©The Author(s) 2025.
World J Hepatol. Dec 27, 2025; 17(12): 113658
Published online Dec 27, 2025. doi: 10.4254/wjh.v17.i12.113658
Published online Dec 27, 2025. doi: 10.4254/wjh.v17.i12.113658
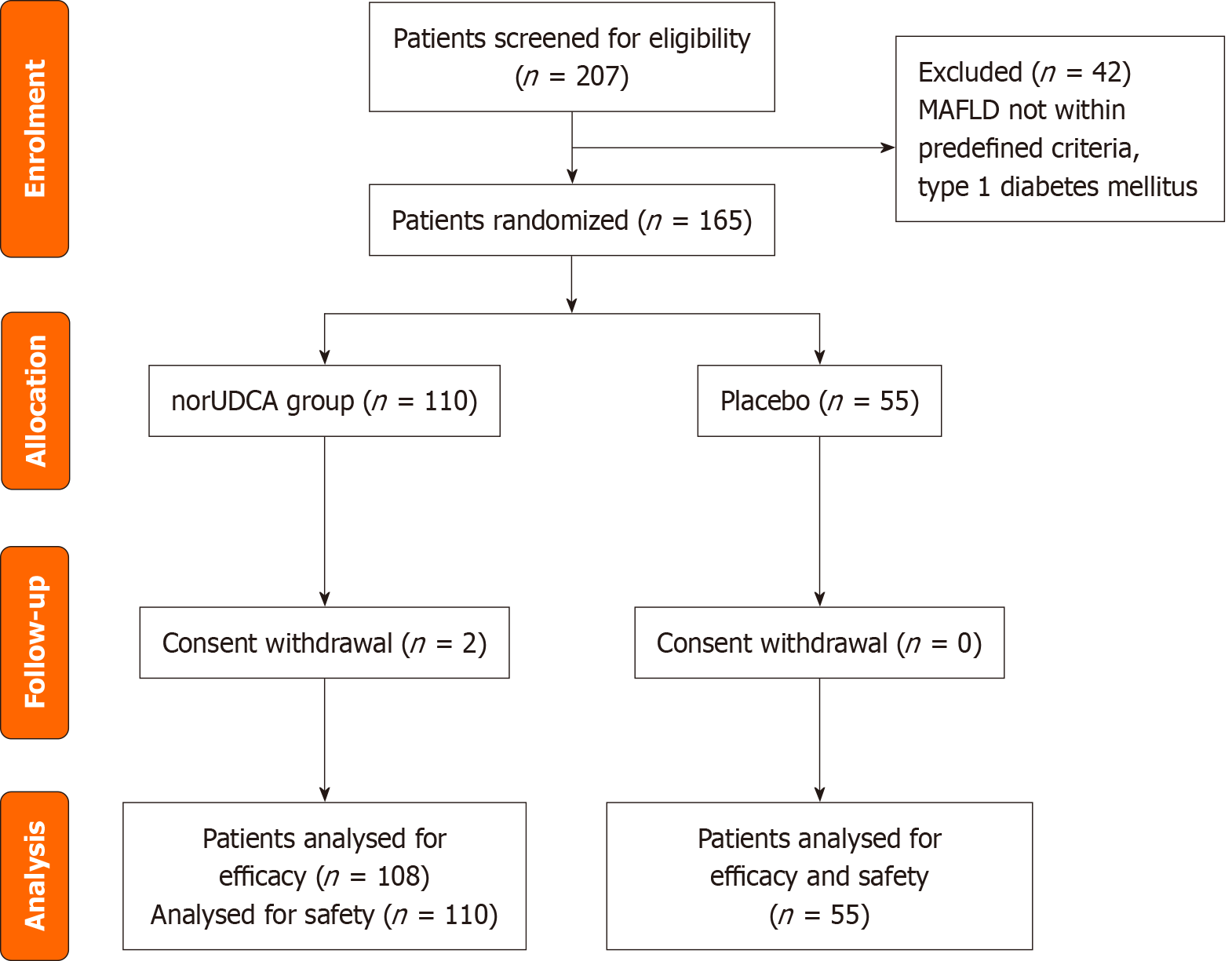
Figure 1 Consort flow chart of subject deposition.
MAFLD: Metabolic dysfunction-associated fatty liver disease; UDCA: Ursodeoxycholic acid.
