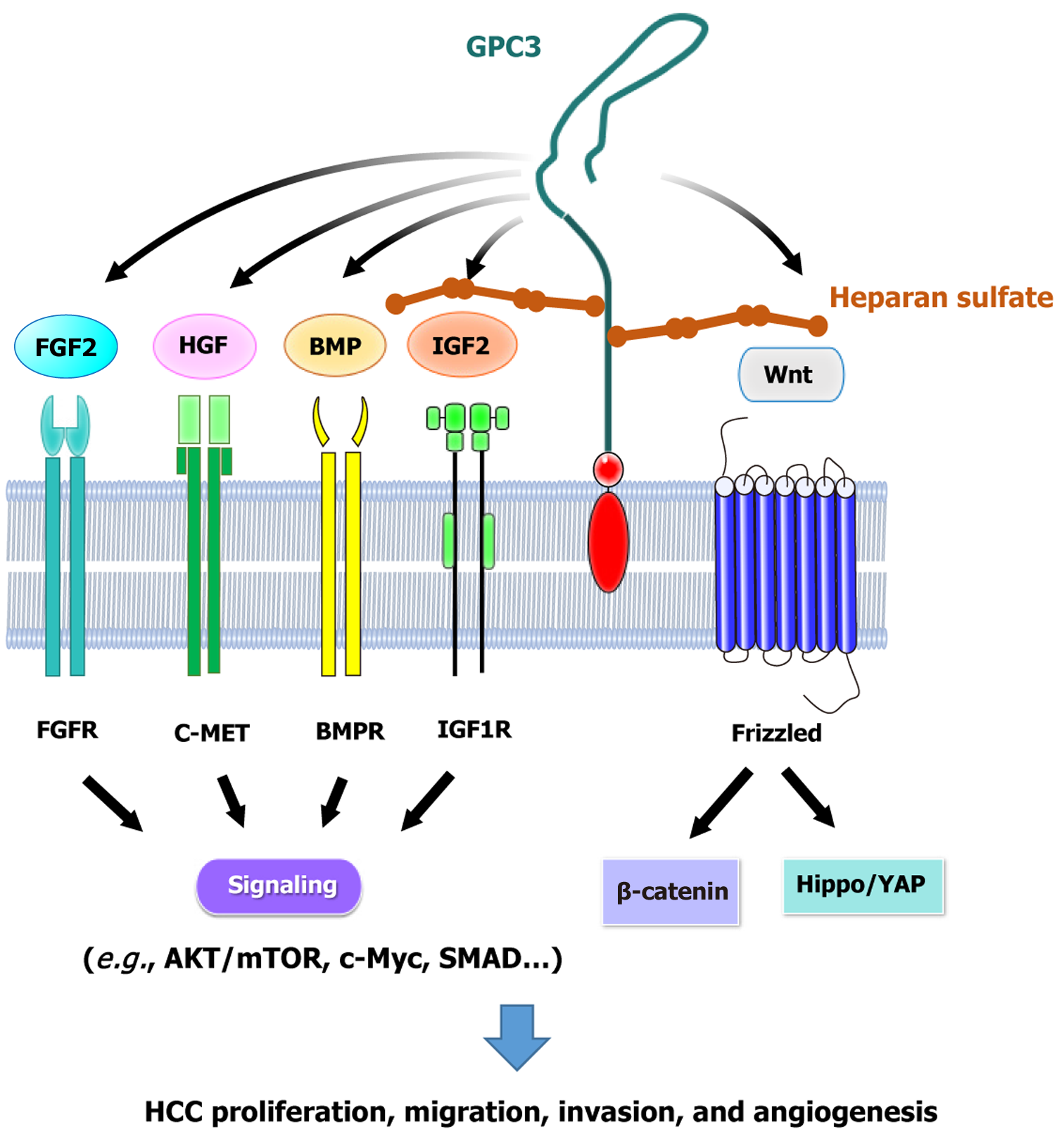
Figure 1 Diverse growth factor signaling pathways coordinated by glypican-3 in hepatocellular carcinoma.
