Copyright
©The Author(s) 2025.
World J Hepatol. Mar 27, 2025; 17(3): 101724
Published online Mar 27, 2025. doi: 10.4254/wjh.v17.i3.101724
Published online Mar 27, 2025. doi: 10.4254/wjh.v17.i3.101724
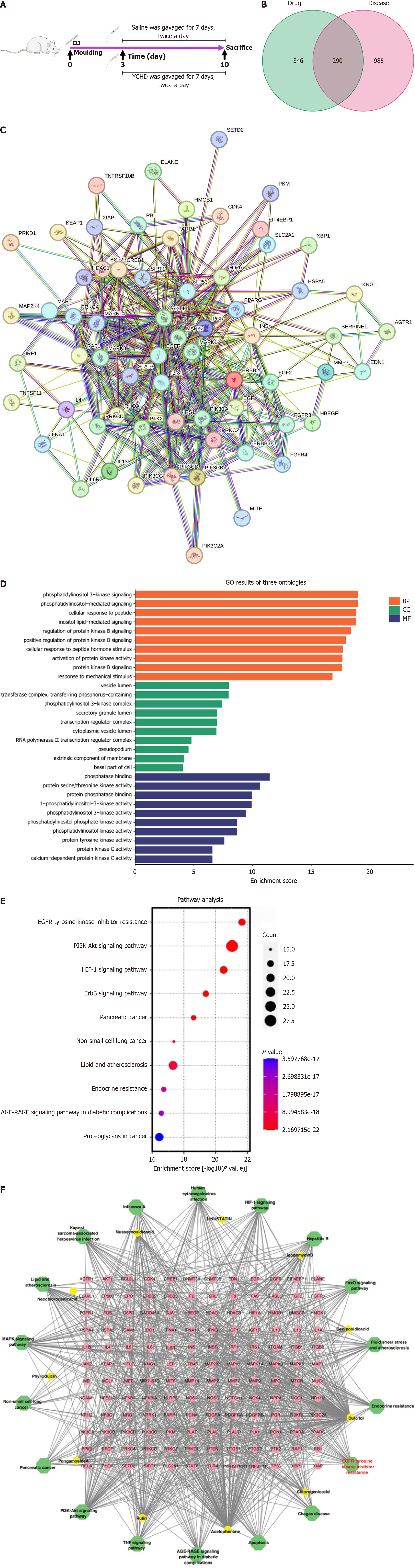
Figure 1 Screening and network pharmacology analysis of Yinchenhao decoction blood components and obstructive jaundice targets.
A: Study workflow: Yinchenhao decoction administered after obstructive jaundice for 3 days, followed by sacrifice and tissue collection on day 10; B: Venn diagrams illustrating the Yinchenhao decoction (YCHD) blood components and potential targets for obstructive jaundice; C: Protein-protein interaction network with 65 nodes and 382 edges; D: The top 10 terms for biological process, molecular function, and cellular component in Gene Ontology functional annotations; E: Kyoto Encyclopedia of Genes and Genomes enrichment analysis of key targets in YCHD; F: Network diagram illustrating the relationship between YCHD components, targets, and pathways, with 175 nodes: 11 components (orange hexagons), 144 targets (pink hexagons), and 20 pathways (green circles), connected by 957 edges. YCHD: Yinchenhao decoction; GO: Gene Ontology; EGFR: Epidermal growth factor receptor.