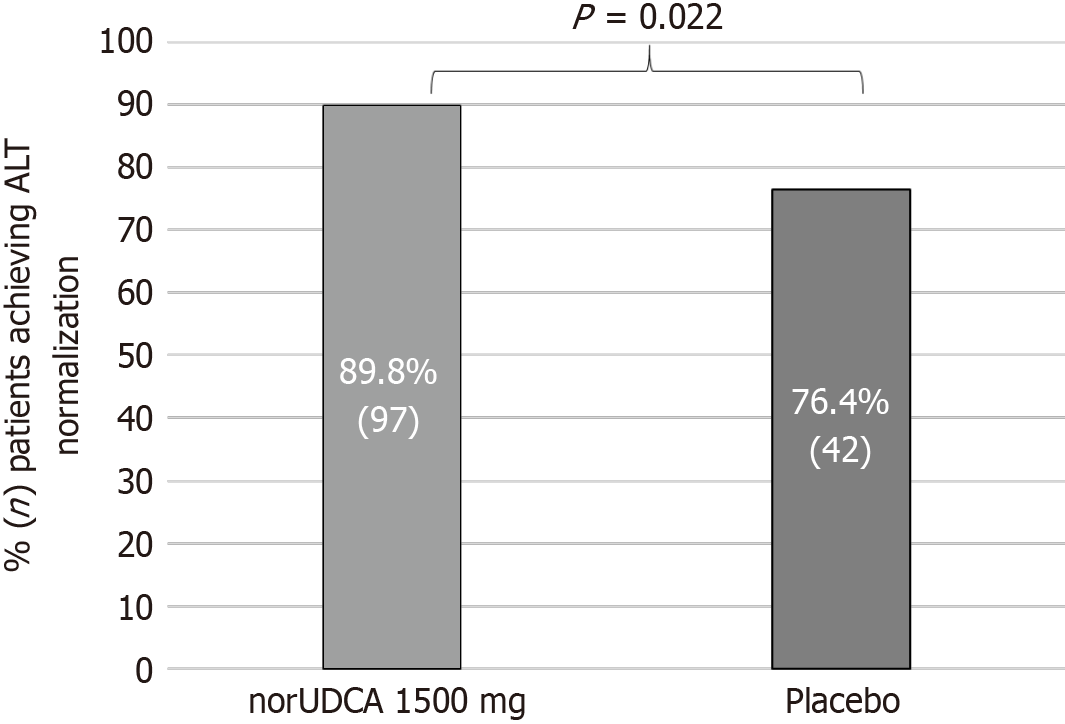
Figure 2 Summary of patients achieving alanine aminotransferase normalization at week 12.
ALT: Alanine aminotransferase; norUDCA: Norursodeoxycholic acid.
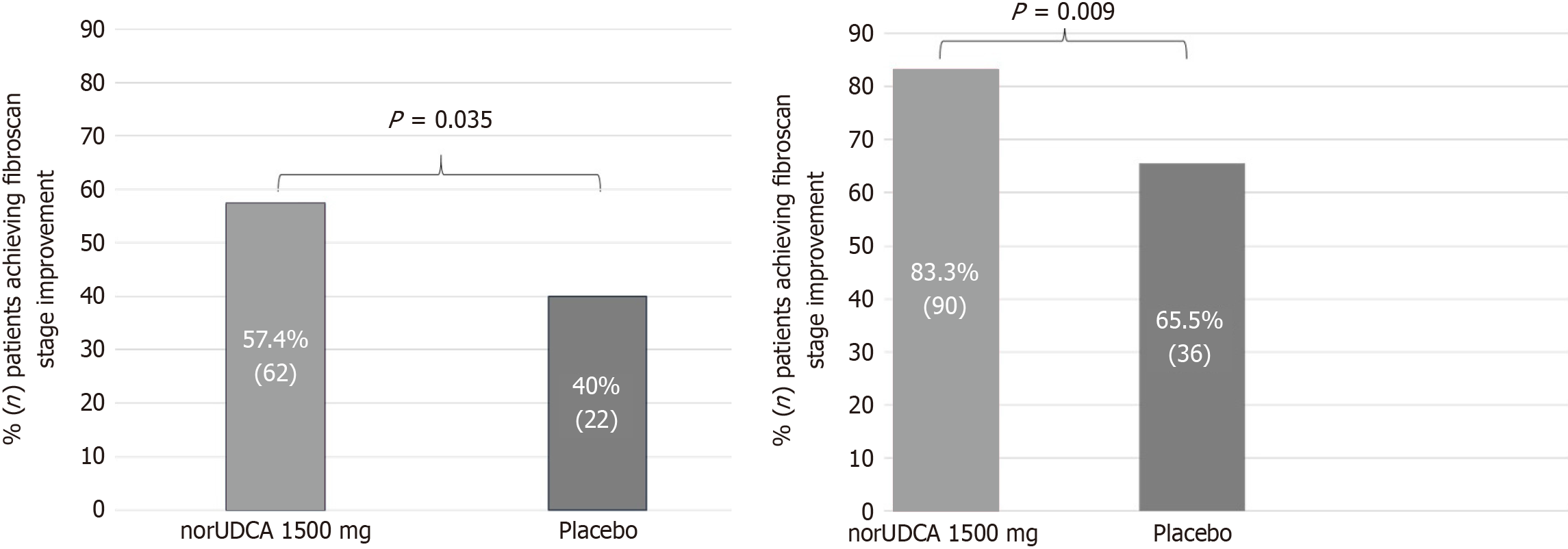
Figure 3 Summary of patients showing improvements in fibrosis.
