Published online Aug 26, 2016. doi: 10.4252/wjsc.v8.i8.243
Peer-review started: April 28, 2016
First decision: June 16, 2016
Revised: June 18, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: August 26, 2016
Processing time: 113 Days and 22.9 Hours
Radiotherapy is a cornerstone of anticancer treatment. However in spite of technical evolutions, important rates of failure and of toxicity are still reported. Although numerous pre-clinical data have been published, we address the subject of radiotherapy-stem cells interaction from the clinical efficacy and toxicity perspective. On one side, cancer stem cells (CSCs) have been recently evidenced in most of solid tumor primary locations and are thought to drive radio-resistance phenomena. It is particularly suggested in glioblastoma, where CSCs were showed to be housed in the subventricular zone (SVZ). In recent retrospective studies, the radiation dose to SVZ was identified as an independent factor significantly influencing overall survival. On the other side, healthy tissue stem cells radio-destruction has been recently suggested to cause two of the most quality of life-impacting side effects of radiotherapy, namely memory disorders after brain radiotherapy, and xerostomia after head and neck radiotherapy. Recent publications studying the impact of a radiation dose decrease on healthy brain and salivary stem cells niches suggested significantly reduced long term toxicities. Stem cells comprehension should be a high priority for radiation oncologists, as this particular cell population seems able to widely modulate the efficacy/toxicity ratio of radiotherapy in real life patients.
Core tip: Radiotherapy is a cornerstone of anticancer treatments. However, significant levels of toxicity and recurrences are still reported. On the one hand, cancer stem cells have been recently suggested to be the root of radio-resistance, with strong pre-clinical rational. One the other hand, convincing pre-clinical data suggesting the importance of healthy tissue stem cells radiation-induced destruction in long term side effects of radiotherapy surfaced. This article provides an overview of the available literature analyzing from the clinical efficacy and toxicity perspective the interactions between stem cells and radiation. Significant improvement of radiotherapy toxicity/efficacy ratio is suggested.
- Citation: Vallard A, Espenel S, Guy JB, Diao P, Xia Y, El Meddeb Hamrouni A, Ben Mrad M, Falk AT, Rodriguez-Lafrasse C, Rancoule C, Magné N. Targeting stem cells by radiation: From the biological angle to clinical aspects. World J Stem Cells 2016; 8(8): 243-250
- URL: https://www.wjgnet.com/1948-0210/full/v8/i8/243.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i8.243
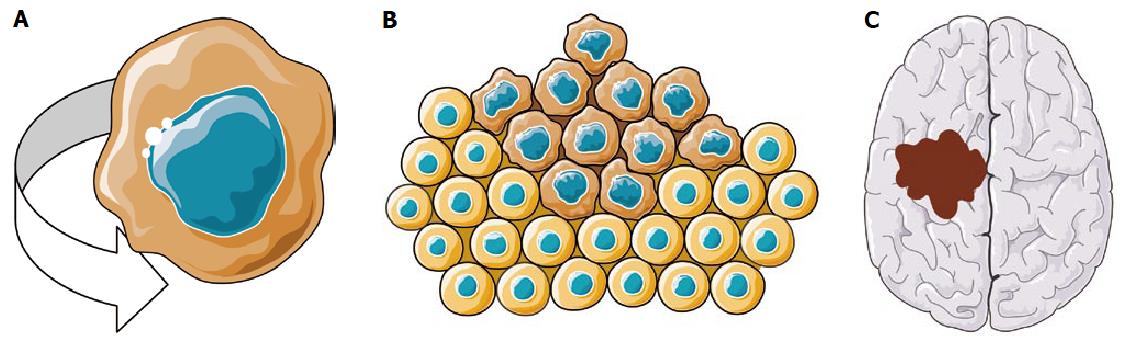
Radiotherapy is a cornerstone of anticancer treatments, since it proved efficacy in various primary tumor location when performed with intent to cure[1-4]. It also proved to be efficient for palliation of bone[5] and brain metastases[6], whatever histologic diagnosis. However, significant rates of failure and of radiation-induced toxicities are still reported in spite of recent technological improvements[1-6]. Radiation resistance seems mainly caused by biological phenomenon driven by cancer stem cells (CSCs)[7]. CSCs have been evidenced in the mid-90s in hematological tumors, but their