Glypican-3 (GPC3) plays a crucial role in hepatocellular carcinoma (HCC) by interacting with multiple ligands through modulation by GPC3-conjugated heparan sulfate. These ligands include Wnt, fibroblast growth factor 2 (FGF 2), hepatocyte growth factor (HGF), bone morphogenetic protein (BMP), and insulin-like growth factor (IGF). Through these interactions, GPC3 regulates key oncogenic signaling pathways, thereby contributing to tumor proliferation, migration, invasion, and angiogenesis. Specifically, FGF2, HGF, BMP, and IGF activate their respective receptors (FGFR, C-MET, BMPR, and IGF1R), triggering downstream signaling cascades such as AKT/mTOR, c-Myc, and SMAD pathways, which ultimately drive tumor progression. Moreover, GPC3 enhances Wnt signaling by facilitating Wnt ligand binding to Frizzled receptors, leading to the activation of the β-catenin and Hippo/YAP pathways, which further promote oncogenic signaling in HCC. GPC3: Glypican-3; HGF: Hepatocyte growth factor; IGF: Insulin-like growth factor.
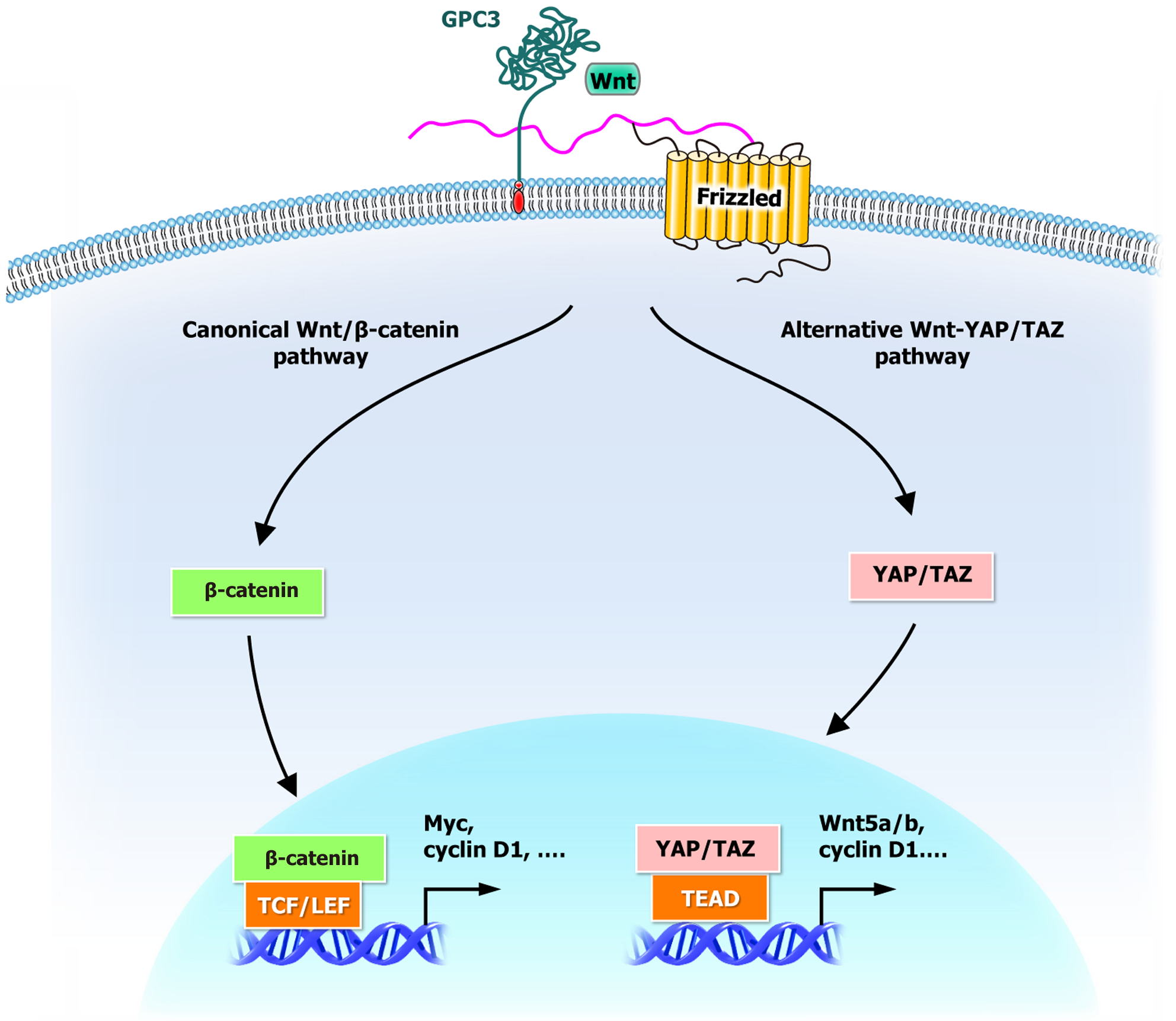
Figure 2 Glypican-3-mediated Wnt/β-catenin and YAP/TAZ signaling axes in hepatocellular carcinoma.