A: At week 12; B: At week 24. norUDCA: Norursodeoxycholic acid.
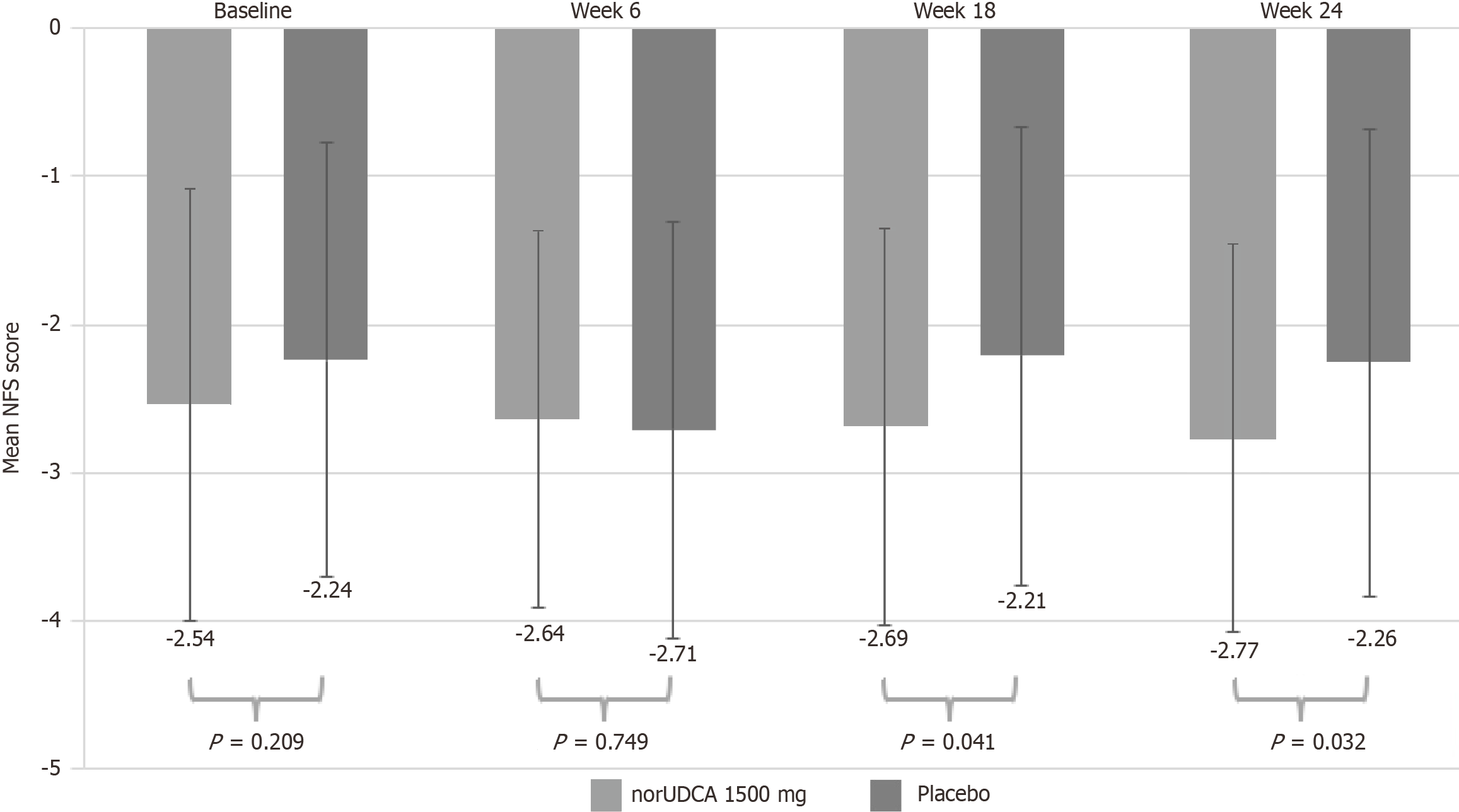
Figure 4 Mean reductions from baseline to end of treatment in nonalcoholic fatty liver disease fibrosis scores.