presence has been proved recently in most of solid tumors (glioblastoma, prostate, breast, rectum, colon, and head and neck cancers)[7]. The CSC is defined by three main characteristics: It can initiate tumorigenesis and endlessly proliferate, it can self-renew, and it can give birth to a high number of progenitor parental cells (Figure 1). Although CSCs account for a very small number of cells considering the whole pool of tumor cells, they are thought to play a leading role in radiation resistance. Pre-clinical data showed that CSCs were able to redirect their cell cycle toward a radiation resistant state (the S-G0 phase), had a considerable capacity of tumor re-population, were not dependant of oxygen, and above it all - possessed hyperactive DNA repair processes[8]. Besides, CSCs seem highly gifted for invasion and migration[9] making them the supposed - main responsible for local and metastatic post-radiotherapy recurrences. Targeting CSCs in order to increase the therapeutic index (efficacy/toxicity ratio) of radiotherapy is a very promising way of research[10]. But from another angle, it might lead to concurrently kill stem cells located in the surrounding healthy tissues, and induce serious radiation-caused toxicities. Ideally, radiotherapy should simultaneously destroy CSCs and spare normal tissue stem cells. Several research approaches actually tried to reach this goal with recent publications regarding the CSC pharmacological targeting[10], the CSC dosimetric targeting, and the healthy tissue stem cells sparing. Interesting potential pharmacological targets have been recently suggested: Wnt/β-caderines pathway inhibitors are currently under clinical investigation[11], with the strong pre-clinical rational that Wnt/β-caderines ex–pression is directly related with radiation-resistance[12], de-differentiation, adhesion, and invasion[13]. Notch-1 (involved in CSC repopulation[14], proliferation and radiation-induced apoptosis resistance[15]), SHH (involved in metastases[16], CSC proliferation, survival, morphogenesis and radioresistance[17]), JAK/STAT (involved in CSC de-differentiation, apoptosis resistance, and proliferation[18]) and PI-3 kinase/Akt (involved in CSC survival after radiation[19]) are pharmacological targets of interest, with inhibitors that are currently tested in pre-clinical studies. Hypoxia is also a major topic of interest, since CSCs are thought to be located in hypoxic niches. In pre-clinical studies, decreasing CSC hypoxia resulted in reduced CSCs self-renewing and multiplication[20,21]. The pharmacological targeting of tumor and vascular stroma (using PDGF inhibitors) seems therefore promising, with the in vitro radio-sensitisation of CSCs that were initially radio-resistant[22]. Contrary to pharmacological targeting, the CSC “dosimetric targeting” (i.e., directly targeting stem cells by radiation) is still at its early stages. However, most of the publications consist in clinical studies with already promising outcomes. The sparing of organs at risk stem cells is also a hot topic, since healthy tissue stem cell death was suggested to be directly related to side effects widely impacting patients’ quality of life, occurring after both curative and palliative radiotherapy. The present article’s objective is to address the radiotherapy/stem cells topic from the clinical efficacy and perspective.