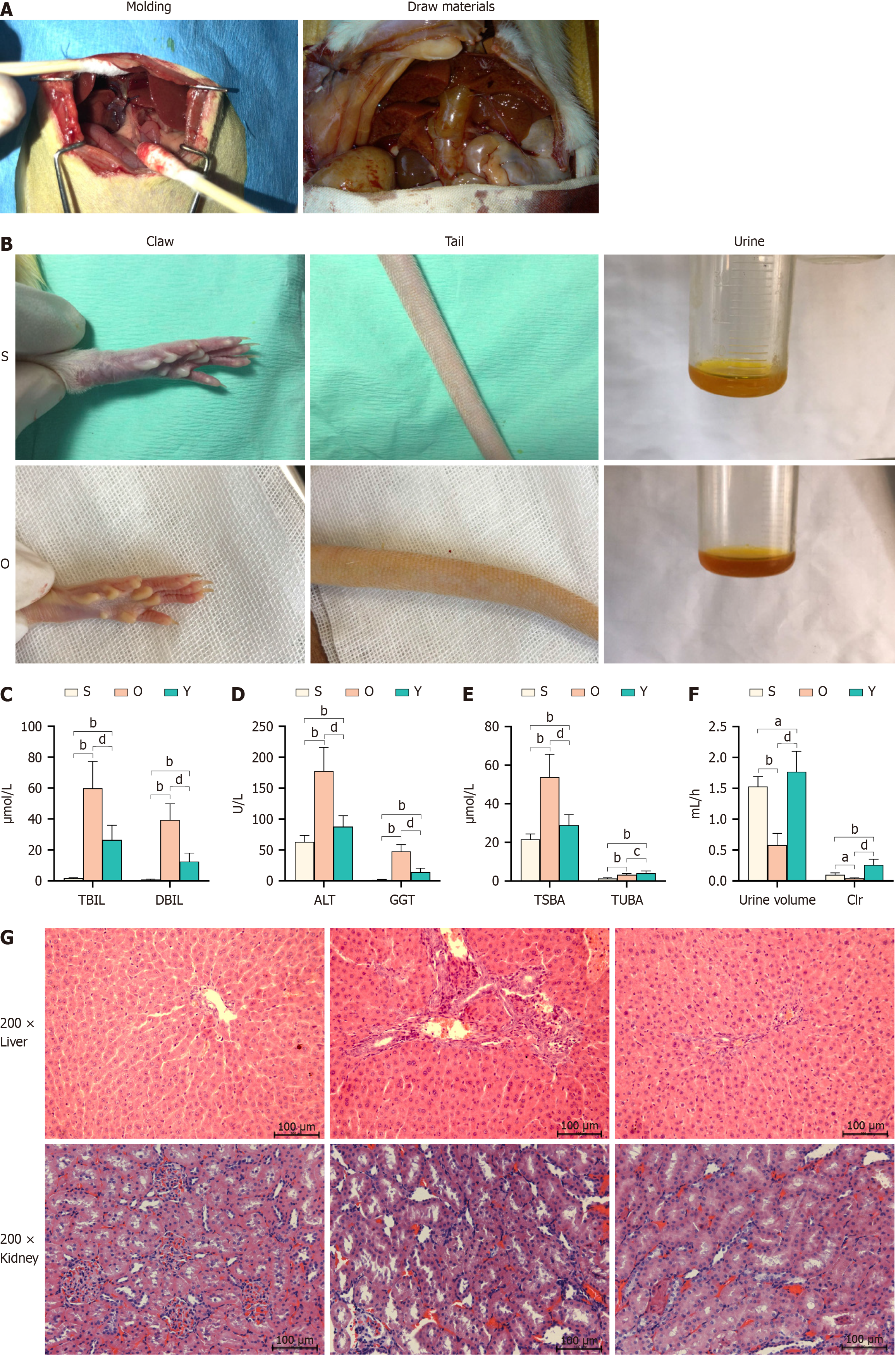
Figure 2 Impact of Yinchenhao decoction on bilirubin, liver function, urine output, and liver cell histology in rats with obstructive jaundice.
A and B: Development of obstructive jaundice (OJ) rat models; C-F: Bilirubin (C), liver function (D), bile acid (E), and urine output (F) in rats with OJ. aP < 0.05 vs group S; bP < 0.01 vs group S; cP < 0.05 vs group O; dP < 0.01 vs group O; G: Histological alterations in liver and kidney tissues of rats with OJ. Group O: Obstructive jaundice model; group S: Sham; Y Group: Yinchenhao decoction-treated; TSBA: Total serum bile acid; DBIL: Direct bilirubin; TBIL: Total bilirubin; AST: Aspartate aminotransferase; GGT: Gamma-glutamyl transferase; Clr: Renal clearance; TUBA: Total urinary bile acid.
