Published online Sep 26, 2015. doi: 10.4252/wjsc.v7.i8.1090
Peer-review started: November 29, 2014
First decision: January 20, 2015
Revised: July 3, 2015
Accepted: July 24, 2015
Article in press: July 27, 2015
Published online: September 26, 2015
Processing time: 302 Days and 8.1 Hours
The complement pathway is best known for its role in immune surveillance and inflammation. However, its ability of opsonizing and removing not only pathogens, but also necrotic and apoptotic cells, is a phylogenetically ancient means of initiating tissue repair. The means and mechanisms of complement-mediated tissue repair are discussed in this review. There is increasing evidence that complement activation contributes to tissue repair at several levels. These range from the chemo-attraction of stem and progenitor cells to areas of complement activation, to increased survival of various cell types in the presence of split products of complement, and to the production of trophic factors by cells activated by the anaphylatoxins C3a and C5a. This repair aspect of complement biology has not found sufficient appreciation until recently. The following will examine this aspect of complement biology with an emphasis on the anaphylatoxins C3a and C5a.
Core tip: This review article provides an overview over the scenarios, where complement activation contributes to tissue repair and regeneration through its effect on stem and progenitor cells, which is an area that needs further investigation.
- Citation: Schraufstatter IU, Khaldoyanidi SK, DiScipio RG. Complement activation in the context of stem cells and tissue repair. World J Stem Cells 2015; 7(8): 1090-1108
- URL: https://www.wjgnet.com/1948-0210/full/v7/i8/1090.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i8.1090
Complement is an effector system present in blood consisting of about 30 soluble proteins and 15 cellular receptors. Although it has been known for over a century that complement is a participant in host immunity, it has recently become generally realized that complement is a contributor to a variety of non-immune functions inclusive of resolution of inflammation, clearance of apoptotic cells, angiogenesis, wound healing, stem cell recruitment and activation, as well as repair processes[1-6].
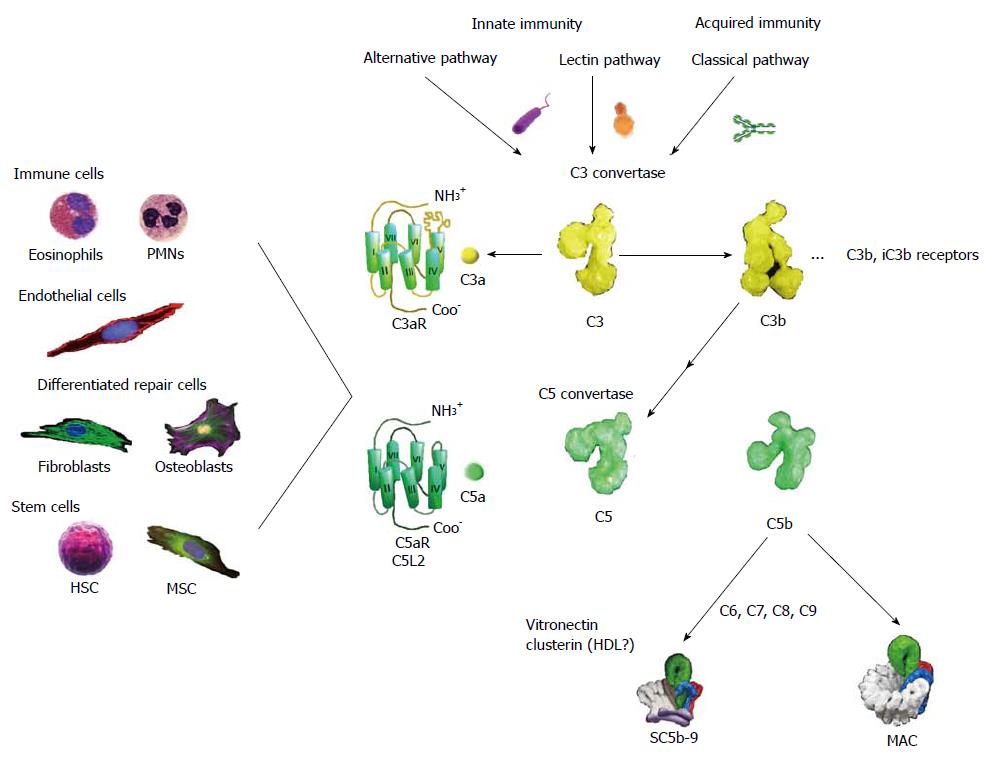
There are three routes of complement activation, the alternative pathway, the lectin pathway, and the classical pathway (Figure 1). All of these converge on the specific cleavage of component C3 (Mr: about 195000) by C3 convertase to yield the split products C3a (Mr: about 9000) and C3b (Mr: about 185000).
The alternative pathway activation is brought about by contact with large complex polysaccharides such as those found on microbial cell walls. This pathway is commenced by a diminished capacity to inactivate C3 convertase on a carbohydrate surface by the control factor H as well as pattern recognition by properdin and possibly contact activation by C3[7-11]. Factor B combines with initially deposited C3b along with the stabilizer properdin to compose this pathway’s C3 convertase, which consists of properdin, C3b, and Bb[12].
The lectin pathway is started by special collagen containing C-type lectins (collectins), namely mannan binding lectin (MBL) and ficolins, which recognize carbohydrate patterns typically characterized by high mannose content, for example mannan that is a component of the coats of a variety of yeast, fungi, and other microorganisms[13]. Proteases, referred to as mannose binding protein associated serine proteases link to fixed MBL to cleave C4 and C2 generating the complex enzyme C3 convertase (C4b, C2a)[14].
The classical pathway can be initiated by IgG subclasses 1, 2, 3 as well as by IgM. Once tagged by immunoglobulins, the collectin, C1q, links to these, along with C1r and C1s to evoke the cleavage of C4 and C2 resulting in the assembly of a C3 convertase (C4b, C2a), which has the same composition as that formed by the lectin pathway[15].