Glioblastoma is a major model of radioresistance since in spite of a multi modal approach (ideally combing surgery, radiotherapy and chemotherapy), the median overall survival time only reaches 12-15 mo, with most of the recurrences located in the radiation fields. The underlying phenomena leading glioblastoma to radioresistance are still misunderstood but it was suggested in animal pre-clinical models that the genesis of glioblastoma was linked to a loss of tumor suppressor gene in neural stem cells (NSCs)[23]. NSCs were shown to be physiologically housed in the subventricular zone (SVZ), an area surrounding the lateral ventricles[24-27]. Therefore, delivering high doses of radiation to niches of “healthy tissue” (i.e., the SVZ) possibly harboring glioblastoma CSCs might allow to overcome its radioresistance. This hypothesis was tested in 2010 by Evers et al[28]. Data of 55 patients treated for a glioblastoma between 2003 and 2009 in California, United States, were retrospectively reviewed. Dosimetric data of radiotherapy were analyzed in order to estimate the dose delivered to the supposed CSC niches (i.e., the SVZ), and correlate it with patient global outcomes. Only patients with histopathologically diagnosed anaplastic glioma (grade 3) or glioblastoma (grade 4), with at least 1 mo of follow-up, and who completed the whole planned radiotherapy were included. SVZ was defined based on previous publications, and doses to the volumes of interest could be a posteriori calculated. The authors estimated that the median dose received by the bilateral SVZ was 43 Gy. They then divided the population into a “low dose group” (receiving less than the median dose, n = 27) and a “high dose group” (receiving more than the median dose, n = 28). The two groups were well balanced on all essential prognosis factors (RPA classification, age, Karnofsky performance scale), but one. Complete resection was less achieved in the “high dose” group (n = 6, 21%) than in the “low dose” group (n = 16, 59%). The mean dose received by bilateral SVZ was 50 Gy ± 2 Gy for the “high dose group” and 27 Gy ± 5 Gy for the “low dose group”. The median progression free survival (PFS, defined as the time between radiotherapy completion and glioblastoma recurrence) was 15 mo for the “high dose group” and 7.2 mo for the “low dose group”. This difference was statistically significant (P = 0.03). Hazard ratio concerning glioblastoma progression was significantly decreased for the “high dose” group (HR = 0.74, 95%CI: 0.567-0.951, P = 0.0019). All other statistical analyses comparing important characteristics could not evidence significant differences, particularly regarding the total dose (P = 0.83), highlighting the high degree of glioblastoma’s radioresistance due to CSCs[29]. No correlation was shown between the total dose and dose to SVZ, since SVZ was most of the time outside of the clinical target volume. Therefore, doses per fraction on the SVZ were limited (1.36 Gy CI: 1.2-1.5). The fact that low doses of radiation could result in an increased radio-sensitivity has already been described in glioblastoma[30,31], but not in CSCs[32]. The underlying biological phenomenon is hypothesized to be the non-detection of DNA damages in case of small doses per fractions, while the CSC radio-resistance is supposedly linked with the over-expression of DNA damage checkpoints[33]. However, CSC high sensitivity to low doses must be studied in prospective clinical studies. Interestingly, when statistical analyses were performed regarding the doses received by the ipsilateral periventricular zone only, no significant difference could be evidenced. Linked with the observation that glioblastoma cells can widely migrate within the healthy brain tissue, causing frequent contralateral recurrences[34], it was hypothesized that ipsilateral CSCs could take shelter in contralateral CSC niches. Targeting radio-resistant CSC might therefore be more efficient if all the possible CSC harbors are damaged, but this hypothesis is still to be demonstrated. In 2012, Gupta et al[35] published outcomes of 40 glioblastoma patients treated between 2008 and 2010 at the Tata Memorial Centre, India. All patients were treated for histologically proven glioblastoma using standard treatment. Dosimetric data were retrospectively reviewed, and doses to SVZ were a posteriori calculated and linked with global outcomes. Median dose to bilateral SVZ was 56.2 Gy, and patients were divided as previously described into a “high dose group” (n = 20, mean dose to ipsilateral, contralateral and bilateral SVZ of 60.1 Gy, 59.9 Gy and 60 Gy respectively) and a “low dose group” (n = 20, mean dose to ipsilateral, contralateral and bilateral SVZ of 57.5 Gy, 47.4 and 52.5 respectively). Most of known prognosis factors were unfavorably distributed in the “high dose group”vs“low dose group”: Patients were older (55 yo vs 46 yo), with higher RPA class (85% of class IV-V vs 55%), with less frequent extensive resection (50% vs 70%), and with more frequent MGMT methylation (55% vs 40%). At a median follow-up of 15 mo, 25 out of the 40 patients experienced progression, with 21 deaths. Age and RPA class (well known prognosis factors) were significantly linked with survival in univariate analysis, as well as the dose to contralateral SVZ (P = 0.05). A Kaplan-meyer analysis showed significantly increased overall survival (P = 0.05) and progression free survival (P = 0.02) for patients with the highest doses to contralateral SVZ. In multivariate analysis, RPA class, Karnofsky performance status and dose to ipsilateral SVZ were identified as independent prognosis factors of overall survival (HR = 0.87, 95%CI: 0.77-0.98, P = 0.025). These results corroborate the efficacy of targeting CSCs by radiation in glioblastoma. However, the ideal target (ipsilateral or contralateral SVZ) and the dose threshold (43 Gy? 50 Gy?) are still to be clarified. The brain model is certainly one of the most interesting models for the CSC dosimetric targeting: Due to its anatomical conception, CSC niches are distinct from differentiated cells, making the result of a precisely delivered radiotherapy easier to interpret.
