©The Author(s) 2025.
World J Hepatol. May 27, 2025; 17(5): 106618
Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.106618
Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.106618
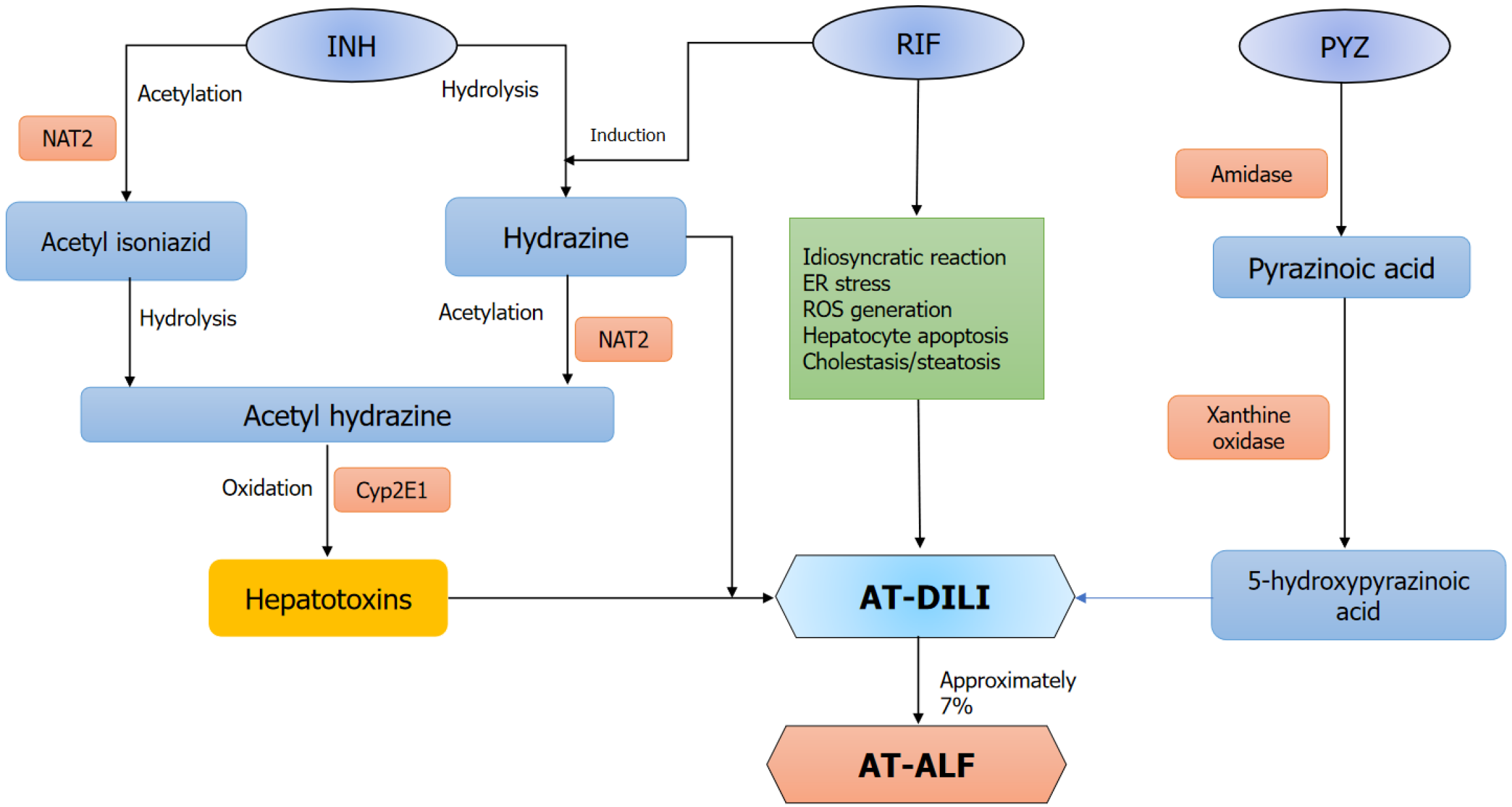
Figure 1 Depicts the possible mechanisms leading to anti-tuberculosis drug-induced acute liver failure.