Additional C3b deposition onto either the alternative or classical pathway C3 convertases changes these into C5 convertases (C3b2, Bb, P or C4b, C3b, C2a)[16,17]. These complex enzymes are now competent to process component C5 (Mr: about 196000) into C5a (Mr: about 11000) and C5b (Mr: about 185000)[18].
The small activation peptides, C3a and C5a, Figure 1 arge in inflammation and germane to this review in wound healing and regeneration[19]. Both C3a and C5a, collectively referred to as anaphylatoxins, cause vasodilation, smooth muscle contraction, and increase vascular permeability[20-22]. Although C3a can be generated in greater abundance than C5a, the latter has greater specific inflammatory potential[21,23]. C5a especially is known for its ability to evoke chemotaxis of immune cells such as neutrophils and eosinophils[24,25]. Both C3a[23,26-28] and C5a[21,24,26,29-31] can stimulate an oxidant burst in granulocytes, but the response of these cells to C3a is considerably weaker and more transient than that to C5a[24,25,30,31]. In particular C3a fails to chemo-attract circulating leukocytes in vivo[25]. Apart from the weak response of leukocytic C3aRs, the response to C3a in vivo would be expected to be limited largely to the interstitial space, since C3a is inactivated by serum carboxypeptidase N (CPN)[32].
The anaphylatoxins are recognized on target cells by G-protein coupled receptors (GPCRs)[33-35] coupled primarily to Gi. Unusually, C3aR has a long second extracellular loop that is important for binding C3a[33,34].
C5a is recognized by two distinct GPCRs, C5aR (CD88) and C5L2, but only the former is coupled to Gi proteins, whereas the latter is enigmatic because it is not connected to a signal transduction pathway, and its biological role has not been established[36]. Several investigations have assigned roles for C5L2 inclusive of an anti-inflammatory function[37] and as a decoy-scavenger receptor[38], but it has also been argued from studies using C5L2 knockout mice that this receptor is important for C5a-mediated signal transduction in neutrophils, macrophages and fibroblasts[39]. Thus the true biological roles of C5L2 to date are not established[40].
The anaphylatoxins are inactivated by plasma CPN (EC 3.4.17.3), a tetrameric protein (Mr: about 260000) that can excise basic amino acids from the carboxyl-termini of C3a, C5a, as well as bradykinin and other polypeptides[32,41,42].
Whereas C3a desArg completely loses its activity[43], C5a desArg retains a small fraction of its specific activity for neutrophil chemotaxis[24,32].
The receptors for the anaphylatoxins are not restricted to immune cells as C3aR and C5aR are found on a variety of non-immune cells[44]. These include differentiated cells that can be important for wound healing and regeneration: mast cells[45], tenocytes[46,47], chondrocytes[48,49], synoviocytes[50], smooth muscle cells[51], endothelial cells[52-54], alveolar epithelial cells[55], mesangial cells[56,57], and regenerating hepatocytes[58]. In addition various stem and progenitor cells express the C3aR and C5aR[2,59-61] including HSC, mesenchymal stem cells (MSC)[61], NSC[2], and dental pulp progenitor cells[62]. Table 1 shows a list of the cell types that express C3aR and C5aR and their function.
| Cells expressing C3aR | Function of C3aR | Cells expressing C5aR | Function of C5aR |
| Neutrophils[244] | Respiratory burst[26], bone marrow retention in vivo[189] | Neutrophils[245] | Respiratory burst[28], chemotaxis[24], enzyme release[127] |
| Eosinophils[30] | Chemotaxis[30], in vitro but not in vivo[25] | Eosinophils[246] | Respiratory burst[27], chemotaxis |
| Monocytes/macrophages[31] | Chemotaxis[247], cytokine/chemokine production[164] | Monocytes/macrophages | Chemotaxis[104,248], cytokine/chemokine production[164] |
| Mast cell | Mediator release[102], chemokine production[249], chemotaxis [100,101] | Mast cell | Mediator release[102], chemokine production[249], chemotaxis[100,101] |
| Small fraction of lymphocytes[250,251] | Complex in vivo functions[252] | Small fraction of lymphocytes[251,253] | Complex in vivo functions |
| Osteoblasts[155,173,254] | Chemotaxis, accelerated osteogenesis, improved bone healing in vivo[174] | Osteoblasts[97,173] | Chemotaxis[97], accelerated osteogenesis[175], improved bone healing in vivo[174] |
| Chondrocytes[172] | Osteogenic differentiation (?) | Chondrocytes[172] | Osteogenic differentiation (?) |
| Tenocytes[46] | Not clear | Tenocytes[46] | Not clear |
| Smooth muscle cells[51] | Increased mediator release from mast cells[255] | Smooth muscle cells[44,51] | Not clear |
| Endothelial cells[52] | Transient ERK and rho activation[52], cytokine production[53] | Endothelial cells[52] | Chemotaxis[52], increased permeability[52] cytokine production[53], proliferation[128] |
| Hepatocytes[88] | Protection from apoptosis[88], liver regeneration in vivo[87,88] | Hepatocytes[44] | Proliferation[58], protection from apoptosis liver regeneration in vivo[87,94] |
| Renal epithelial cells[256] | Chemokine production[257], EMT under stress conditions[258] | Renal epithelial cells | EMT under stress conditions[259] |
| Neurons[193] | Protection from cell death[193,199] | Neurons[194] | Protection from cell death[193-195] |
| Astrocytes[260] | Indirect neuroprotection[198], NGF expression[200] | Astrocytes[261] | Cytokine and NGF expression[200,262] |
| MSC[61,90] | Chemotaxis[61], protection from apoptosis[61], production of angiogenic factors[91] | MSC[61,90] | Chemotaxis[61], protection from apoptosis[61], production of angiogenic factors[91] |
| HSPC[59] | Enhanced effects of SDF-1[263], improved bone marrow engraftment[60,188] | Not expressed | Indirect: decreased mobilization[192]; indirect: improved bone marrow engraftment[191] |
| CSPC[182] | Chemotaxis[182], proliferation[182] | CSPS[182], | Chemotaxis[182], proliferation[182] cardiac dysfunction in C5/C5aR -/- mice[180] |
| NSPC[2] | Increased neurogenesis[2], chemotaxis and differentiation[89] | NSPC[2] | Increased neurogenesis[2] |
| ESC | Not expressed | ESC | Prevents differentiation[168] |
While the C3b portion of C3 binds to the surface of pathogens leading to greater internalization by phagocytic cells, C5b, the remaining split product of C5, assembles with complement C6, C7, C8, and polymeric C9 to form the membrane-spanning membrane attack complex (MAC), which lyses bacteria, but which can also damage eukaryotic cells. Finally, a C3b cleavage produce, iC3b can bind to the β 2-integrins CR3 (CD11b/CD18) and CR4 (CD11C/CD18) on phagocytic cells facilitates the clearance of apoptotic cells.
