©The Author(s) 2025.
World J Hepatol. Oct 27, 2025; 17(10): 110848
Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110848
Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110848
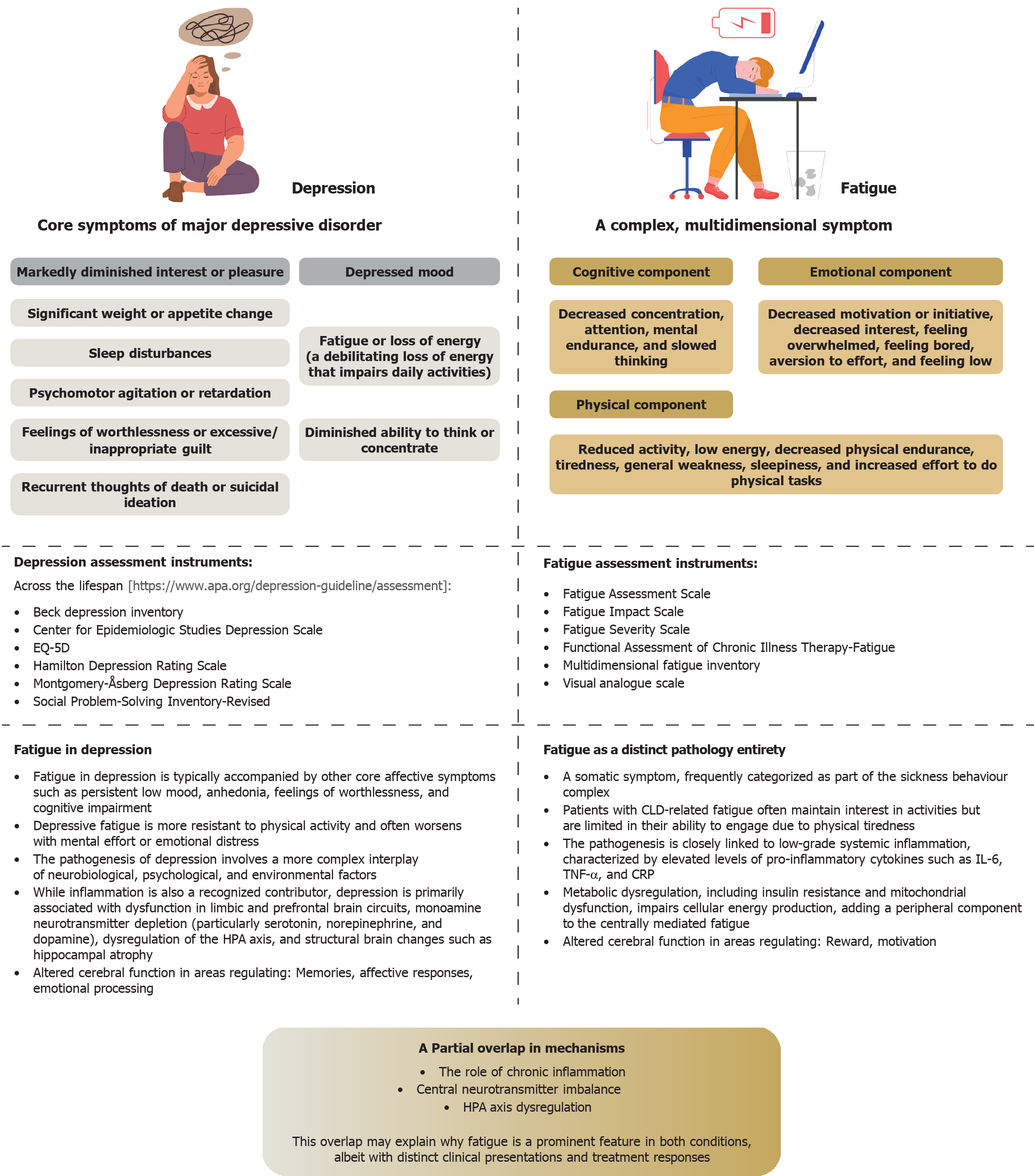
Figure 1 Differentiating fatigue from depression: Major symptoms, commonly used diagnostic tools, and key pathogenic mechanisms.
EQ-5D: European Quality of Life 5-Dimension 5-Level Questionnaire; HPA: Hypothalamic-pituitary-adrenal; CLD: Chronic liver disease; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α; CRP: C-reactive protein.