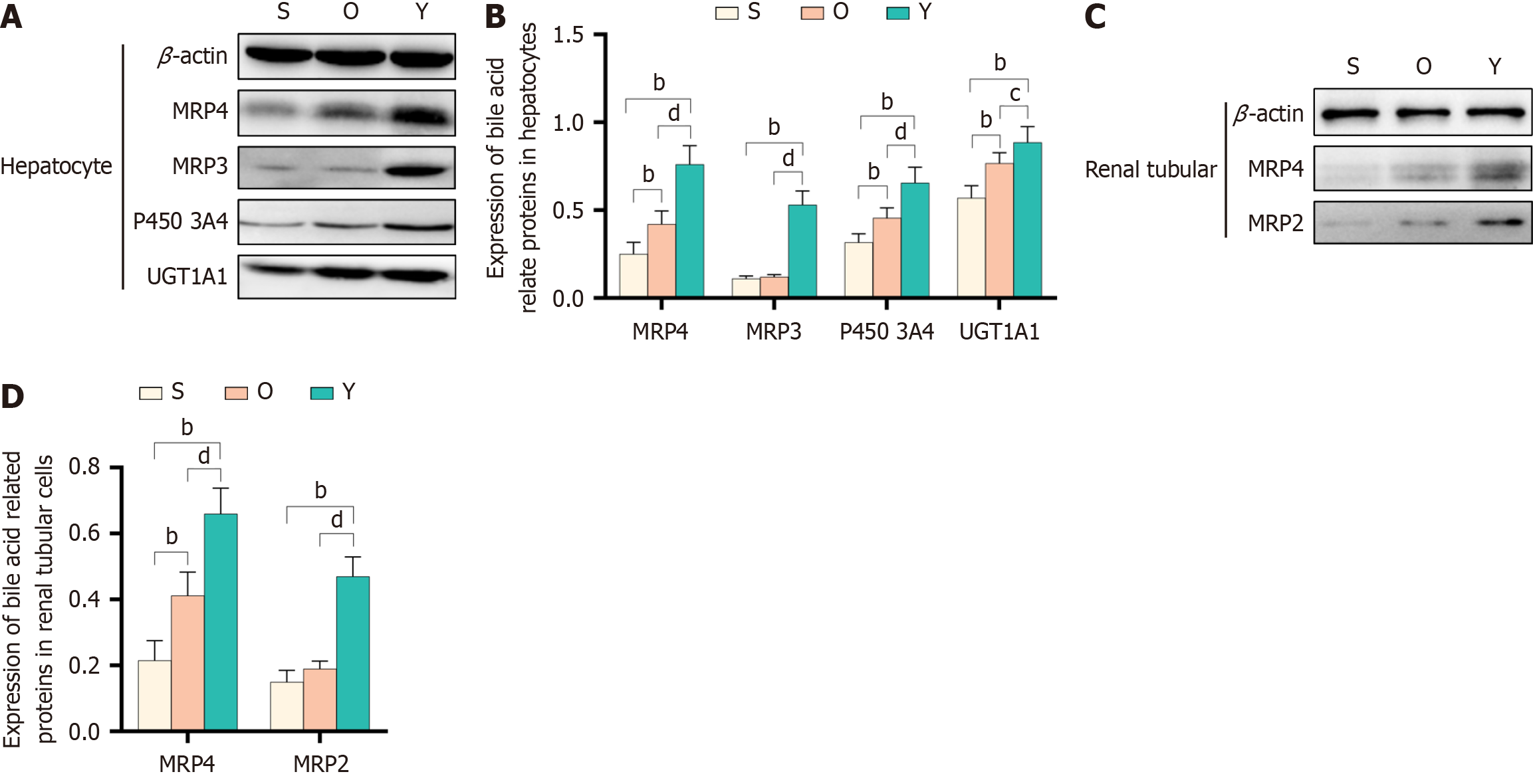
Figure 3 Effect of Yinchenhao decoction on bile acid active transporters in rats with obstructive jaundice.