There are multiple modalities which inhibit complement activation or the formation of the MAC; these include the plasma proteins factor H and C4b-binding protein and the membrane-anchored complement receptor 1 (CR1/CD35), membrane cofactor protein (CD46), decay accelerating factor or CD55, and MAC-inhibitory protein (CD59). As the plethora of inhibiting factors indicates complement activation has to be fine-tuned to provide optimal protection from infection without causing inflammatory tissue injury.
While complement proteins in the circulation are primarily produced by the liver except for the late acting complement components in particular C7 which are produced by monocytes/macrophages[63,64], it has become apparent that production and activation of complement proteins can happen in a localized fashion in many different parts of the body[65-68], and one would expect prolonged activation by the anaphylatoxins C3a and C5a under such conditions because of the absence of CPN in the interstitial space.
The important role of complement in the defense against infection comes, however at a price: excessive complement activation plays a role in numerous disease processes ranging from ischemic reperfusion injury[69-71] to asthma[72], acute lung injury[73,74], glomerulonephritis[75], rheumatoid arthritis[76], Alzheimer’s disease[77], multiple sclerosis and demyelination in general[78,79], and age-related and genetic macular degeneration[80-83]. In some instances the specific injurious complement pathway components have not been distinguished[75,76], in others C5a[69-71,77,80] or the MAC are the clear culprits[79]. A role for C3a was only seen in a mouse asthma model[72] and a mouse model of laser-induced macular degeneration, where the presence of the C3aR was associated with increased angiogenesis[80], which is detrimental in the retina, but which could support repair following ischemic insults in other tissues.
It should be noted here that C5a appears the major culprit responsible for most of the observed pathologies, and that specific C5/C5a inhibition preserving the early steps of complement activation could be highly advantageous in some circumstances.
While the inflammatory aspect of complement activation has long been emphasized, it has been largely ignored that complement activation contributes also to resolution of inflammation and tissue repair with few reviews covering this aspect[4,84-86].
In particular, C3a has anti-inflammatory and regenerative effects[2,61,87-91]. In fact the regenerative potential of C3/C3a dates way back phylogenetically, as its expression is prominently up-regulated in mesenchymal cells in the regeneration zone in amphibians undergoing limb regeneration[92]. Furthermore, recent findings indicate that the C3aR on mesenchymal cells plays an important migration-directing role during early vertebrate development in zebra-fish[93]: Neural crest cells mutually attract each other via C3a and the C3aR forming clusters of migratory mesenchymal cells. Such collective cell migration is a phenomenon crucial for morphogenesis. It remains to be seen, whether C3a and the C3aR play the same role during mammalian embryonic development.
While C5a also has regenerative effects for instance by its effects on the liver[94,95], neurons[96], osteoblasts[97], and dental pulp progenitors[62], these properties are often overshadowed by the strong inflammatory reaction caused by the activation of leukocytic C5a receptors, which are involved in most of the pathologic conditions described above.
However, it should also be considered that inflammation itself constitutes a first step in wound healing. C3a and C5a can lead to an increase in vascular permeability[21,98], which is important for wound healing as it aids the flow of chemical and cellular entities necessary for repair and regeneration while facilitating waste removal[99].
Although swelling is traditionally seen as a characteristic of inflammation, edema is also necessary for the resolution of inflammation and restoration of functional tissue because an increase in vascular permeability facilitates entry of repair and restorative cells. Specific to this theme is the function of histamine. C3a and C5a both are chemotactic for mast cells and both are inducers from these cells of histamine release[100-102]. Histamine due to its potent vasodilation activity can induce swelling, but histamine is also required for skin wound healing as demonstrated using Kit mutant mice that are mast cell deficient. These animals are unable to secrete mast cell derived histamine, and the animals were found to have a defective response to cutaneous wound healing[103].
The increase in vascular permeability facilitates the recruitment of monocytes that can respond to C5a mediated chemotaxis gradients[104], and these cells are crucial for “cleanup” functions. Today it is understood that clearance of debris and apoptotic cells is an important activity necessary for subsequent wound healing, and complement along with pentraxins have been shown to participate in this activity[105,106]. Indeed the clearance function was probably the original function of the complement system dating all the way back to metazoans[107].
The collectins C1q and MBL are important for enhanced phagocytosis by monocytes and macrophages of modified lipoprotein complexes, immune complexes, and apoptotic cells[108-111]. Apoptotic cells present exteriorized phosphatidyl serine that can be recognized at an early stage by the lectin domains of members of the collectin family[112-114]. Apoptotic cells, debris or immune complexes tagged by C1q or MBL are identified by monocytes and macrophages bearing CD91 that can be in complex with a collectin receptor, calreticulin[115-117]. The facilitated uptake of these “disposables” has been referred to as macropinocytosis[118].