These two publications also reflect the need for reliable imaging of CSC niches. The recent development of spectroscopy (identifying the specific metabolic profile of glioblastoma CSCs) is certainly a very promising technique that could allow a precise dosimetric targeting of CSCs in the future[36,37]. Out of the glioblastoma model, the CSC imaging systems are mainly based on hypoxia[38]. Hypoxia is thought to be a cornerstone of radiation resistance since it was clearly proven that the biological effects of conventional radiotherapy (i.e., the DNA damages caused by chain oxidization) are potentiated by oxygen. In case of hypoxia, the efficacy of radiotherapy is de facto significantly reduced. It also seems clear that tumor hypoxic niches harbor CSCs (in glioblastoma but also in other solid tumors[39]) and therefore represent a target of interest for radiotherapy: The most radioresistant cells are housed in a micro-environment enhancing radioresistance. Imaging the hypoxic niches and targeting them by radiation might be the key to overcome cancers radioresistance since higher doses could induce the destruction and the re-oxygenation of these niches, initiating a virtuous cycle. The challenge of properly imaging hypoxia is still ongoing. Efficient nitroimidazole-based tracers were developed during the past 30 years, based on the fact that hypoxia induces a transformation of nitro-imidazole intermediates into alkylating agents that bind to cell component[40]. These elements could be then coupled with positron emitting radionuclides (18F, 64Cu, 60Cu) in order to be detected by positron emission tomography (PET) imaging devices. (18F) Fluoromisonidazole and (18F) 1-(5-fluoro-5-deoxy-α-Darabinofuranosyl)-2-nitroimidazole were validated (regarding specificity) by invasive gold standard methods and can be now clinically used. However, sensitivity is still limited due to low tumor-to-plasma ratios and poor spatial resolution of PET imaging systems[38]. Techniques based on magnetic resonance imaging (MRI) have been developed, resolving the issue of spatial resolution (Blood oxygen dependent MRI imaging, Mapping of Oxygen by Imaging Lipid Relaxation Enhancement, and Dynamic-Contrast-Enhanced MRI), but sensitivity issues remained[38]. Moreover, recent data suggested that CSC were not necessarily located in the most hypoxic areas[41], making multi-modal imaging methods absolutely needed (coupled PET-MRI, or imaging techniques detecting CSC surface marker). In this field, nanoparticles are very promising theragnostic tools, since they can be used both as MRI contrast agents, and as radiotherapy targets[42,43]. Finally, the ideal solution might be a radiotherapy technique capable of destroying as well CSC as differentiated cancer cells. Hadrontherapy (carbon or proton-based radiotherapy) seems to fulfill these criteriae, showing in vitro the ability to kill with the same efficacy CSCs and conventional cancer cells, thanks to the absence of oxygen effect[44]. However, the high cost of this technique might be a clear drawback to its routine application. Moreover, radio-resistance phenomena have been very recently described in vitro and need to be fully investigated to evaluate their possible clinical impact[45].