GPC3 acts as a central signaling molecule that regulates two key pathways in hepatocellular carcinoma. One is the canonical Wnt/β-catenin pathway, where activated β-catenin translocates to the nucleus and induces the expression of oncogenic genes. The other is the alternative Wnt–YAP/TAZ pathway, in which activated YAP/TAZ translocate to the nucleus and promote tumor progression through transcriptional regulation. GPC3: Glypican-3.
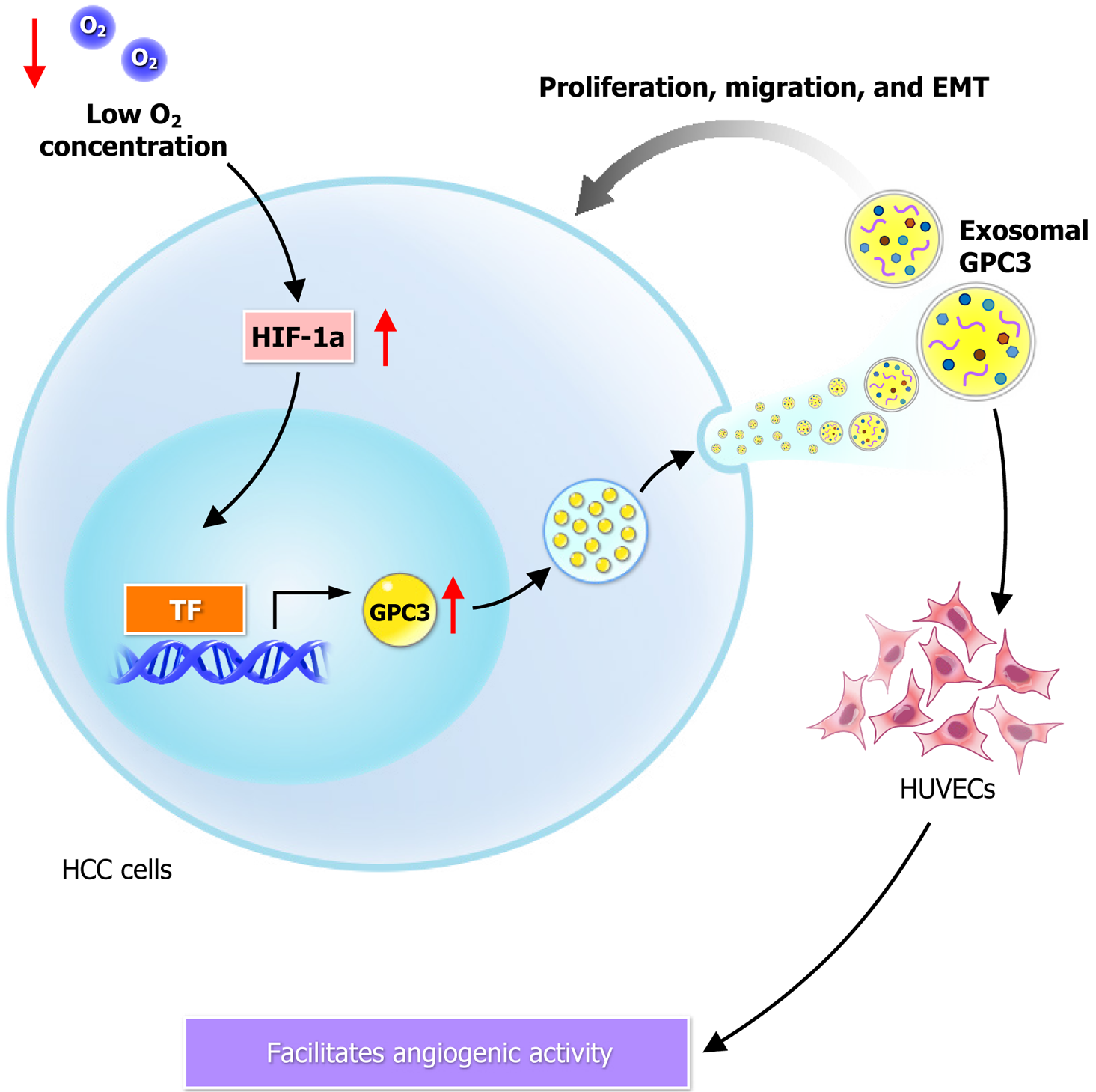
Figure 3 Hypoxia-induced exosomal release of glypican-3 and its effects on hepatocellular carcinoma progression and angiogenesis.
Under hypoxic conditions, hepatocellular carcinoma cells upregulate glypican-3 expression and release it via exosomes, contributing to tumor progression and angiogenesis in the tumor microenvironment. GPC3: Glypican-3; HCC: Hepatocellular carcinoma; EMT: Epithelial-mesenchymal transition.