In addition to recognition of pathogens, debris and dead cells by members of the collectin family, fragments of C3 are important for clearance functions. C3b is susceptible to processing by Factor H and I to iC3b that can be cleaved further into C3d and C3c[119]. C3 fragments are recognized by receptors such as CR1 (CD35), CR2 (CD21), CR3 ( CD11b/CD18), CR4 (CD11c/CD18), and CRIg found on Kupffer cells, monocytes and macrophages, which are immune adherence receptors that facilitate removal of opsonized microorganisms, immune complexes and apoptotic cells[120,121].
The importance of angiogenesis in wound healing and regeneration has been clearly understood[122]. The process has been categorized in three continuous overlapping phases: inflammatory, proliferative, and remodeling[122].
Some aspects of participation in inflammation inclusive of increase in vascular permeability induced by C3a and C5a have already been discussed, but these mediators have additional functions that indirectly support angiogenesis. C5a but not C3a has been shown to induce an upregulation of gene expression on endothelial cells for adhesion molecules E-selectin, ICAM-1, and VCAM-1[123,124]; the upregulation of these adhesion molecules facilitates extravasation of immune cells inclusive of monocytes that are important for debridement, remodeling and angiogenic mediator secretion[125]. Angiogenesis requires restructuring of the extracellular matrix by controlled proteolysis, and the anaphylatoxins were reported to increase the levels of MMP-1 and MMP-9 in monocytes[126] and to be secretagogues of MMP-9 from granulocytes[127].
Both C3aR and C5aR are found on cultured endothelial cells, but these mediators use different signal transduction pathways and the response to C3a is more transient[52]. Both the anaphylatoxins up-regulates chemokine production in endothelial cells[53], but only C5a is chemotactic for human umbilical vein endothelial cells (HUVECs)[52] and microvascular endothelial cells[54]. Moreover, it was reported that C5a could induce not only migration of cultured microvascular endothelial cells but proliferation and ring formation as well[128].
C3a and C5a were found to increase vascular endothelial cell growth factor (VEGF) in human culture retinal pigment epithelial cells, and when the anaphylatoxins were injected intravitreously into normal mice, an increase in VEGF within the retinal pigment epithelial-choroid layer of the retina was observed[80]. Others found that C5a but not C3a induced VEGF synthesis and secretion from a retinal pigment epithelial cell line[129]. Furthermore, both C3a and C5a were reported to induce production and secretion of VEGF from MSC[91]. Although there is no in vitro evidence that C3a and C5a are directly angiogenic, they have been shown to be angiogenic in in vivo situations[80,130,131], perhaps in response to angiogenic factors that the anaphylatoxins induce in cells in the proximity as just described.
In summary, C3a and C5a can contribute to the inflammatory and proliferative phases of angiogenesis, and thus the anaphylatoxins can be viewed as factors with indirect angiogenic potential; however, it is necessary to mention that one publication is in apparent contradiction to this view, namely investigators studying experimental retinal neovascularization published that C5a is anti-angiogenic[132]; however, these investigators were examining murine models of retinopathy of prematurity and hypoxia induced retinal vascularization, and these observations though correct may not be of a general nature.
Although tissue regeneration is very limited in mammals, the mammalian liver has retained an amazing capacity for regeneration following viral infection, exposure to toxins or surgical resection. This regeneration can occur at the hepatocyte level in cases of acute liver injury, although liver stem and progenitor cells appear to contribute in more chronic conditions.
The complement activation products C3a and C5a play an essential role in regeneration of the liver parenchyma[87,95]. After experimental CCl4 induced liver toxicity or partial hepatectomy, mice deficient in C3 or C5 exhibited defective regeneration and a higher frequency of mortality[87]. Furthermore, C5a was demonstrated to be a growth factor for regenerating hepatocytes, and blockage of the C5aR in experimental liver regeneration experiments resulted in the inability of hepatocytes to proliferate leading to defective liver restoration[58,94].
However, the role of complement activation is a double-sided sword in hepatic regeneration and the MAC was found to be the principle mediator of hepatic ischemia reperfusion injury[133], which creates a dilemma, since the early components of complement activation, C3a, and C5a are necessary for liver regeneration. However, targeted inhibition of MAC formation with CR2-CD59 significantly improved survival after partial hepatectomy in mice[133], while retaining the benefit of complement activation and anaphylatoxin production.
MSC are rare, often perivascular cells found in all tissues that are able to differentiate into all types of connective tissue lineages including osteoblasts, adipocytes and chondrocytes. Furthermore, these cells produce a variety of angiogenic and trophic factors[134,135] and possess anti-inflammatory properties[136-138]. Owing to the immune-evasive properties of MSC, allogeneic MSC transplantation is generally accepted. Because of all these properties MSC have started to find clinical application in a variety of diseases ranging from myocardial infarction[139] to graft vs host disease[140] and have found attention in the context of acute lung injury[141].
However, in the rush to the clinic, survival of the transplanted MSC has not been sufficiently considered, and there have been failed clinical trials using MSC - in spite of promising results in animal models[142-146], and the full regenerative potential of these cells has not been harnessed due to poor tissue homing and limited cell survival following transplantation. Successful clinical trials will require additional information about the mechanisms by which MSC repair injured tissues, about the optimal route of administration, and about means of increasing their survival at a site of tissue injury. It is surprising, how little there is known about MSC recruitment and survival in vivo for a cell type that is being investigated in numerous clinical trials. Various means of improving MSC homing[147], growth factor production[148,149] and survival[150] are being pursued as ways to improve the therapeutic efficacy of MSC, but usually different means are used to achieve each one of these goals. It is hypothesized here that C3a can improve all of these functions, since we postulate that the C3a-dependent regenerative capacity of MSC seen in amphibians[92] has been preserved in mammalian tissue repair.
