Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110849
Revised: July 11, 2025
Accepted: September 18, 2025
Published online: October 27, 2025
Processing time: 132 Days and 21.8 Hours
Hepatic encephalopathy (HE) affects more than 30% of patients with cirrhosis. Extrahepatic portosystemic shunt (EHPSS) has been suggested to be a con
To evaluate the effects of shunt embolization on mortality and HE recurrence.
In this retrospective case-control study, 16 cirrhotic patients with HE treated at a tertiary care center from January 2012 to August 2022 were included. Outcomes in eight patients who underwent embolization of EHPSS were compared with those in eight patients receiving standard care without embolization. Data on baseline characteristics, HE recurrence, and overall survival were collected and analyzed using Kaplan-Meier and log-rank tests.
Baseline characteristics were comparable between the groups. The 1-year overall survival rate was significantly higher in the treatment group (0.50) than in the control group (0.33). The HE recu
EHPSS embolization significantly improves 1-year survival and prevents recurrence of HE in cirrhotic patients. Routine computed tomography and early embolization are clinically beneficial.
Core Tip: Extrahepatic portosystemic shunt (EHPSS) is an underrecognized contributor to hepatic encephalopathy (HE) recurrence and mortality in patients with cirrhosis. In this study, EHPSS embolization significantly improved 1-year survival (50% vs 33%) and prevented HE recurrence (100% vs 17%) compared with no intervention. Embolization also extended both survival and recurrence-free duration. These findings highlight the importance of routine abdominal computed tomo
- Citation: Park JW, Kim Y, Lee JS, Jung IS, Kim KB, Chae HB. Improved clinical outcomes following embolization of extrahepatic portosystemic shunts in cirrhotic patients with recurrent hepatic encephalopathy. World J Hepatol 2025; 17(10): 110849
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/110849.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.110849
Hepatic encephalopathy (HE) is common in patients with chronic liver disease, with a prevalence of 20%-80% in patients with liver cirrhosis[1]. Some patients with HE experience chronic and recurrent disease, which impacts not only the quality of life of these patients but also places a burden on health economics due to high resource utilization[2]. Gut-derived neurotoxins are not removed by the liver owing to vascular shunting; decreased hepatic mass allows these toxins to reach the brain. Many of these patients have spontaneous portosystemic shunts that are often large enough to divert a major portion of the portal blood flow[2-4]. Previous reports have suggested that 40%-70% of patients with refractory HE demonstrate an extrahepatic portosystemic shunt (EHPSS) upon radiological screening[5]. These shunts were regarded as a compensatory safety valve in progressive portal hypertension, diverting blood flow and thereby partially decom
An et al[7] further corroborated these findings, showing that EHPSS embolization not only reduced HE recurrence but also enhanced liver function and overall survival of patients with relatively preserved hepatic reserve (MELD score < 15). However, an improvement in overall survival was observed only in a limited subgroup within the overall study population. They emphasized the importance of timely intervention and restoration of hepatic portal perfusion to mitigate the effects of portosystemic shunting. Moreover, recent studies, including meta-analysis by Song et al[8], have confirmed the therapeutic benefit of EHPSS embolization in reducing HE recurrence, and supported its role as an effective treatment in selected cirrhotic patients[9,10]. However, the survival benefit of the procedure was not well demonstrated. In this study, we aimed to compare the therapeutic efficacy and safety between embolization and control groups and to retrospectively determine the therapeutic efficacy and safety of embolization.