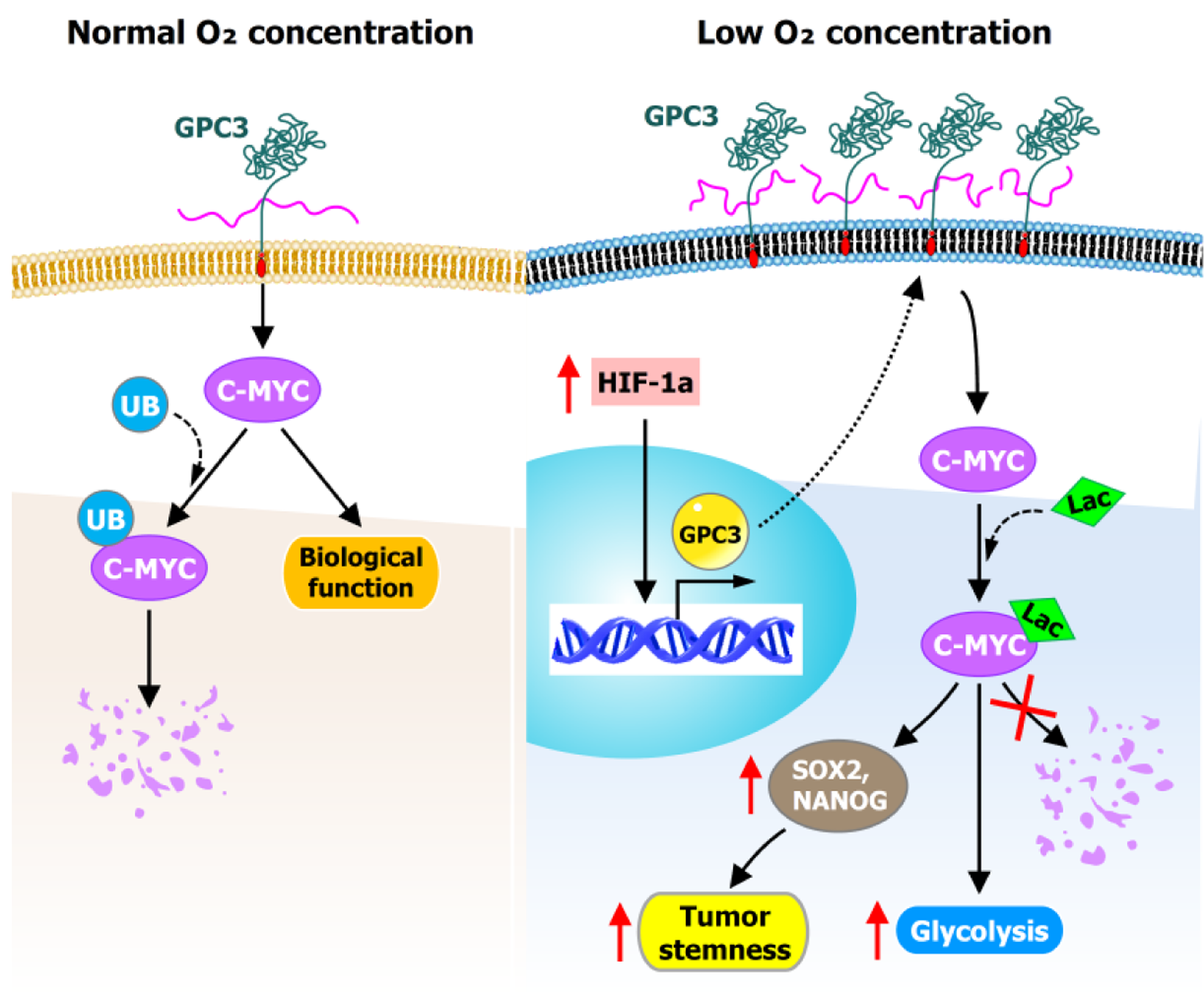
Figure 4 Glypican-3-mediated regulation of c-Myc stability and metabolic adaptation under hypoxia.
Glypican-3 (GPC3) regulates tumor metabolic adaptation and stemness by modulating c-Myc protein stability under normoxic and hypoxic conditions. Under normoxic conditions, c-Myc is more prone to ubiquitination and degradation when GPC3 expression is low. Under hypoxia, GPC3 is upregulated and promotes global protein lactylation, which specifically prevents c-Myc degradation, thereby stabilizing c-Myc protein and enhancing glycolysis and stemness. GPC3: Glypican-3.
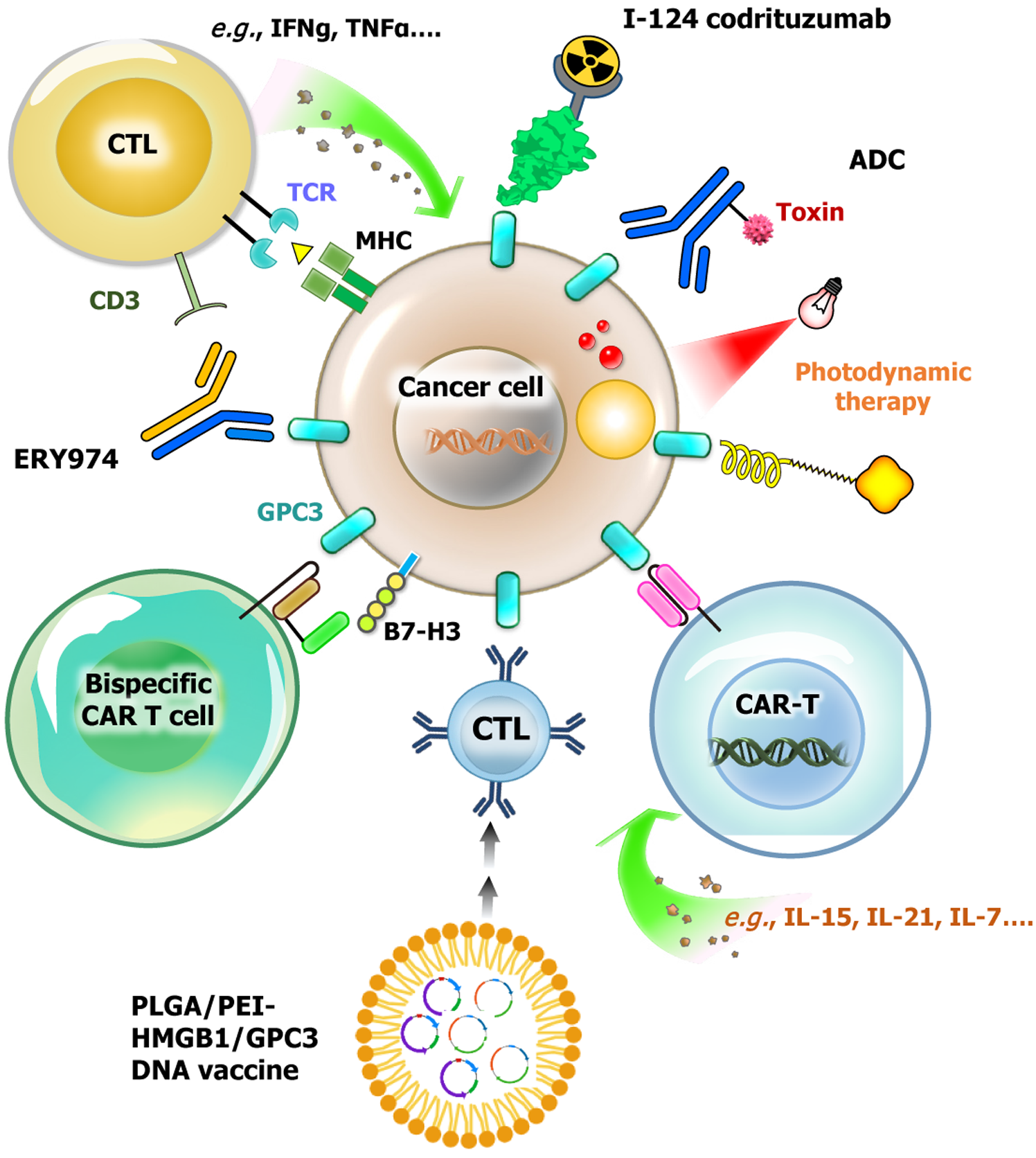
Figure 5 Applications of glypican-3-targeted therapies.
Glypican-3 (GPC3) serves as a promising target for hepatocellular carcinoma (HCC) therapy, with various therapeutic strategies under development. I-124 codrituzumab, a radiolabeled