Although MSC have various anti-inflammatory and immune-evasive properties[151] - including the ability to inhibit the proliferation of allogeneic T cells, low levels of expression of MHC class I and II proteins, the ability to convert inflammatory M1-type macrophages to repair-type M2 macrophages, and secretion of the complement-inhibitory factor H[152], - they are not fully protected from complement induced injury themselves, and complement activation appears to be involved in the demise of MSC following allogeneic transplantation[151,153]. One would wish that such basic complement biology had been considered before using allogeneic MSC in clinical trials. Incubation of MSC with complement active human plasma resulted in the deposition of C3c and iC3b on the cell surface of the MSC and C3a and soluble C5b-9 detection in the supernatant[90], indicative of complement activation, which could be prevented by various means of complement inhibition.
In addition, MSC as well as osteoblasts express components of the complement cascade themselves[154] including C3, C5[155], the C3aR and C5aR[61] and the cell surface complement regulators CD46, CD55, and CD59[155]. Furthermore, MSC engineered to up-regulate CD46, CD55, and CD59 protected these cells from complement-mediated cell lysis in vitro and in vivo[156].
MSC show tropism for areas of tissue damage[157,158], but it is controversial which chemotactic factors are responsible for this. In leukocytes a large degree of cell recruitment to an area of tissue injury depends on chemokines and C5a, but the role of chemokines in trafficking of MSC is unclear with widely contradictory findings[158-162]. Since MSC are chemo-attracted by C3a and C5a in vitro[61], we hypothesize that complement activation is an important player in attracting MSC to an area of tissue damage in vivo. C3a and C5a can be locally generated at the surface of MSC which contact serum[90] in close proximity of C3aRs and C5aRs expressed by MSC; it is possible that this may circumvent access to CPN-mediated inactivation of the anaphylatoxins C3a and C5a. In addition to being potent chemoattractants for MSC[61], C3a and C5a increase the survival of MSC under conditions of oxidative stress[61], which would be encountered in an area of tissue injury. Indeed C3/C3a may be a survival factor for MSC[163]. Furthermore, C3a, - and to a lesser degree C5a, - induce the production of trophic and angiogenic factors by MSC including VEGF, basic fibroblast growth factor (bFGF), platelet derived growth factor, IL-6, and IL-8[91], and supernatants from C3a-stimulated MSC are angiogenic for HUVECs in vitro[91]. The increased production of growth factors by MSC stimulated with C3a or C5a was largely due to activation of NFκB[91], but in contrast to other cell types, in which C3a and C5a cause NFκB activation such as monocytes/macrophages[164], this does not lead to the concomitant release of the inflammatory cytokines TNF-α and IL-1β, thus converting a normally inflammatory pathway into one that supports regenerative processes. For TNF-α this occurs through promoter inactivation[165], while IL-1β production in MSC appears to be blocked at the level of protein processing.
We propose that C3a and C5a play a physiological role in MSC-dependent tissue repair by recruiting MSC to an area of tissue injury, by increasing MSC survival under challenging conditions, and by increasing the production of trophic, angiogenic and anti-inflammatory factors by these cells. It is also suggested that pretreatment of MSC with C3a, -C5a is considered too inflammatory - prior to transplantation may increase the repair capacity of MSC by augmenting the ability of the MSC to survive in an area of tissue damage and by inducing increased production of angiogenic and anti-inflammatory factors.
In addition, it has also been reported that complement C1q is a chemoattractant for MSC[166].
It is also worth mentioning that C3a and C5a cause prolonged activation of the ERK[61], Akt[61], NFκB[91], and Stat3[167] pathways in MSC and other stem cells, which are the same pathways that are activated by bFGF albeit with differing routes of activation, and it will remain to be seen whether C3a or C5a have a similar effect as bFGF in maintaining stem cells in the undifferentiated state as has been suggested for C5a in embryonic stem cells (ESC)[168].
MSC are not the only mesenchymal cells expressing complement components and responding to complement activation. Myoblasts express the complement components of both the alternative and classical pathways (C1q, C1r, C1s, C2, C3, C4, factor B, factor H, factor I[169,170], as well as the C3aR, and they spontaneously activate allogeneic complement, but are themselves protected from self-killing due to expression of high levels of CD46 and CD59[171]. Finally, scratch-injured tenocytes showed increased proliferation and survival in the presence of C3a[46].
Consistent with the role of complement activation during limb regeneration in amphibians[92] described above, it has been suggested some time ago that complement activation may be important in cartilage-bone transformation during fracture healing and that the alternate complement activation pathway may be involved[172]. Like their MSC precursors, osteoblasts are able to express the key complement proteins C3 and C5[155] and express the C3aR and C5aR, which both mediate osteoblast migration[97]. Expression of the C5aR was highly up-regulated during osteogenic differentiation[97], but later during osteoblast to osteocyte differentiation complement genes were greatly down-regulated[173].