The precise mechanisms underlying anti-tuberculosis drug-induced liver injury remain incompletely understood. The metabolism of isoniazid (INH) by NAT2 produces metabolites like acetyl diazine and reactive acetyl free radicals, which cause liver injury. Additionally, INH can be directly converted into INH hydrazine, causing hepatocellular damage. Notably, co-administration with rifampicin can significantly amplify this pathway. Pyrazinamide is metabolized to 5-hydroxy pyrazinoic acid and pyrazinoic acid, which are responsible for the hepatotoxicity. RIF: Rifampicin; INH: Isoniazid; PYZ: Pyrazinamide; ER: Endoplasmic reticulum; ROS: Reactive oxygen species; AT-DILI: Anti-tuberculosis drug-induced liver injury; AT-ALF: Anti-tuberculosis drug induced acute liver failure.
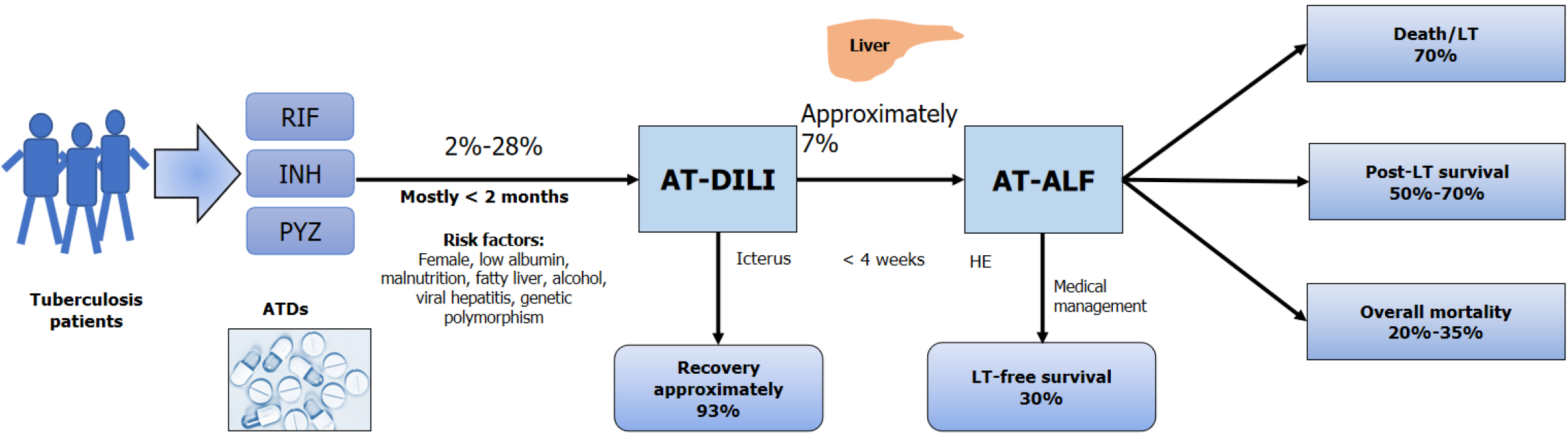
Figure 2 Diagrammatic representation of the magnitude of anti-tuberculosis drug-induced liver injury, its progression to anti-tuberculosis drug-induced acute liver failure, and subsequent patients’ outcomes.
RIF: Rifampicin; INH: Isoniazid; PYZ: Pyrazinamide; ATD: Anti-tuberculosis drug; AT-DILI: Anti-tuberculosis drug-induced liver injury; AT-ALF: Anti-tuberculosis drug induced acute liver failure; LT: Liver transplantation; HE: Hepatic encephalopathy.
- Citation: Kumar R, Kumar A, Kumar S. Acute liver failure from anti-tuberculosis drug-induced liver injury: An update. World J Hepatol 2025; 17(5): 106618
- URL: https://www.wjgnet.com/1948-5182/full/v17/i5/106618.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i5.106618