A and B: Levels of expression of the cytochrome P450 family 3 subfamily A member 4 (P4503A4), UDP glucuronosyltransferase family 1 member A1 (UGT1A1), multidrug resistance-associated protein (MRP) 3, and 4 in the liver tissue; C and D: Multidrug resistance-associated protein (MRP) 2, and 4 in the kidney tissues; and levels of expression of MRP 2, 4 in the renal tissue. aP < 0.05 vs group S; bP < 0.01 vs Sham (group S); cP < 0.05 vs OJ model (group O); dP < 0.01 vs group O. Group O: Obstructive jaundice model; Group S: Sham; Y group: Yinchenhao decoction-treated.
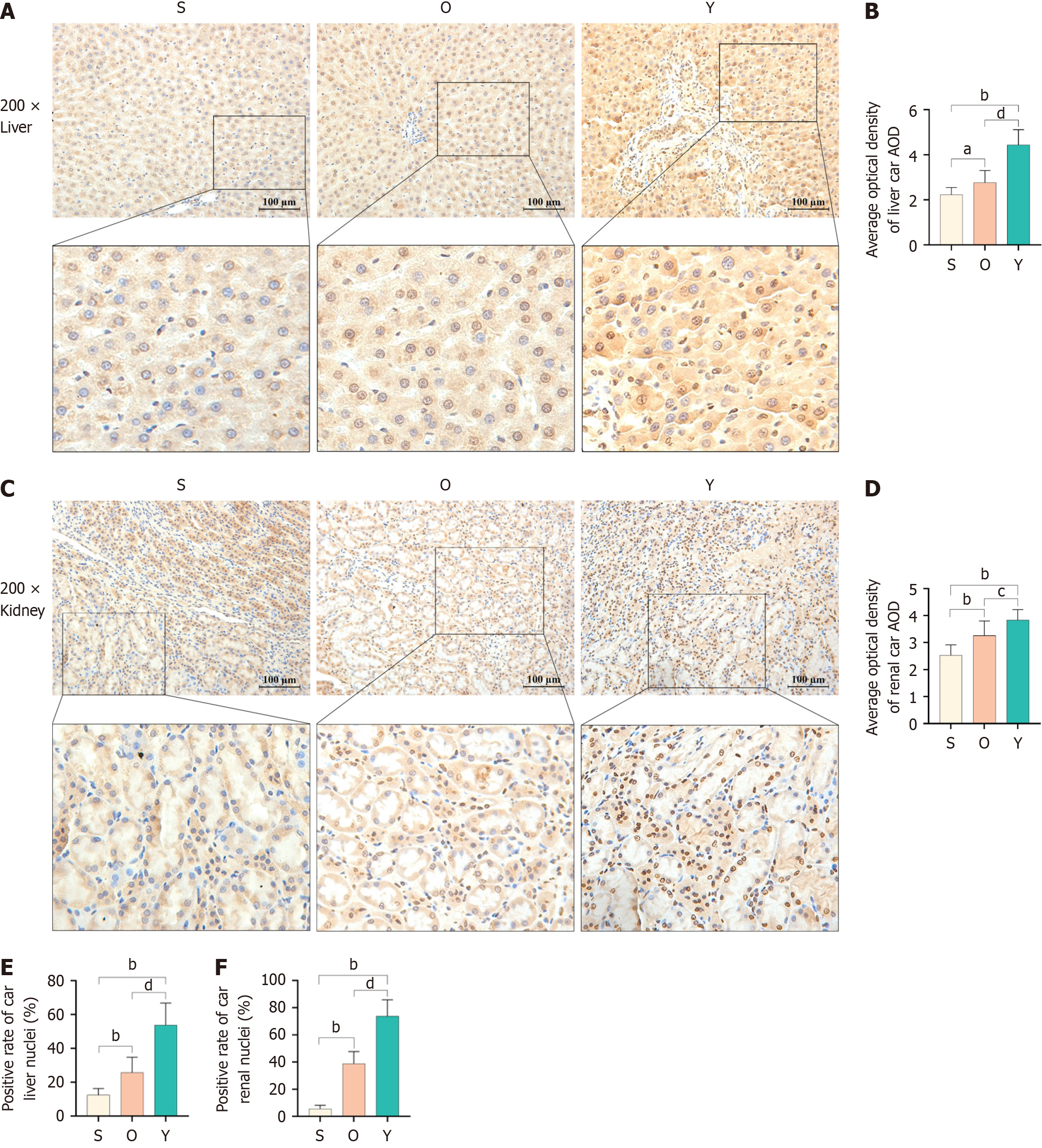
Figure 4 Effect of Yinchenhao decoction on constitutive androstane receptor expression and its nuclear heterotopy in liver and kidney tissues of rats with obstructive jaundice.