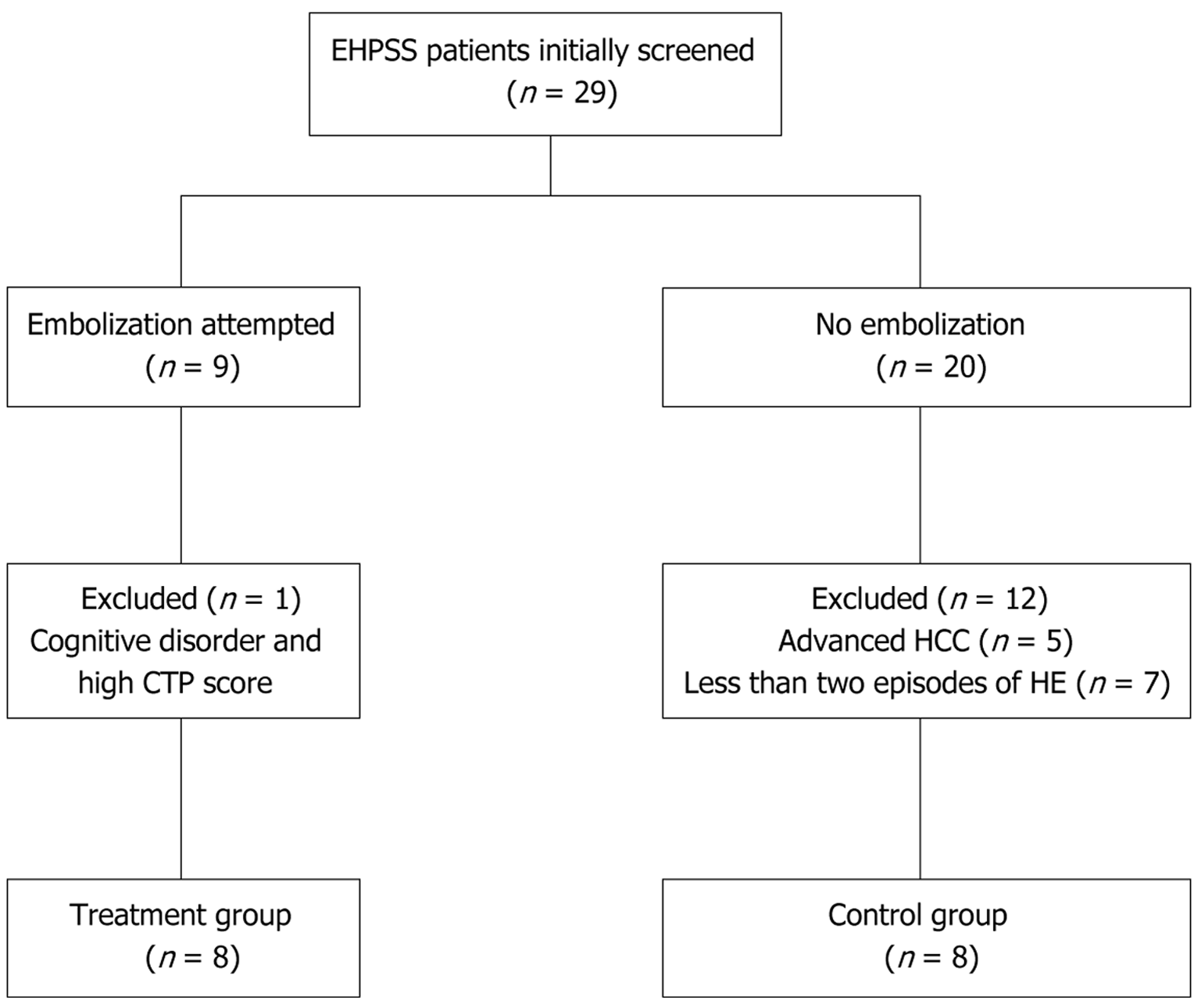
Nine patients underwent portosystemic shunt embolization between January 2012 and August 2022. Patients in the treatment group experienced more than two episodes. Twenty patients in the control group were also screened during the same period. We retrospectively reviewed the patients’ medical records. This study was approved by the Institutional Review Board of Chungbuk National University Hospital, and the requirement for written informed consent was waived.
The inclusion criteria were: History of admission, age between 19 and 80 years, liver cirrhosis with not less than two episodes of HE, and early-stage and cured hepatoma. The exclusion criteria were: Age < 19 or > 80 years, Child-Turcotte-Pugh (CTP) score > 13, advanced-stage hepatoma (not less than stage II), large esophago-gastric varices (grade > 2), recipient with surgical portocaval shunt, transjugular intrahepatic portal systemic shunt, liver transplantation, and other cognitive disorders such as cerebrovascular accident, Parkinson’s disease, and dementia.