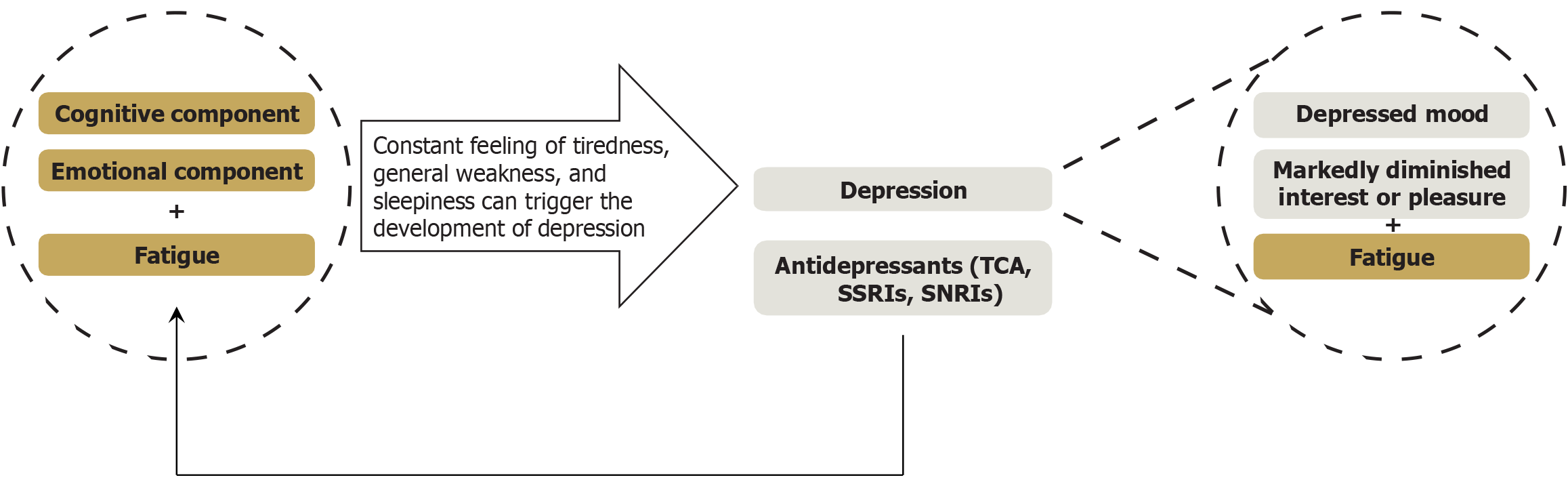
Figure 2 The relationship between fatigue and depression.
In the context of chronic liver disease, fatigue and depression are generally regarded as distinct pathological entities. However, the persistent feelings of tiredness, general weakness, and sleepiness characteristic of fatigue can precipitate the development of depression. In such cases, a patient experiencing fatigue should also exhibit either a depressed mood or markedly diminished interest or pleasure in most activities for a minimum duration of two weeks. Conversely, certain medications, such as tricyclic antidepressants, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors, can independently induce fatigue. Therefore, in patients with metabolic dysfunction-associated steatotic liver disease and depression, fatigue may be caused or exacerbated by these treatments. Under these circumstances, fatigue often manifests as a complex, multidimensional condition involving not only physical but also cognitive and emotional components. TCAs: Tricyclic antidepressants; SSRIs: Selective serotonin reuptake inhibitors; SNRIs: Serotonin-norepinephrine reuptake inhibitors.