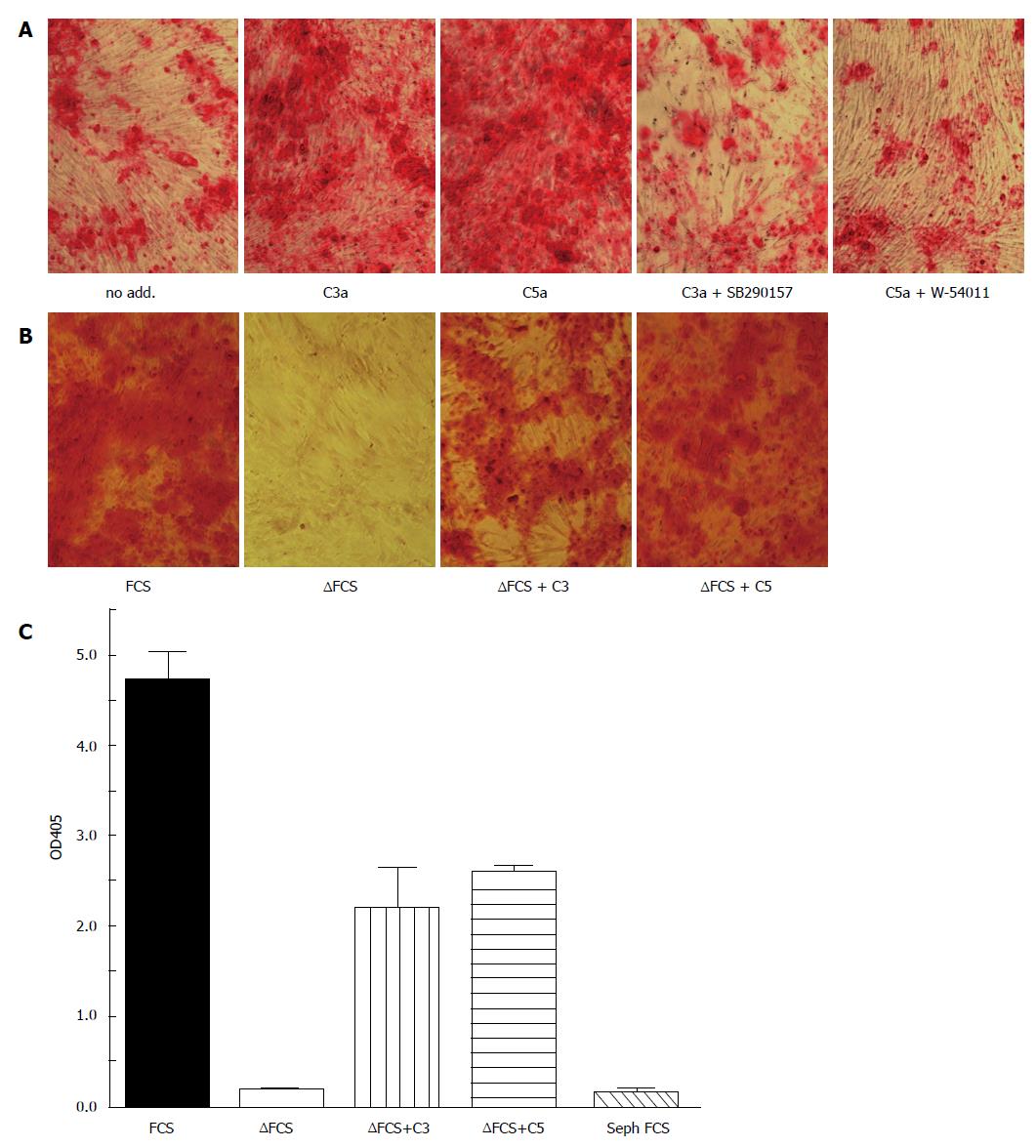
Although osteogenic differentiation of MSC can occur in the absence of C3a or C5a, it is accelerated in the presence of C3a or C5a in a C3aR and C5aR-specific fashion as shown with receptor-specific inhibitors in Figure 2A: After two weeks of osteogenic differentiation in the presence of fetal calf serum (FCS) that was not heat-inactivated, i.e., complement proteins had not been inactivated, Alizarin red staining of calcium salt deposits indicated moderate staining in FCS, which was significantly augmented, when C3a or C5a had been added to the media. However, by 3 wk the difference between these groups was largely diminished (results not shown). If heat-inactivated FCS (FCS) replaced the FCS, osteogenic differentiation was still further delayed but the addition of C3 or C5 partially substituted for the presence of serum complement components (Figure 2B) indicating that the differentiating cells themselves must have provided the necessary complement components.
Consistent with these in vitro findings, delayed fracture healing was observed in C3 or C5-deficient mice which received a standardized femur osteotomy[174]. C5-deficiency also resulted in poor quality bone[174], indicating that complement activation plays an important role in fracture healing. However, under chronic conditions the osteogenic effect of complement activation is a double-edged sword, because it can also result in vascular calcification during the atherosclerotic process, where MSC-derived C5aR participation has been shown recently[175].
Following cardiac infarction extensive necrosis of ischemic cardiomyocytes activates complement. The ensuing infiltration of the infarct zone with neutrophils and monocytes serves to clear the injured site from dead cells and debris, and initiates reparative pathways.
However, there is little doubt that complement activation plays an injurious role in the acute phase of myocardial infarction mostly in the context of C5a-mediated reperfusion injury and neutrophil influx[176-178], but clinical trials inhibiting at the level of C5 have been unsuccessful[179] indicating that even in this early phase, complement activation is not all deleterious. Furthermore, even C5a appears to be protective in several models of cardiac hypertrophy, where C5/C5aR knockout mice fared worse than wild type mice[180].
The beneficial effect of complement activation becomes more apparent in the more chronic situation, where complement activation contributes to tissue repair[181]: C3-deficiency in C3 knockout mice exacerbated myocardial dysfunction four weeks after coronary artery ligation showing more scar tissue, and decreased cardiac stem/progenitor cells (CSPC) in the infarct zone[181]. Both murine and human CSPC express C3aR and C5aR, are chemo-attracted by C3a and C5a, and show greater proliferation in the presence of the anaphylatoxins[182]. It remains to be seen, whether they also produce more angiogenic factors as described above for MSC stimulated with C3a or C5a, which would be a further advantage in the context of cardiac repair. In CSPC C3a or C5a also induced several genes associated with - unwanted - myofibroblast differentiation in vitro[182], but it remains to be seen, whether this is relevant in vivo.
Like any tissue damage, myeloablation by radiation or chemotherapy activates complement resulting in the generation of the complement activation peptides C3a and C5a[59,60]. Following bone marrow transplantation fast and efficient homing to and engraftment in the bone marrow is important. In this scenario SDF-1 is the most important chemotactic factor, which chemo-attracts hematopoietic stem and progenitor cells (HSPC) to the bone marrow and retains them there through the CXC chemokine receptor 4 on these cells[183,184].