Each episode of HE was graded with the West Haven criteria[11]. Only episodes classified as grade 2 or higher were counted, and patients who had at least one such episode were eligible for inclusion. Because the study population consisted solely of individuals with overt HE, psychometric and neurophysiological assessments were not performed. Grade 2 HE was defined by the presence of one or more of the following: Disorientation in time, place, or person; bizarre behavior; obvious asterixis; or impaired serial-subtraction performance. All participants underwent portal-venous-phase multidetector computed tomography to identify and characterize portosystemic shunts. Extra-hepatic shunts considered relevant were splenorenal communications and recanalized para-umbilical veins. Shunt embolization was considered eligible in patients with an EHPSS diameter greater than 8 mm. Before embolization, every patient received optimized medical therapy - including correction of precipitating factors and administration of lactulose and/or rifaximin - acc
EHPSS embolization was attempted angiographically in nine patients; the shunts were completely occluded in all patients (treatment group). One 31-year-old female patient was excluded from the treatment group due to other cognitive disorders and a CTP score of 13. Twenty patients who did not undergo EHPSS embolization were initially screened. Five of these patients were excluded because they had advanced hepatocellular carcinoma; the other seven were excluded because they had less than two episodes of HE. Finally, eight patients were included in the control group (Figure 1). The baseline was defined as the date of the first shunt in the treatment group and the date of the second hospitalization at our institution for the management of the control group. The patients were followed up for survival, HE recurrence, liver function parameters, and liver volume. The HE recurrence was defined as hospitalization or emergency room visits for management. The occurrence or worsening of ascites or esophagogastric varices was also assessed. All clinical and labo
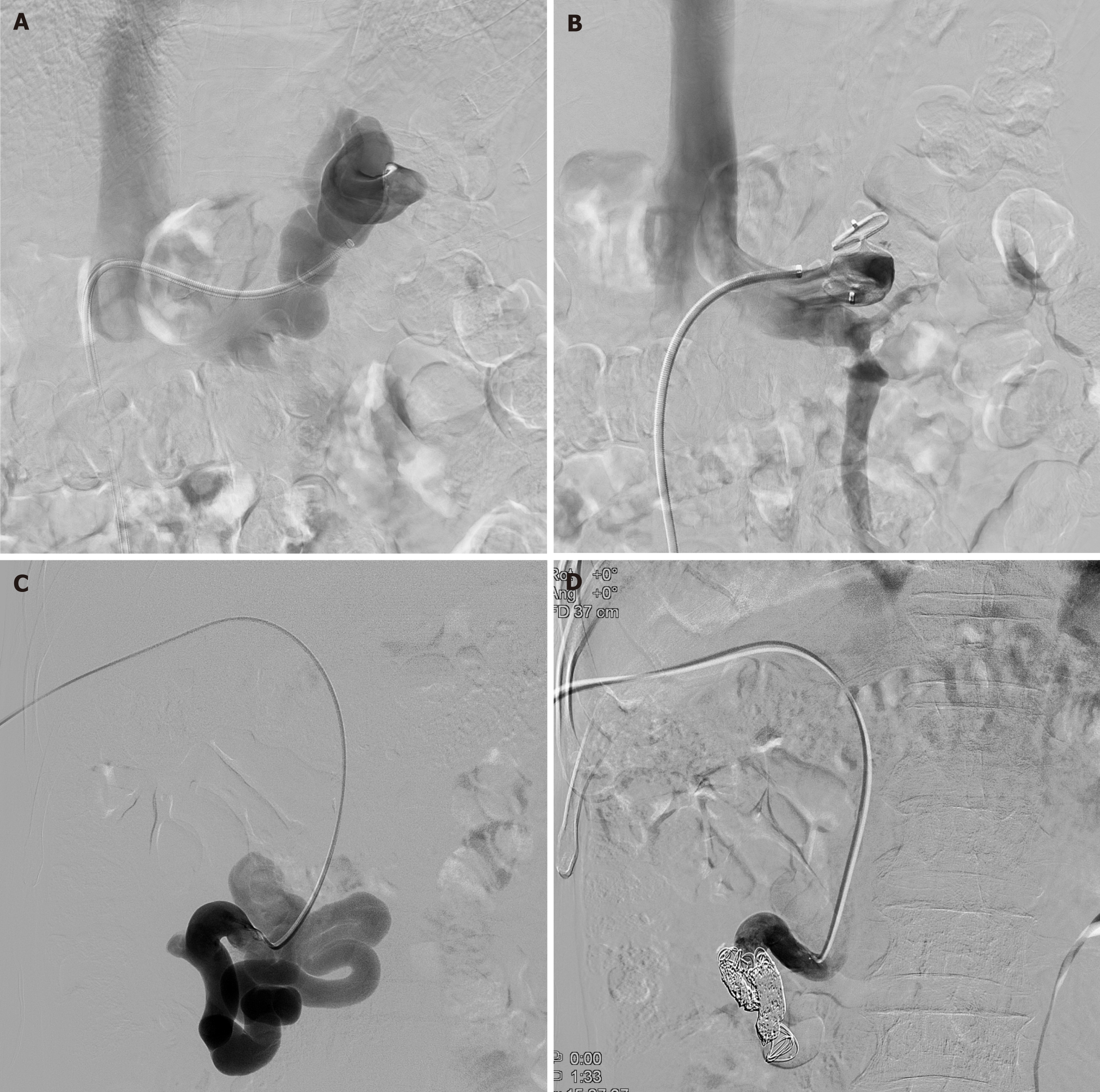
In all cases, large (20-40 mm) shunts were suspected on computed tomography and confirmed via arteriography. Ultrasonography revealed splenorenal shunts. Angiographic access for embolization was performed via the femoral vein in seven splenorenal and one mesocaval shunts. All procedures were carried out under local anesthesia. Shunt occlusion was achieved with either an Amplatzer vascular plug (AVP) (Medical, Golden Valley, MN, United States) or a combination of detachable coils and gelatin-sponge (Gelfoam, Upjohn, Kalamazoo, MI, United States) with up to six steel coils of 5-10 mm in diameter. The coils were placed within the sinus lumen through a femoral catheter in all patients.