NFS: Nonalcoholic fatty liver disease fibrosis; norUDCA: Norursodeoxycholic acid.
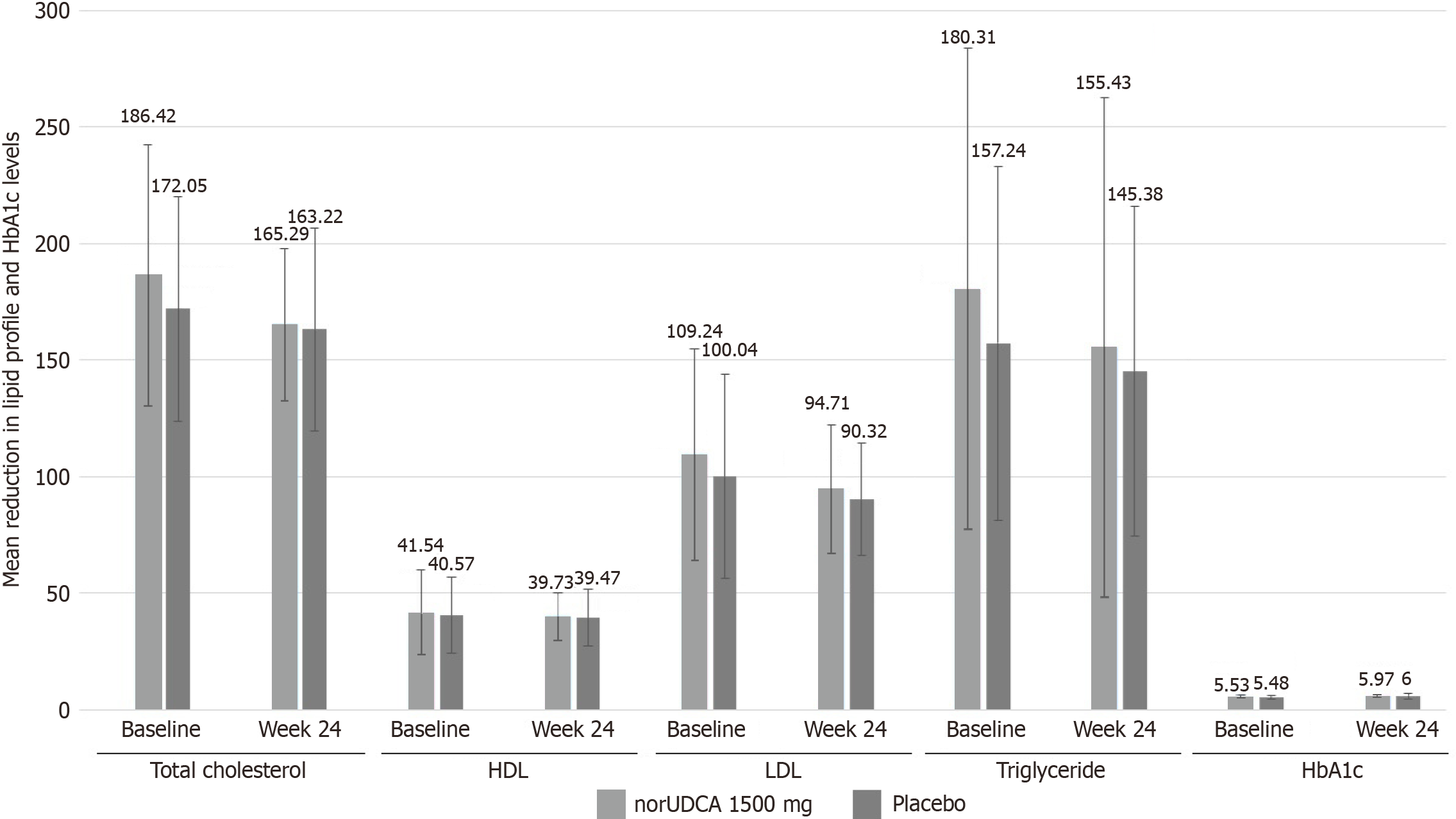
Figure 5 Mean reductions in lipid profile and glycosylated hemoglobin at baseline and end of the treatment.
HbA1c: Glycosylated hemoglobin; norUDCA: Norursodeoxycholic acid; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
- Citation: Panuganti VK, Alluri CV, Mohammad J, Dundigalla MR, Madala PK, KSSVV S, Shaik A. Phase III, multicenter, randomized, double-blind, placebo-controlled study of norursodeoxycholic acid in metabolic dysfunction-associated steatotic liver disease patients. World J Hepatol 2025; 17(12): 113658
- URL: https://www.wjgnet.com/1948-5182/full/v17/i12/113658.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i12.113658