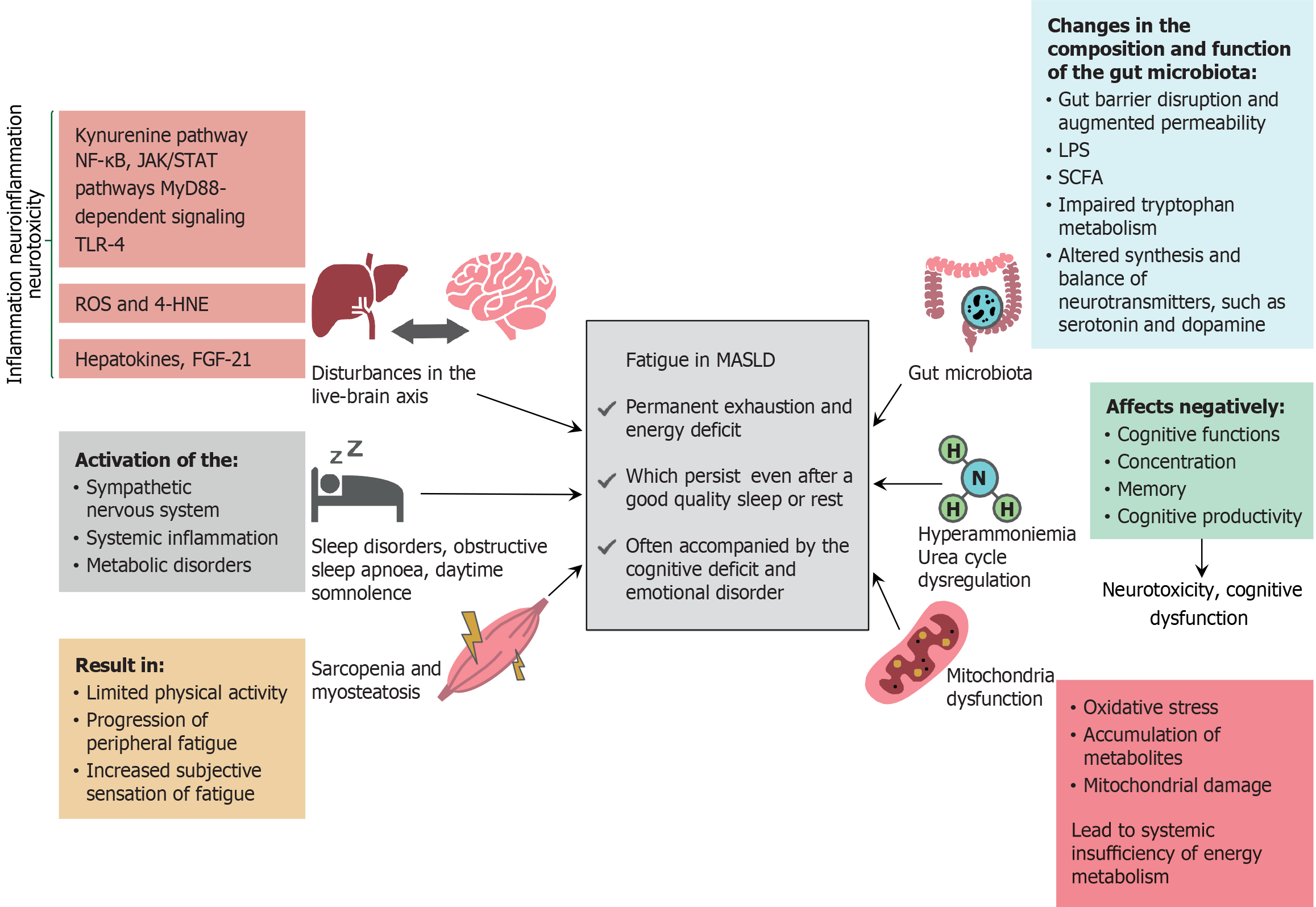
Figure 3 Pathophysiological mechanisms of fatigue in metabolic dysfunction-associated steatotic liver disease.
The pathogenesis of fatigue in metabolic dysfunction-associated steatotic liver disease is complex and may involve the following factors: (1) Dysfunction of the liver-brain axis and neuroinflammation; (2) Sleep disorders, particularly obstructive sleep apnea and daytime somnolence; (3) Skeletal muscle disorders, such as sarcopenia and myosteatosis; and (4) Hyperammonemia and urea cycle dysregulation, which may be at least partially attributed to mitochondrial dysfunction and disturbances in gut microbiota composition and function. These mechanisms contribute to systemic and neuroinflammation, alter neurotransmitter synthesis and balance, induce neurotoxicity, and impair systemic energy metabolism. All the aforementioned pathological processes may be interrelated, for example, sarcopenia may contribute to or exacerbate hyperammonemia, thus highlighting the multifactorial nature of fatigue in metabolic dysfunction-associated steatotic liver disease. NF-κB: Nuclear factor-κB; JAK/STAT: Janus kinase/signal transducers and activators of transcription; MyD88: Myeloid differentiation primary response protein 88; TLR-4: Toll-like receptor 4; ROS: Reactive oxygen species; 4-HNE: 4-hydroxynonenal; FGF-21: Fibroblast growth factor-21; MASLD: Metabolic dysfunction-associated steatotic liver disease; LPS: Lipopolysaccharide; SCFA: Short-chain fatty acid.
- Citation: Sheptulina AF, Golubeva JA, Kiselev AR, Drapkina OM. Clinical significance and pathogenic mechanisms of fatigue in metabolic dysfunction-associated steatotic liver disease. World J Hepatol 2025; 17(10): 110848
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/110848.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.110848