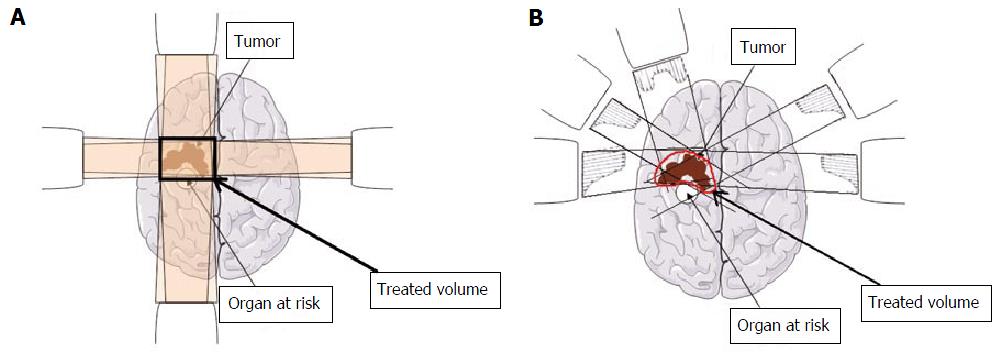
Memory disorders are a well known long term side effect of whole brain radiotherapy (WBRT), performed in case of multiple brain metastases. Radio-damaged neural stem cells (NSCs) located in the subgranular zone of the hippocampal dentate gyrus[46] have been hypothesized to cause the reported cognitive decline following WBRT[47]. Thanks to the development of the intensity modulated radiotherapy (IMRT), Gondi et al[48] showed the feasibility of a WBRT avoiding (i.e., reducing the delivered dose of ≥ 80% to) the hippocampal NSC niches, without impairing the quality of coverage of the remaining brain. IMRT offers the possibility to spare areas that could not be spared with conventional radiotherapy indeed, thanks to highly conformal dose painting (Figure 2). Gondi et al[49] published in 2014 the outcomes of an international single-arm phase II trial, comparing the results of a WBRT sparing hippocampal NSCs with the results of a 2003 phase III trial using conventional WBRT for brain metastasis. Patients treated using WBRT for solid tumor brain metastasis were assessed for standardized cognitive assessments [Hopkins Verbal Learning Test-Revised Delayed Recall (HVLT-R DR)] at baseline, 2-, 4- and 6-mo follow-up, with a primary endpoint being the HVLT-R DR at 4 mo. At 4 mo, the mean relative decline in HVLT-R DR score from baseline was of 30% in the 2003 control trial. In the experimental trial, hippocampal NSC niches definition was standardized and based on MRI fusion with planning computed tomography-scan. Standard (and similar to the control trial) fractionation scheme was delivered, with 30 Gy in 10 fractions. Doses were limited to 9 Gy to the entire hippocampus, with a maximum focal dose of 16 Gy. Between 2011 and 2012, 113 patients were included, with 42 patients being analyzable for the primary endpoint. At 4 mo, the mean relative HVLT-R DR impairment was significantly lower in the population who experienced the hippocampal NSC protection compared to the population who did not (7% vs 30%, P < 0.001). Interestingly, if most of patients experienced intracranial progression, with a mean overall survival of 6.8 mo, only 4.5% of patients developed intra-hippocampal progression. The authors concluded that the avoidance of hippocampal NSC was significantly related to memory preservation, bringing a direct clinical evidence that hippocampal NSC niche was implicated in the pathophysiology of radiotherapy-induced memory decline. Of course, the main limitation of this article is the absence of direct control group, but phase III trials have been approved and will clarify the place and efficacy of NSC avoidance during WBRT.
Xerostomia is one of the most quality of life-impacting late side effects of head and neck radiotherapy. Oral dryness frequently ruins patients’ everyday life inducing ulcerations, speech, taste and swallowing difficulties. Even with modern radiotherapy techniques minimizing mean dose to salivary glands, important rates of mucosal complications (15% to 40% of treated patients) are still reported[50,51]. It was clearly demonstrated that the xerostomia was linked with the irradiation of salivary glands, because of the high radiosensitivity of stem cells niches located in the salivary glands[50,52,53]. Xerostomia seemed to be proportionally linked with the dose delivered to salivary gland stems cells niches, determining the quantity of post radiotherapy viable salivary stem cells[52,54]. However, the clinically relevant threshold dose of radiotherapy damaging stem cells is still undetermined and only techniques delivering doses as low as reasonably achievable to parotid stem cells-rich regions were tested. Moreover, the exact location of these areas is still debated, the strongest hypothesis being they could be located in the larger excretory ducts[55]. Based on animal models, van Luijk et al[56] suggested that the centre of the parotid (containing the major ducts) was certainly rich in stem cells, since its restricted irradiation leaded to long term saliva production collapse. This hypothesis was recently tested in humans[55]. Salivary and dosimetric data of 74 patients treated for a head and neck cancer without salivary gland involvement were retrospectively reviewed. Spatial dose distribution inside the parotid could be correlated to salivary flows 1 year after radiotherapy completion (with a dose-dependent effect relationship), defining a stem cell region located near the dorsal edge of the mandible, at the occurrence of the first branching of Stensen’s duct, in concordance with animal stem cells locations. Doses delivered to this area were more predictive of salivary flow than (routinely used) parotid mean dose. Moreover, after radiotherapy, only cells provided by biopsies of