All patients underwent initial diagnostic angiography under local anesthesia and analgesics via the transfemoral approach in four splenorenal shunts and the transhepatic approach in four mesocaval shunts using a 5-F vascular sheath. The shunts were catheterized with a 5-F angiographic catheter (Cobra, Cook, Bloomington, IN, United States) and a 2.4-F microcatheter (Progreat, Terumo, Somerset, NJ, United States) in four patients, whereas a 9-F guiding sheath (Flexor Ansel, Cook Medical, Bloomington, IN, United States) was introduced to deploy the vascular plug in four patients. Embolization was performed using vascular plugs (AVP; Medical, Golden Valley, MN, United States) or coils (Interlock, Boston Scientific Co., Natick, MA, United States) combined with gelatin sponges (Gelfoam, Upjohn, Kalamazoo, MI, United States). The AVP II was utilized. Furthermore, correctly sized detachable coils (average 11.1 ± 5.3, range 5-22) were deployed.
The primary outcome was overall survival. The secondary outcome was the recurrence of overt HE for 3 months. HE of grade 2 or higher was considered a recurrence, regardless of the presence of an identifiable precipitant. Changes in liver function, plasma ammonia levels, and safety laboratory tests were evaluated before and after the procedure. MELD scores and CTP scores were assessed before and 1 year after the shunt embolization.
Since the data did not follow a normal distribution and sample sizes of both the treatment (n = 8) and control groups (n = 8) were small, a non-parametric statistical analysis was applied. Categorical variables were compared and analyzed using Fisher’s exact test, and continuous variables were compared using the Mann-Whitney U test. Kaplan-Meier analyses were performed for survival and cumulative HE recurrence rates, and differences between the two groups were compared and tested using the log-rank test. The Cox proportional hazards model was used to identify the risk factors associated with patient mortality and HE recurrence. The variables included in the Cox hazard model were group (treatment vs control), age, sex, previous HE episodes within 3 months, HE grades (II vs III/IV), albumin, total bilirubin, creatinine, PT-international normalized ratio, CTP score (B vs C), and MELD score. Statistical significance was defined as P < 0.05; all analyses were performed using R version 4.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
The baseline characteristics of the treatment (n = 8) and control (n = 8) groups were comparable across all demographic and biochemical parameters (Table 1). The etiology of cirrhosis in the treatment group included four, one, and two cases of alcoholic cirrhosis, autoimmune hepatitis, and cryptogenic disease, respectively. The control group had one case of hepatitis B virus and seven cases of alcoholic cirrhosis, respectively. The median age was 63 years (range, 45-74) in the treatment group and 57 years (range, 42-71) in the control group (P = 0.27). The incidence of esophageal varices was 50% in the treatment group and 87.5% in the control group (P = 0.11). Albumin levels were similar between the groups, measuring 3.10 g/dL (range, 2.85-3.25) in the treatment group vs 3.15 g/dL (range, 2.75-3.55) in the control group (P = 0.64). The bilirubin levels were 2.51 mg/dL (range, 0.26-4.76) and 2.88 mg/dL (range, 0.85-4.91) in the treatment and control groups, respectively (P = 0.35). Creatinine levels were 0.93 mg/dL (range, 0.48-1.38) in the treatment group and 1.38 mg/dL (range, 0.17-2.59) in the control group (P = 0.67). The international normalized ratio was 1.42 (range, 1.12-1.72) in the treatment group and 1.41 (range, 1.22-1.60) in the control group (P = 0.875). The MELD scores were 11.08 (range, 5.32-16.84) vs 14.08 (range, 7.57-20.59) (P = 0.25). The CTP scores in the two groups were 8.88 (range, 7.33-10.43) vs 9.38 (range, 8.08-10.68) (P = 0.43).