While HSPC express the C3aR, C3a itself does not appear to be a direct chemo-attractant, but it augments the chemotactic responsiveness of HSPC to gradients of SDF-1 as well as to sphingosine-1-phospate and ceramide-1-phosphate[59,60,185,186]. In vivo, mice deficient in complement C3 exhibited delayed engraftment of HSPC[60]. This effect was specifically mediated by the C3aR as shown when HSPC from C3aR-/- mice were injected into irradiated wild type mice, which resulted in a significant delay in recovery of leukocytes and platelets and decreased committed progenitors in the bone marrow[187]. Similarly, engraftment of human CD34+ cells treated with a C3aR inhibitor showed impeded engraftment in nonobese diabetic/severe combined immune deficiency mice[187].
C3a also contributes to the retention of HSPC in the bone-marrow as C3-/- or C3aR-/- mice showed accelerated mobilization of HSPC into the peripheral blood following administration of granulocyte colony-stimulating factor (G-CSF)[188]. This retention mechanism is not limited to HSPC, but also applies to their neutrophil progeny, and indeed the C3aR protects from ischemic intestinal injury due to reduced neutrophil mobilization, and increased neutrophil accumulation causes exacerbated injury in C3aR deficient mice[189]. Indeed, decreased neutrophil mobilization in wild type vs C3aR-/- mice may explain the increased mortality observed in C3aR-/-mice in an endotoxin shock model[190], although the mechanism was not reported for this model.
C5-deficient mice also exhibited impaired HSPC engraftment: In this scenario the role of C5 cleavage leading to the formation of soluble MAC resulted in increased adhesion of HSPC to bone marrow stromal cells and augmented secretion of SDF-1 by the bone marrow stroma[191]. However, HSPC do not express the C5aR themselves, and C5 deficient mice show reduced HSPC mobilization following the administration of G-CSF[192], which causes complement activation. Apparently, granulocytes, which are released into the circulation in response to C5a formation, pave the way for HSPC to egress from the bone marrow perhaps due to MMP9 release, which facilitates HSPC mobilization[192].
It has been known for some time that neurons express both C3aR[193] and C5aR[194], and that these two receptors protect from neural cell death[193-195]. This protective effect is not limited to differentiated neurons, but already functions in neural stem and progenitor cells, which express both C3aR and C5aR. C3-deficient mice showed deficits in both basal and ischemia-induced neurogenesis[2], and C3aR expression was essential for basal neurogenesis[2], while C5aR expression made no difference in this respect[196]. Consistent with these results, C3a protected from ischemic insult-induced memory impairment in neonatal mice[197].
In vitro, C3a could induce neuronal differentiation of neural progenitor cells[89], and increased the chemotactic response to low concentrations of SDF-1[89] similar to the situation with HSPC. In addition, C3a protected from NMDA neurotoxicity, but only in the presence of astrocytes[198], which suggests that C3a-stimulated astrocytes, which express the C3aR[199], were the primary target, and that they in turn protected through the production of NGF and other neurotrophic factors[200]. However, in a mouse model of ischemic reperfusion injury, C3aR inhibition had the opposite effect resulting in increased neuroprogenitor proliferation and suppressed T cell infiltration[201]. The reasons for such opposing results are not clear, although it is possible that the last model includes a larger inflammatory response that may cancel out any direct effect of C3a on neuronal progenitors and/or astrocytes. Specific pathways by which complement activation protect neural stem and progenitor cells await further elucidation.
Interestingly complement C1q, - in the absence of other components of the complement cascade increased neuron viability and neurite outgrowth and prevented α-amyloid-induced neuronal death in vitro[202] and in vivo[203]. Neuroprotection was promoted by activation of the transcription factor cAMP responsive element binding protein and by increasing LRP1B and GPR6 expression[203]. Furthermore, in retinal neurons, TGF-β signaling regulates C1q expression, which in turn is necessary for synaptic pruning[204]. Indeed, complement activation plays a role during a process called synaptic elimination in new-born mice[205], where either C1q or C3 deficiency resulted in failure of synaptic elimination[205], implying the classical complement cascade in this process. Interestingly, C1q-/- mice presented with signs of epilepsy due to increased excitatory synaptic connectivity[206].
ESC only express a limited number of proteins of the complement cascade including C6, C7, C8, C9, factor I, H, properdin/factor D, and complement component 1r, s and q receptor, and beta polypeptide[207,208]. However, a recent report indicates that they may also express C5 and the C5a receptor[168] and more importantly that C5a promotes survival and maintenance of the pluripotent state of ESC in the absence of bFGF[168], the standard addition to maintain human ESC in the undifferentiated state. While this report awaits further validation, it highly suggests that complement activation presumably with the support of maternal complement components plays a role in embryonic development from the very beginning.
It is known that the maternal complement system plays a crucial role starting early on during fetal development and that it is essential for the maintenance of fetomaternal tolerance. In mice Cr1l/Crry (complement regulatory protein) deficiency is embryonically lethal, but the embryos are rescued in C3-/- mothers[209]. Indeed, ESC are more susceptible to complement mediated cell lysis than differentiated cells, and this pathway may contribute protection from teratocarcinoma formation during pregnancy[210]. Complement activation has, however to be finely regulated during pregnancy, since excessive activation of this pathway in later pregnancy is associated with miscarriage[211] and preeclampsia[212-215].
There is limited knowledge about the role of complement in early vertebrate development with much of the information derived from lower vertebrates. While further investigation using mammalian models is surely required, the existence of these complement pathways during amphibian development indicates that complement activation is a phylogenetically preserved ancient process during embryogenesis. In xenopus complement components are extensively expressed during development starting during the gastrula/early neurula stage[216] with organ-specific expression patterns during early organogenesis. C1qA, C3 and C9 are strongly expressed in the early neural plate, while C1qR and C6 are expressed at the periphery of the neural plate presumably in the neural crest[216,217] preceding the development of hematopoiesis. At this point C3 and C3aR show a predominantly mesodermal expression. Interestingly, neural crest cells, a multipotent embryonic cell population undergo epithelial to mesenchymal transition (EMT) in xenopus and zebrafish in a fashion reminiscent of metastasizing cancer cells and it is following this EMT transition that they express both C3 and the C3aR[93]. These cells form cohesive clusters of migrating cells that are co-attracted via C3a and the C3aR and this process is necessary for collective migration of these cells[93] suggesting that C3aR/C3a contribute to the intricate mass cell movements of the developing embryo.