these zones could be grown in vitro. A feasibility study was performed in 22 patients, showing that the preservation of the parotid stem cell niche seemed feasible with IMRT, even in case of impossible avoidance of the whole parotid. Other areas of parotid have been suggested to house stem cells capable of salivary long-term regeneration. It was suggested in one retrospective cohort derived from an important phase III study that sparing the superficial lobe of the two parotid glands could induce a better salivary preservation than complete contralateral parotid gland sparing[57]. These data need to be validated in larger patient cohorts, but might be a significant progress in order to limit radiation-induced xerostomia. The main limitation of these articles (out of their retrospective nature) is that the link between salivary flow and xerostomia is still unclear: The major salivary glands (parotid glands, submandibular glands and sublingual glands) produce 90% of saliva, but minor salivary glands (thousands of small glands located in the oral cavity) secrete the major quantity of mucin, the saliva lubricating agent. Mucin is also secreted for a small account by submandibular glands and sublingual glands. Therefore, only shielding parotids stem cells might insufficient to guarantee the restoration of good quality saliva after radiotherapy. Pre-clinical and clinical data are certainly needed concerning the radio-sensitivity and the location of stem cells in the submandibular and minor salivary glands. Currently, no reliable biological or imaging markers have been validated to precisely locate salivary stem cells, making progresses difficult to be made.
If the interaction between radiotherapy and CSCs is an en vogue topic[58], targeting CSC by radiation is at its early stage of development. Combining radiotherapy with biological drugs targeting CSC could be an efficient mean to overcome local and metastatic recurrences, with various agents that are currently tested based on solid pre-clinical rationales[59]. But directly targeting CSC using radiation is also a promising anticancer therapy with already interesting clinical results. The evolution of modern techniques of radiotherapy might widely depend of the imaging progresses in term of sensitivity. In order to increase the therapeutic index of radiotherapy, sparing stem cells of healthy tissue is also a major topic of interest since significant improvements regarding quality of life-impacting side effects following radiotherapy can be achieved. More than ever, prospective trials with solid methodologies are needed to confirm or infirm the suggested trends. Finally, both cancer and normal tissue stem cells seem to be central elements modulating the toxicity and the efficacy of radiotherapy. A better comprehension of stem cells location and their intrinsic radio-sensitivity is crucial, and permanent return trips between pre-clinical and clinical data are mandatory.
| 1. | Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-2106. [PubMed] |
| 2. | Wallis CJ, Saskin R, Choo R, Herschorn S, Kodama RT, Satkunasivam R, Shah PS, Danjoux C, Nam RK. Surgery Versus Radiotherapy for Clinically-localized Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;70:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Eberhardt WE, De Ruysscher D, Weder W, Le Péchoux C, De Leyn P, Hoffmann H, Westeel V, Stahel R, Felip E, Peters S. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26:1573-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 301] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 4. | Gupta T, Agarwal J, Jain S, Phurailatpam R, Kannan S, Ghosh-Laskar S, Murthy V, Budrukkar A, Dinshaw K, Prabhash K. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol. 2012;104:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, Howell D, Konski A, Kachnic L, Lo S. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 574] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 6. | Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol. 2012;109:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Moncharmont C, Levy A, Gilormini M, Bertrand G, Chargari C, Alphonse G, Ardail D, Rodriguez-Lafrasse C, Magné N. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett. 2012;322:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Guy JB, Rancoule C, Méry B, Espenel S, Wozny AS, Simonet S, Vallard A, Alphonse G, Ardail D, Rodriguez-Lafrasse C. [Radiosensitivity and/or radioresistance of head and neck cancers: Biological angle]. Bull Cancer. 2016;103:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Moncharmont C, Levy A, Guy JB, Falk AT, Guilbert M, Trone JC, Alphonse G, Gilormini M, Ardail D, Toillon RA. Radiation-enhanced cell migration/invasion process: a review. Crit Rev Oncol Hematol. 