| Group | Control group (n = 8) | Treatment group (n = 8) | P value |
| Age (years) | 57 (42-71) | 63 (45-74) | 0.269 |
| Male | 6 (75.0) | 3 (37.5) | 0.143 |
| Etiology | |||
| HBV | 1 (12.5) | 0 | 0.044 |
| Alcohol | 7 (87.5) | 5 (62.5) | |
| Others | 0 | 3 (37.5) | |
| Number of HE episodes during the previous 12 months | |||
| 1-2 | 4 (50.0) | 5 (62.5) | 0.626 |
| ≥ 3 | 4 (50.0) | 3 (37.5) | |
| HE grades | |||
| II | 4 (50.0) | 5 (62.5) | 0.626 |
| III-IV | 4 (50.0) | 3 (37.5) | |
| Esophageal varix (%) | 87.5 | 50.0 | 0.106 |
| Albumin (g/dL), median (IQR) | 3.15 (2.75-3.55) | 3.10 (2.85-3.25) | 0.635 |
| Bilirubin (mg/dL), median (IQR) | 2.88 (0.85-4.91) | 2.51 (0.26-4.76) | 0.345 |
| Creatinine (mg/dL), median (IQR) | 1.38 (0.17-2.59) | 0.93 (0.48-1.38) | 0.674 |
| INR, median (IQR) | 1.41 (1.22-1.60) | 1.42 (1.12-1.72) | 0.875 |
| MELD × 10, median (IQR) | 14.08 (7.57-20.59) | 11.08 (5.32-16.84) | 0.248 |
| CTP score, median (IQR) | 9.38 (8.08-10.68) | 8.88 (7.33-10.43) | 0.431 |
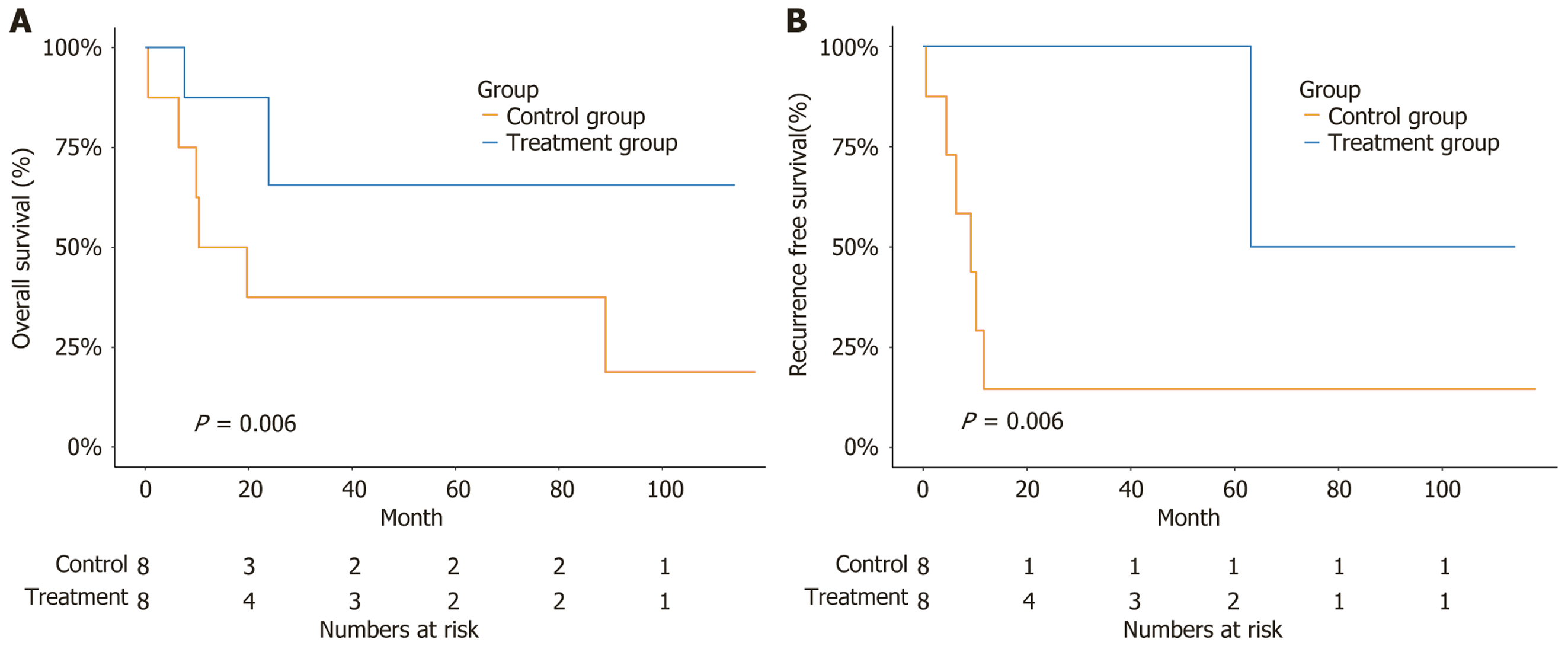
The 1-year overall survival rate was significantly higher in the treatment group than in the control group (0.50 vs 0.33). The median survival duration was longer in the treatment group than in the control group {not reached (NR) [95% confidence interval (CI): 23.84 to not available (NA)] vs 15.02 months (95%CI: 9.86 to NA)} (P = 0.006) (Figure 2A). The recurrence-free rate was significantly higher in the treatment group than in the control group (1.00 vs 0.17). The recurrence-free duration was longer in the treatment group than in the control group [63.09 months (95%CI: 63.09 to NR) vs 9.21 months (95%CI: 4.47 to NR)] (P = 0.006) (Figure 2B).