monoclonal antibody targeting GPC3, enables tumor imaging and potential radioimmunotherapy applications. Antibody-drug conjugates utilize GPC3-targeting antibodies conjugated with cytotoxic agents to selectively eliminate cancer cells. Photodynamic therapy, an emerging approach, employs light-activated GPC3-targeting molecules to induce localized cytotoxicity. GPC3-specific chimeric antigen receptor (CAR)-T cells have been engineered to target tumor cells, with their efficacy further enhanced by the secretion of cytokines such as IL-15, IL-21, and IL-7, which improve CAR-T cell persistence and strengthen antitumor activity. Bispecific CAR-T cells, designed to simultaneously recognize GPC3 and B7-H3, offer a dual-targeting strategy that enhances tumor specificity, boosts immune activation, and mitigates antigen escape, ultimately improving the effectiveness of CAR-T-cell therapy. In DNA vaccine development, PLGA/PEI nanoparticles are utilized to encapsulate and deliver GPC3 and HMGB1, aiming to stimulate immune responses and activate cytotoxic T lymphocyte. ERY974, a bispecific T cell-redirecting antibody, is designed to target both GPC3 on tumor cells and CD3 on T cells, facilitating potent T cell-mediated tumor killing. Collectively, these GPC3-targeted strategies offer significant potential for advancing HCC treatment by leveraging multiple mechanisms to enhance tumor specificity, immune activation, and therapeutic efficacy. CTL: Cytotoxic T lymphocyte; CAR-T: Chimeric antigen receptor-T.
- Citation: Wu CS, Lee TY, Chao HW. Targeting glypican-3 as a new frontier in liver cancer therapy. World J Hepatol 2025; 17(9): 107671
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/107671.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.107671