2014;92:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Méry B, Guy JB, Espenel S, Wozny AS, Simonet S, Vallard A, Alphonse G, Ardail D, Rodriguez-Lafrasse C, Magné N. Targeting head and neck tumoral stem cells: From biological aspects to therapeutic perspectives. World J Stem Cells. 2016;8:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Jang GB, Hong IS, Kim RJ, Lee SY, Park SJ, Lee ES, Park JH, Yun CH, Chung JU, Lee KJ. Wnt/β-Catenin Small-Molecule Inhibitor CWP232228 Preferentially Inhibits the Growth of Breast Cancer Stem-like Cells. Cancer Res. 2015;75:1691-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 13. | Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1377] [Cited by in RCA: 1461] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 14. | Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R’s of radiobiology revisited. Stem Cells. 2010;28:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Avila JL, Kissil JL. Notch signaling in pancreatic cancer: oncogene or tumor suppressor? Trends Mol Med. 2013;19:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Gu D, Liu H, Su GH, Zhang X, Chin-Sinex H, Hanenberg H, Mendonca MS, Shannon HE, Chiorean EG, Xie J. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Owens DM, Watt FM. Contribution of stem cells and differentiated cells to epidermal tumours. Nat Rev Cancer. 2003;3:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 236] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Levy DE, Darnell JE. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2485] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 19. | Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 20. | Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1056] [Article Influence: 62.1] [Reference Citation Analysis (10)] |
| 21. | Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1640] [Cited by in RCA: 1583] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 22. | Gomez-Casal R, Bhattacharya C, Ganesh N, Bailey L, Basse P, Gibson M, Epperly M, Levina V. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol Cancer. 2013;12:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Llaguno SA, Chen J, Kwon CH, Parada LF. Neural and cancer stem cells in tumor suppressor mouse models of malignant astrocytoma. Cold Spring Harb Symp Quant Biol. 2008;73:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879-11883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 762] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 25. | Goldman SA, Sim F. Neural progenitor cells of the adult brain. Novartis Found Symp. 2005;265:66-80; discussion 82-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565-4574. [PubMed] |
| 27. | Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3870] [Cited by in RCA: 3867] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 28. | Evers P, Lee PP, DeMarco J, Agazaryan N, Sayre JW, Selch M, Pajonk F. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Yu VY, Nguyen D, Pajonk F, Kupelian P, Kaprealian T, Selch M, Low DA, Sheng K. Incorporating cancer stem cells in radiation therapy treatment response modeling and the implication in glioblastoma multiforme treatment resistance. Int J Radiat Oncol Biol Phys. 2015;91:866-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Short SC, Mitchell SA, Boulton P, Woodcock M, Joiner MC. The response of human glioma cell lines to low-dose radiation exposure. Int J Radiat Biol. 1999;75:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Beauchesne PD, Bertrand S, Branche R, Linke SP, Revel R, Dore JF, Pedeux RM. Human malignant glioma cell lines are sensitive to low radiation doses. Int J Cancer. 2003;105:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Zamulaeva IA, Matchuk ON, Selivanova EI, Andreev VG, Lipunov NM, Makarenko SA, Zhavoronkov LP, Saenko AS. [Increase in the number of cancer stem cells after exposure to low-LET radiation]. Radiats Biol Radioecol. 2014;54:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4467] [Cited by in RCA: 4845] [Article Influence: 242.3] [Reference Citation Analysis (0)] |