Using univariate analysis, EHPSS embolization treatment was a significant predictor of reduced HE recurrence in all patients [hazard ratio (HR) = 0.09; 95%CI: 0.01-0.75; P = 0.026]. The presence of severely decompensated cirrhosis - CTP C was a significant predictor of HE recurrence (HR = 5.61; 95%CI: 1.02-30.9; P = 0.048). No significant predictors of mortality were identified in univariate analysis (Table 2). Because only a limited number of HE-recurrence events occ
| Characteristic | Mortality | HE recurrence | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Group | ||||||
| Control group | - | - | - | - | ||
| Treatment group | 0.30 | 0.06-1.49 | 0.139 | 0.09 | 0.01-0.75 | 0.026a |
| Age (years) | 0.97 | 0.91-1.04 | 0.430 | 0.95 | 0.88-1.02 | 0.147 |
| Sex | ||||||
| Female | - | - | - | - | ||
| Male | 1.45 | 0.34-6.15 | 0.611 | 1.54 | 0.34-6.99 | 0.573 |
| Number of HE episodes during the previous 3 months | 0.00 | 0.00, infinity | 0.999 | 0.98 | 0.34-2.89 | 0.978 |
| HE grades | ||||||
| II | - | - | - | - | ||
| III/IV | 0.77 | 0.18-3.24 | 0.721 | 2.86 | 0.55-15.0 | 0.213 |
| Albumin | 0.35 | 0.09-1.37 | 0.132 | 0.98 | 0.19-5.06 | 0.979 |
| Total bilirubin | 1.13 | 0.80-1.61 | 0.479 | 1.20 | 0.88-1.64 | 0.245 |
| Creatinine | 0.71 | 0.24-2.10 | 0.531 | 1.12 | 0.55-2.28 | 0.760 |
| INR | 3.07 | 0.15-61.8 | 0.464 | 0.92 | 0.03-25.3 | 0.961 |
| MELD | 0.99 | 0.32-3.08 | 0.991 | 1.19 | 0.39-3.67 | 0.758 |
| CTP class | ||||||
| B | - | - | - | - | ||
| C | 2.35 | 0.50-11.0 | 0.277 | 5.61 | 1.02-30.9 | 0.048a |
Four patients with splenorenal shunts were approached via the transfemoral route; a coil was used for embolization in three of them, and a vascular plug was used in one patient. Four patients with mesocaval shunts were approached transhepatically; a coil was used for embolization in two patients, and a vascular plug was used in the remaining two patients. Embolization was successful in all patients during the first attempt. No procedure-related complications occurred. No encephalopathy or variceal bleeding was observed after embolization. The transfemoral approach was used in four splenorenal patients, and the transhepatic approach was used in four patients with mesocaval shunts (Figure 3). Plasma ammonia levels decreased substantially after embolization, with the median falling from 161.5 μmol/L to 86 μmol/L (Table 3).
| Subject number | Plasma ammonia level before embolization | Plasma ammonia level after embolization | ||
| Date of measurement | Lab result (μmol/L)1 | Date of measurement | Lab result (μmol/L)1 | |
| 1 | August 10, 2023 | 100 | August 17, 2023 | 92 |
| 2 | May 10, 2022 | 130 | February 17, 2022 | 90 |
| 3 | May 22, 2021 | 218 | June 16, 2021 | 60 |
| 4 | December 26, 2020 | 213 | December 29, 2020 | 95 |
| 5 | January 17, 2020 | 143 | March 12, 2020 | 118 |
| 6 | September 8, 2017 | 180 | October 11, 2017 | 62 |
| 7 | April 4, 2014 | 237 | April 18, 2014 | 45 |
| 8 | June 27, 2023 | 83 | July 27, 2023 | 82 |
| Median (161.5) | Median (86) | |||
| mean ± SD (163.0 ± 57.5) | mean ± SD (80.5 ± 23.5) | |||
HE is a debilitating complication of cirrhosis. Alteration of mental status leads to the inability to make correct judgments and eventual job loss. Standard medical treatments cannot prevent recurrence. The mainstays of treatment include non-absorbable disaccharides such as lactulose and antibiotics such as rifaximin, which decrease ammonia production in the gut[12]. These treatments effectively reduce the incidence and severity of HE, improve patient outcomes, and reduce healthcare costs[13]. There remains an unmet need for a more concrete or definitive treatment method before liver transplantation[13]. A retrospective cohort study of 1729 patients showed that EHPSS were linked to higher rates of gas
Our study showed that the embolization procedure could reduce overall mortality and HE recurrence. The 1-year survival rates in the embolization and control groups were 75% and 38%, respectively. No safety concerns were observed in the embolization group. These findings align with those in previous reports. Ke et al[14] demonstrated improved HE-free survival following embolization in a multicenter cohort using an inverse probability of treatment weighting analysis, with safety confirmed in appropriately selected patients. Similarly, Rajesh et al[15] showed 1-year survival of 87% and a significant reduction in HE recurrence. However, concerns remain that occluding a large shunt may acutely raise portal pressure, thereby aggravating ascites or gastroesophageal varices and increasing the risk of variceal bleeding in a subset of patients, which may necessitate the addition of non-selective β-blockers or prophylactic endoscopic therapies. Accordingly, the worldwide uptake of EHPSS embolization as routine therapy is still limited, and further prospective, controlled studies are needed to define which patients derive net benefit[2].