A: Immunohistochemical results of constitutive androstane receptor (CAR) in liver tissues of rats with obstructive jaundice (OJ); B: The average optical density of CAR protein in liver cells; C: Immunohistochemical results of CAR in kidney tissues of rats with OJ; D: The average optical density of CAR protein in renal tubular epithelial cells; E: The expression rate of CAR protein in hepatocyte nuclei; F: CAR protein expression rate in renal tubular epithelial cell nuclei. aP < 0.05 vs Sham (group S); bP < 0.01 vs group S; cP < 0.05 vs OJ model (group O); dP < 0.01 vs group O; AOD: Average optical density; Group O: Obstructive jaundice model; group S: Sham; Y Group: Yinchenhao decoction-treated.
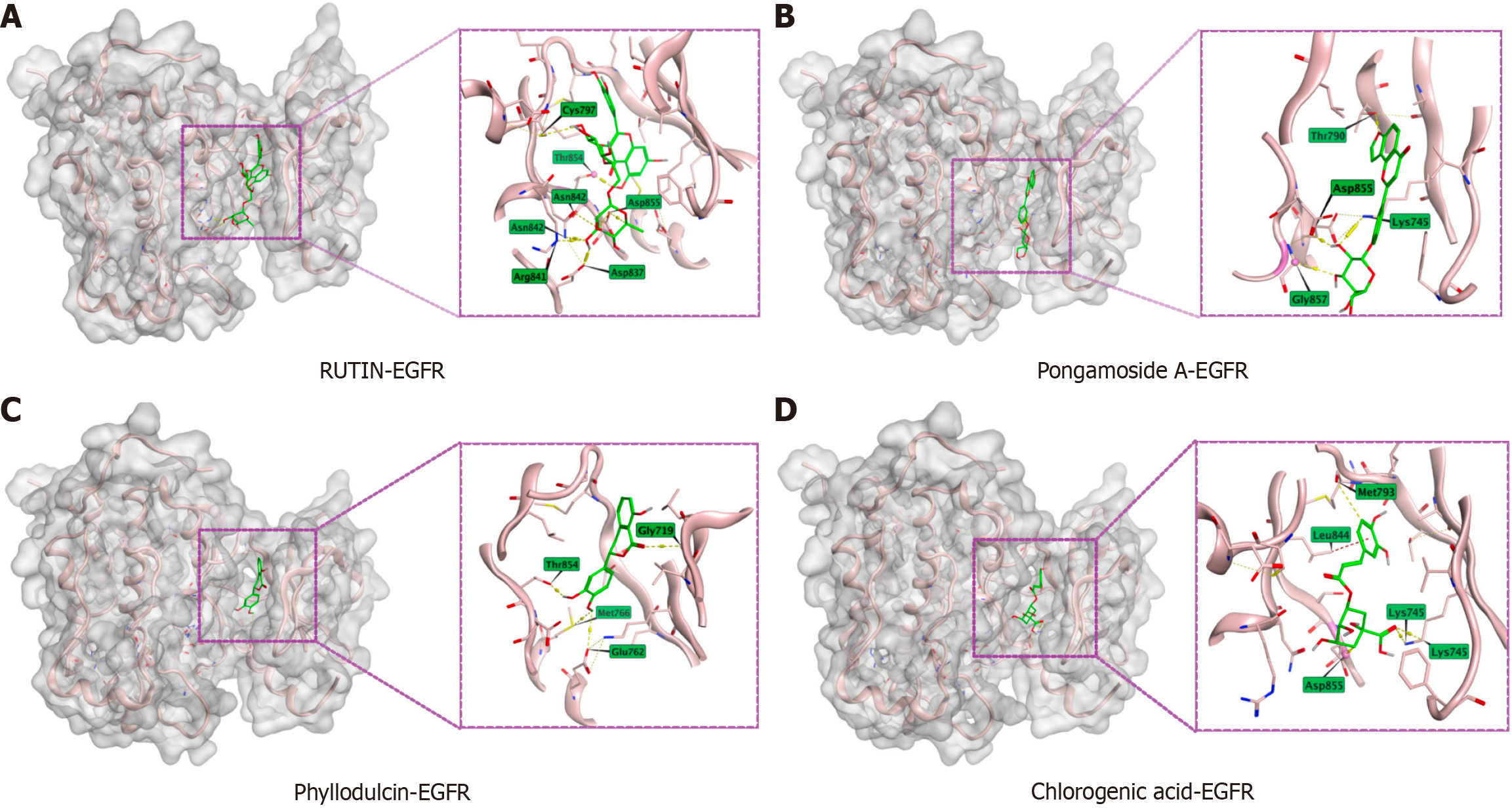
Figure 5 Results of Yinchenhao decoction enrollment component and epidermal growth factor receptor molecular docking.
Docking of epidermal growth factor receptor with RUTIN, Pongamoside A, Phyllodulcin, and Chlorogenic acid. A: RUTIN; B: Pongamoside A; C: Phyllodulcin; D: Chlorogenic acid. EGFR: Epidermal growth factor receptor.
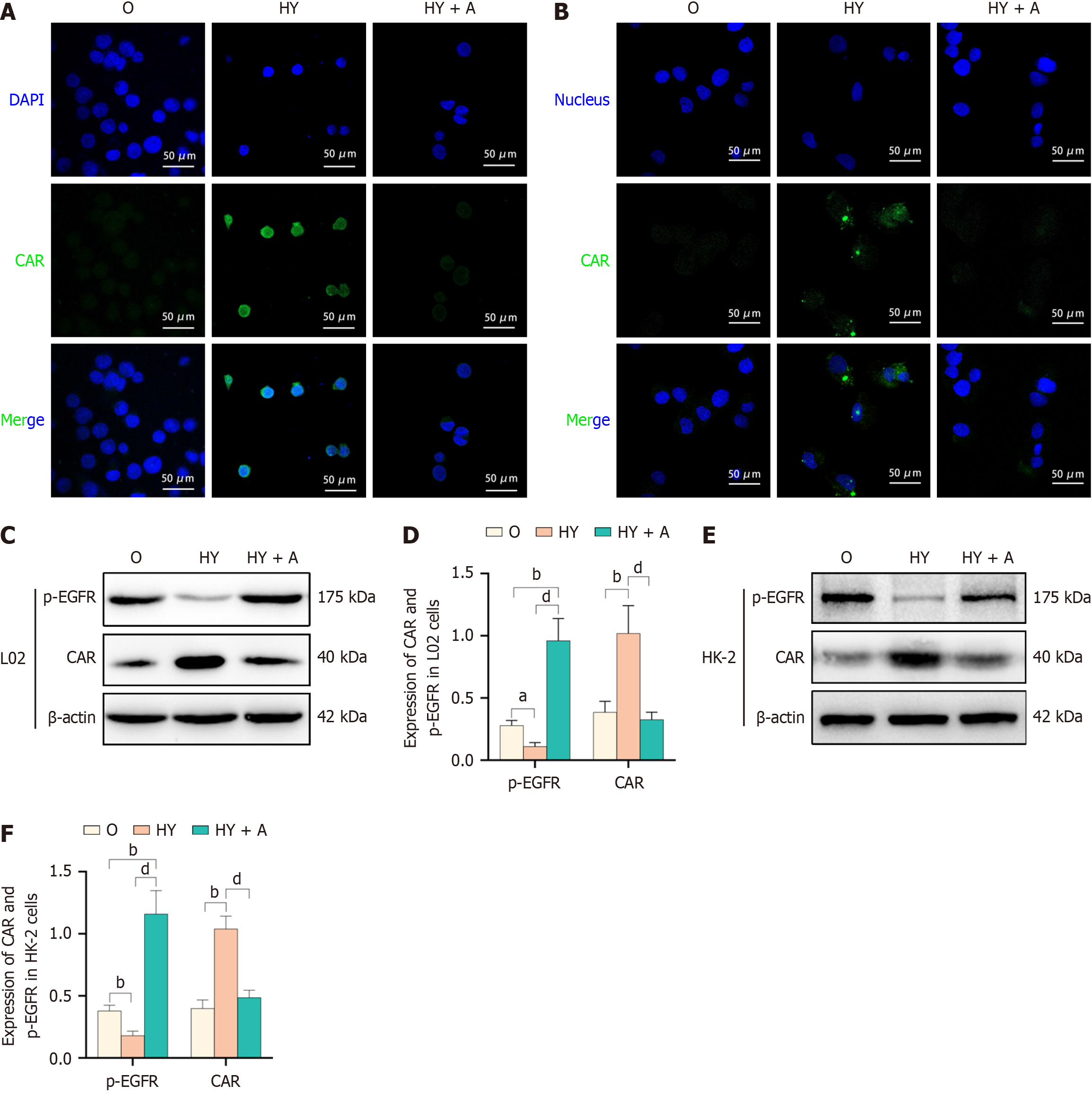
Figure 6 Yinchenhao decoction-induced regulation of constitutive androstane receptor expression and its nuclear heterotopy via epidermal growth factor receptor inhibition.
A and B: Constitutive androstane receptor (CAR) expression and its nuclear heterotopia were assessed using immunofluorescence analysis in L02 cells (A) and HK-2 cells (B); C-F: Phosphorylated p-epidermal growth factor receptor and CAR levels of expression in L02 cells (C and D) and HK-2 cells (E and F). aP < 0.05 vs Sham (group S); bP < 0.01 vs group S; cP < 0.05 vs obstructive jaundice (OJ) model (Group O); dP < 0.01 vs group O; CAR: Constitutive androstane receptor; EGFR: Epidermal growth factor receptor; HY group: 5% OJ serum + 5% Yinchenhao decoction (YCHD)-containing serum + 5% FBS; HY + A group: 5% OJ + 5% YCHD-containing serum + 5% FBS + 10 μM NSC228155; Group O: Obstructive jaundice model.