| 34. | Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, Amistà P, Morandi L, Spagnolli F, Ermani M. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009;27:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 35. | Gupta T, Nair V, Paul SN, Kannan S, Moiyadi A, Epari S, Jalali R. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol. 2012;109:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Uckermann O, Galli R, Anger M, Herold-Mende C, Koch E, Schackert G, Steiner G, Kirsch M. Label-free identification of the glioma stem-like cell fraction using Fourier-transform infrared spectroscopy. Int J Radiat Biol. 2014;90:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Ramm P, Bettscheider M, Beier D, Kalbitzer HR, Kremer W, Bogdahn U, Hau P, Aigner L, Beier CP. 1H-nuclear magnetic resonance spectroscopy of glioblastoma cancer stem cells. Stem Cells Dev. 2011;20:2189-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Peitzsch C, Perrin R, Hill RP, Dubrovska A, Kurth I. Hypoxia as a biomarker for radioresistant cancer stem cells. Int J Radiat Biol. 2014;90:636-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Chargari C, Moncharmont C, Lévy A, Guy JB, Bertrand G, Guilbert M, Rousseau C, Védrine L, Alphonse G, Toillon RA. [Cancer stem cells, cornerstone of radioresistance and perspectives for radiosensitization: glioblastoma as an example]. Bull Cancer. 2012;99:1153-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49 Suppl 2:129S-148S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 41. | Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 407] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 42. | Rancoule C, Magné N, Vallard A, Guy JB, Rodriguez-Lafrasse C, Deutsch E, Chargari C. Nanoparticles in radiation oncology: From bench-side to bedside. Cancer Lett. 2016;375:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Miladi I, Aloy MT, Armandy E, Mowat P, Kryza D, Magné N, Tillement O, Lux F, Billotey C, Janier M. Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomedicine. 2015;11:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Cui X, Oonishi K, Tsujii H, Yasuda T, Matsumoto Y, Furusawa Y, Akashi M, Kamada T, Okayasu R. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. 2011;71:3676-3687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Bertrand G, Maalouf M, Boivin A, Battiston-Montagne P, Beuve M, Levy A, Jalade P, Fournier C, Ardail D, Magné N. Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiation. Stem Cell Rev. 2014;10:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 46. | Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313-1317. [PubMed] |
| 47. | Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97:370-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 48. | Gondi V, Tolakanahalli R, Mehta MP, Tewatia D, Rowley H, Kuo JS, Khuntia D, Tomé WA. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 49. | Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810-3816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 839] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 50. | Toledano I, Graff P, Serre A, Boisselier P, Bensadoun RJ, Ortholan C, Pommier P, Racadot S, Calais G, Alfonsi M. Intensity-modulated radiotherapy in head and neck cancer: results of the prospective study GORTEC 2004-03. Radiother Oncol. 2012;103:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Vallard A, Guy JB, Mengue Ndong S, Vial N, Rivoirard R, Auberdiac P, Méry B, Langrand-Escure J, Espenel S, Moncharmont C. Intensity-modulated radiotherapy or volumetric-modulated arc therapy in patients with head and neck cancer: Focus on salivary glands dosimetry. Head Neck. 2016;38:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, Elting LS, Langendijk JA, Coppes RP, Reyland ME. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78:983-991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 53. | Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells. 2008;26:2595-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 55. | van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, Baanstra M, van der Laan HP, Kierkels RG, van der Schaaf A. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med. 2015;7:305ra147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 56. | van Luijk P, Faber H, Schippers JM, Brandenburg S, Langendijk JA, Meertens H, Coppes RP. Bath and shower effects in the rat parotid gland explain increased relative risk of parotid gland dysfunction after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:1002-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Buettner F, Miah AB, Gulliford SL, Hall E, Harrington KJ, Webb S, Partridge M, Nutting CM. Novel approaches to improve the therapeutic index of head and neck radiotherapy: an analysis of data from the PARSPORT randomised phase III trial. Radiother Oncol. 2012;103:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Méry B, Rancoule C, Guy JB, Espenel S, Wozny AS, Simonet S, Vallard A, Alphonse G, Ardail D, Rodriguez-Lafrasse C. [Cancer stem cells: Radiotherapeutic features and therapeutic targets]. Bull Cancer. 2016;103:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Gilormini M, Malesys C, Armandy E, Manas P, Guy JB, Magné N, Rodriguez-Lafrasse C, Ardail D. Preferential targeting of cancer stem cells in the radiosensitizing effect of ABT-737 on HNSCC. Oncotarget. 2016;7:16731-16744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kita K, Liu J, Liu L, Nechifor G S- Editor: Ji FF L- Editor: A E- Editor: Li D