Our findings are in agreement with those in earlier investigations, demonstrating that neurological symptoms and HE recurrence were reduced after treatment when shunt embolization was combined with standard therapy for recurrent HE accompanied by EHPSS[16]. An et al[7] reported that embolization prevented HE recurrence and improved survival in patients with preserved liver function, whereas no benefit was observed in those with advanced liver dysfunction. Seven patients showed poor outcomes after embolization, with four deaths due to sepsis or variceal bleeding, and only two patients survived, one of whom required liver transplantation[5]. A European multicenter study involving 37 patients found that embolization of EHPSS controlled HE in 60% of cases at three months; half of the cohort maintained an HE-free status two years after the procedure[2]. Two predictors may affect recurrence. Severely decompensated cirrhosis - CTP C and embolization were significant predictors of HE recurrence in both patient groups. Many studies have shown that hepatic reserve function is an important predictor of therapeutic outcomes[2,7,17].
In this study, embolization was performed by an interventionist at the authors’ institution. This procedure was successful on the first attempt, after which no early or late procedure-related complications were observed. There were two types of EHPSS: Four splenorenal and four mesocaval shunts. We suggest that the findings in our study were more consistent and robust than those reported by other institutions, because variations in interventional expertise may have biased treatment outcomes. The types of shunts were diverse in each study. The most common type was splenorenal, ranging between 21% and 100%[18-21].
In the embolization group, one patient was ineligible due to a high CTP score. This patient, who showed HE recurrence after embolization, already had six HE episodes in the year before embolization, a MELD score of 14.71, and a CTP score of 13. The effectiveness of shunt embolization may be reduced in patients with HE; this might have severely reduced baseline liver function. This study confirmed that the prognosis after embolization in patients with liver cirrhosis and HE symptoms could be predicted based on preprocedural hepatic reserve function. Univariate analysis showed that severely decompensated cirrhosis - CTP C and embolization were significant predictors of HE recurrence in all patients. Several previous studies have suggested that the effectiveness of embolization might decrease depending on the baseline cirrhosis status before shunt embolization. An et al[7] analyzed patients with MELD score < 15 and no hepatocellular carcinoma, finding a markedly superior survival rate in the embolization group (100% vs 60%) among those who did not undergo the procedure. Inoue et al[19] reported that serum albumin levels < 2.8 mg/dL were linked to decreased sur
This study has several limitations. First, the small sample size of eight patients in each group reflects the rarity of HE due to EHPSS and might limit the generalizability of our findings. Hence, this issue requires further study, especially in prospective controlled trials. A prospective study would require approximately 33 patients to detect a significant difference in 6-month recurrence-free survival rates of 45% in the control group and 22% in the embolization group. Second, as a single-center study conducted in a medium-sized tertiary hospital, external validity might be limited. Third, although all procedures were performed by a single interventionist, this ensured consistency in technique throughout the study. Given that the procedure does not require highly specialized equipment or exceptional expertise, it may be reproducible in similar clinical settings. Finally, the retrospective design introduces potential selection bias. Nonetheless, we applied strict and consistent inclusion criteria and matched controls by age, sex, and CTP class to improve comparability and reduce confounding.
Shunt embolization improves 1-year survival and HE recurrence-free rates in patients with cirrhosis. Embolization and baseline decompensated cirrhosis are associated with HE recurrence. Early detection and timely embolization of EHPSS might improve outcomes in selected patients.
We would like to thank Dr. Suk Ran Kim for her work in editing this manuscript.