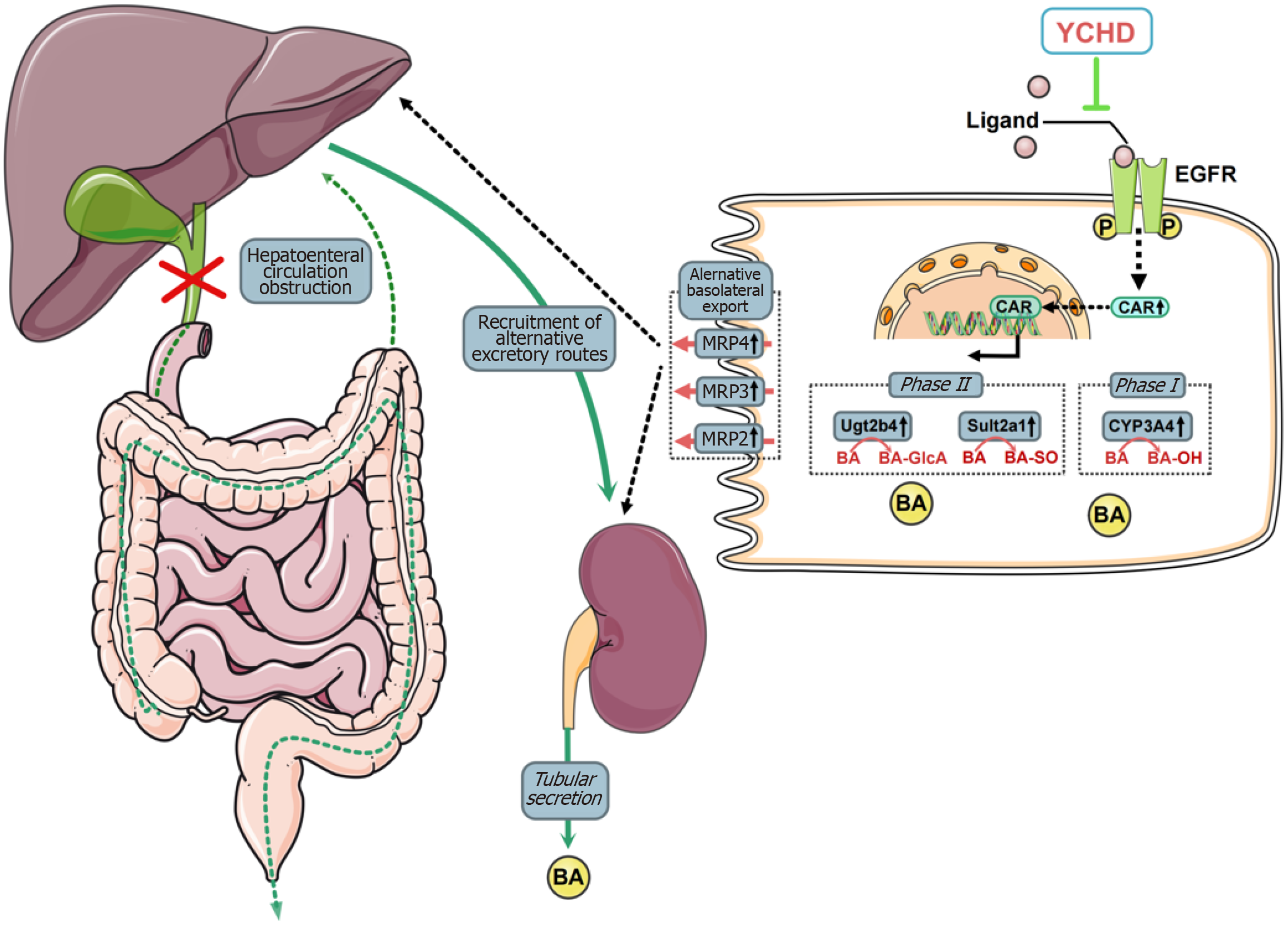
Figure 7 The schematic representation of the potential mechanisms underlying the therapeutic effects of Yinchenhao decoction in the treatment of obstructive jaundice.
Yinchenhao decoction (YCHD) enhances bile acid metabolism and active transport within liver cells and increases bile acid excretion by proximal renal tubular epithelial cells. The therapeutic effects of YCHD are likely due to its inhibition of epidermal growth factor receptor activation, which promotes constitutive androstane receptor (CAR) expression and facilitates CAR nuclear heterotopia. YCHD: Yinchenhao decoction; EGFR: Epidermal growth factor receptor.
- Citation: Liu JJ, Mei HW, Jing YY, Li ZL, Wu SG, Yuan HX, Zhang XB. Yinchenhao decoction alleviates obstructive jaundice liver injury by modulating epidermal growth factor receptor and constitutive androstane receptor signaling. World J Hepatol 2025; 17(3): 101724
- URL: https://www.wjgnet.com/1948-5182/full/v17/i3/101724.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i3.101724