| 1. | Adams RD, Foley JM. The neurological disorder associated with liver disease. Res Publ Assoc Res Nerv Ment Dis. 1953;32:198-237. [PubMed] |
| 2. | Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, Villalba J, Garcia-Pagan JC, Barrufet M, Jalan R, Brookes J, Thalassinos E, Burroughs AK, Cordoba J, Nevens F; EASL-CLIF-Consortium. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57:2448-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Riggio O, Efrati C, Catalano C, Pediconi F, Mecarelli O, Accornero N, Nicolao F, Angeloni S, Masini A, Ridola L, Attili AF, Merli M. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology. 2005;42:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Ohnishi K, Sato S, Saito M, Terabayashi H, Nakayama T, Saito M, Chin N, Iida S, Nomura F, Okuda K. Clinical and portal hemodynamic features in cirrhotic patients having a large spontaneous splenorenal and/or gastrorenal shunt. Am J Gastroenterol. 1986;81:450-455. [PubMed] |
| 5. | Zidi SH, Zanditenas D, Gelu-Siméon M, Rangheard AS, Valla DC, Vilgrain V, Pelletier GM. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int. 2007;27:1389-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Simón-Talero M, Roccarina D, Martínez J, Lampichler K, Baiges A, Low G, Llop E, Praktiknjo M, Maurer MH, Zipprich A, Triolo M, Vangrinsven G, Garcia-Martinez R, Dam A, Majumdar A, Picón C, Toth D, Darnell A, Abraldes JG, Lopez M, Kukuk G, Krag A, Bañares R, Laleman W, La Mura V, Ripoll C, Berzigotti A, Trebicka J, Calleja JL, Tandon P, Hernandez-Gea V, Reiberger T, Albillos A, Tsochatzis EA, Augustin S, Genescà J; Baveno VI-SPSS group from the Baveno Cooperation. Association Between Portosystemic Shunts and Increased Complications and Mortality in Patients With Cirrhosis. Gastroenterology. 2018;154:1694-1705.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 7. | An J, Kim KW, Han S, Lee J, Lim YS. Improvement in survival associated with embolisation of spontaneous portosystemic shunt in patients with recurrent hepatic encephalopathy. Aliment Pharmacol Ther. 2014;39:1418-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Song J, Lu W, Yang S, Wu F, Zhao Z, Ji J. Effects of shunt embolization on hepatic encephalopathy recurrence in patients with major portosystemic shunts: A systematic review and meta‑analysis. Biomed Rep. 2025;22:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Lv Y, Chen H, Luo B, Bai W, Li K, Wang Z, Xia D, Guo W, Wang Q, Li X, Yuan J, Cai H, Xia J, Yin Z, Fan D, Han G. Concurrent large spontaneous portosystemic shunt embolization for the prevention of overt hepatic encephalopathy after TIPS: A randomized controlled trial. Hepatology. 2022;76:676-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Zhang L, Wei B, Wang Z, Tong H, Wu H. Treatment of refractory hepatic encephalopathy induced by spontaneous portosystemic shunt: Selective splenic vein embolization versus shunt embolization. Dig Liver Dis. 2023;55:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1496] [Article Influence: 124.7] [Reference Citation Analysis (2)] |
| 12. | Caraceni P, Vargas V, Solà E, Alessandria C, de Wit K, Trebicka J, Angeli P, Mookerjee RP, Durand F, Pose E, Krag A, Bajaj JS, Beuers U, Ginès P; Liverhope Consortium. The Use of Rifaximin in Patients With Cirrhosis. Hepatology. 2021;74:1660-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Nardelli S, Gioia S, Faccioli J, Riggio O, Ridola L. Hepatic encephalopathy - recent advances in treatment and diagnosis. Expert Rev Gastroenterol Hepatol. 2023;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 14. | Ke Q, He J, Cai L, Lei X, Huang X, Li L, Liu J, Guo W. Safety and efficacy of interventional embolization in cirrhotic patients with refractory hepatic encephalopathy associated with spontaneous portosystemic shunts. Sci Rep. 2024;14:14848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Rajesh S, Philips CA, Ahamed R, Singh S, Abduljaleel JK, Tharakan A, Augustine P. Clinical outcomes related to portal pressures before and after embolization of large portosystemic shunts in cirrhosis. SAGE Open Med. 2023;11:20503121231208655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Bajaj JS, Sanyal AJ, Bell D, Gilles H, Heuman DM. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther. 2010;31:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Lynn AM, Singh S, Congly SE, Khemani D, Johnson DH, Wiesner RH, Kamath PS, Andrews JC, Leise MD. Embolization of portosystemic shunts for treatment of medically refractory hepatic encephalopathy. Liver Transpl. 2016;22:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Inoue H, Emori K, Toyonaga A, Oho K, Kumamoto M, Haruta T, Mitsuyama K, Tsuruta O, Sata M. Long term results of balloon-occluded retrograde transvenous obliteration for portosystemic shunt encephalopathy in patients with liver cirrhosis and portal hypertension. Kurume Med J. 2014;61:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Naeshiro N, Kakizawa H, Aikata H, Kan H, Fujino H, Fukuhara T, Kobayashi T, Honda Y, Miyaki D, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Hyogo H, Ishikawa M, Awai K, Chayama K. Percutaneous transvenous embolization for portosystemic shunts associated with encephalopathy: Long-term outcomes in 14 patients. Hepatol Res. 2014;44:740-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Philips CA, Kumar L, Augustine P. Shunt occlusion for portosystemic shunt syndrome related refractory hepatic encephalopathy-A single-center experience in 21 patients from Kerala. Indian J Gastroenterol. 2017;36:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/