In rats C3 derived from the visceral yolk sac is an embryotrophic factor between days 9.5 to 11.5 post conception[218], - however no further details have been elucidated.
Evidence for a role of C3a in fetal tissue regeneration comes from studies on embryonic chick retina regeneration. In this model C3a can induce complete regeneration of the ablated chick retina from stem/progenitor cells via Stat3 mediated up-regulation of IL-6, IL-8, and TNF-α[219]. However, there was an optimal concentration of C3a that induced regeneration, while very high concentrations caused apoptosis, indicating that fine-tuning of the C3a/C3aR axis is necessary, perhaps not surprising since the cytokines produced by C3a stimulation may serve as growth factors at low concentrations, but become highly inflammatory at higher concentrations.
Beyond the early effect of C5a stimulation on ESCs mentioned above, C5a and the C5aR play a continued important role during mammalian development: They are both expressed during the period of neurolation in mice and humans[220], and while C5aR knockout mice show no congenital defects under normal pregnancy conditions, they present with a wide variety of congenital malfunctions due to neural tube defects ranging from anencephaly to scoliosis and anophthalmia, if the mothers are folate deficient[220].
Complement evolved to destroy microorganisms, and one effector outcome of complement activation is the assembly within target cell membranes of a multiprotein complex referred to as the MAC. This consists of one molecule each of C5b, C6, C7, C8 and multiple copies of C9 (6 or more). In its complete form the MAC creates a transmembrane pore of 100 Ǻ that destroys the functional integrity of cellular membranes[221,222].
In the absence of proximal phospholipid membranes the terminal components of complement form a soluble complex referred to as Soluble complement C5b to 9 (SC5b-9), which was initially described as having a composition of one molecule each of C5b through C8 and three units of C9 and vitronectin[223]. Later it was also shown to contain clusterin (apolipoprotein J)[224], which is known to be a component of a subclass of high-density lipo-protein (HDL) particles[225]. Although the term “SC5b-9”, as originally conceived designated a soluble form of the terminal complement complexes, it is probable that these assemblies are heterogeneous with some containing vitronectin and others clusterin presumably associated with HDLs. Whether heterogeneous or not, indications exist that these macromolecular composites may be adaptive for recovery from injury.
Vitronectin, a known matrix and adhesive protein, circulates in human plasma in an inactive state in which its heparin linkage region and integrin binding site, containing the canonical Arg-Gly-Asp sequence, are buried[226,227]; however, as a consequence of oligomerization and conformational change these regions on the protein can interact with glycosaminoglycans (GAG) and integrins[228,229]. GAGs are a fundamental constituent of the extracellular matrix that will necessarily become exposed upon tissue damage. Furthermore, vitronectin binding integrins, αζβ1,3,5,8 and IIbβ3, are found on a variety of cells responsive to injury inclusive of platelets, fibroblasts, myoblasts, vascular smooth muscle cells, and endothelial cells[230-232].
Thus incorporation of plasma derived vitronectin into damaged ECM can be seen as a beneficial response that facilitates wound healing because this arrangement can help dock and anchor restorative cells. Furthermore, because vitronectin is known to bind growth factors such as insulin like growth factor[233], it may be speculated that vitronectin in context of SC5b-9 could deliver the growth mediators to a wound site.
It is also conceivable that complexes of SC5b-9 containing clusterin may also contribute to host recovery from injury. Clusterin is found in HDL containing apolipoprotein A-I but not apolipoprotein A-II[224,234-237]. HDL particles are highly heterogeneous, and whereas HDLs were originally ascribed to function for reverse cholesterol transport, it is now realized that these operate for a diversity of biological roles inclusive of transport of hormones and bioactive lipids, inflammation regulation, clearance, and immune defense against parasites and microorganisms[238-241].
Although investigations about the interface of HDLs and stem/progenitor cell biology are just commencing, a few publications suggest that this will be a fruitful topic for future research. For example HDL can promote MSC proliferation by interaction with Scavenger receptor class B member 1[242]. Also HDL have been shown to advance endothelial cell precursor migration and proliferation[243].
We leave it an open question as to whether HDL-associated SC5b-9 can facilitate wound healing through influence on stem and progenitor cells.
Although complement is best known for its role in inflammation, increasing evidence has accumulated that emphasizes that complement activation and in particular the complement split products C3a and C5a play a role in many scenarios of tissue repair. Table 1 shows a compilation of cell types expressing C3aR and C5aR and the function of these receptors on any particular cell. However there are still many gaps in our understanding of the role of complement activation outside the inflammatory axis. A more complete understanding of the effects of complement activation in stem cell biology will contribute to improve the therapeutic potential of these cells.
The human mesenchymal stem cells employed in this work were provided by the Tulane Center for Regenerative Medicine, now the Texas A and M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott and White through a grant from National Center for Research Resources of the National Institute of Health, Grant # P40RR017447.
| 1. | Carroll MV, Sim RB. Complement in health and disease. Adv Drug Deliv Rev. 2011;63:965-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Rahpeymai Y, Hietala MA, Wilhelmsson U, Fotheringham A, Davies I, Nilsson AK, Zwirner J, Wetsel RA, Gerard C, Pekny M. Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J. 2006;25:1364-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Fang S, Parsa AT. Complement and the central nervous system: emerging roles in development, protection and regeneration. Immunol Cell Biol. 2010;88:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Mastellos DC, Deangelis RA, Lambris JD. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin Immunol. 2013;25:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Mastellos D, Lambris JD. Complement: more than a ‘guard’ against invading pathogens? Trends Immunol. 2002;23:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2932] [Cited by in RCA: 2863] [Article Influence: 178.9] [Reference Citation Analysis (0)] |
| 7. | Fearon DT, Austen KF. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci USA. 1977;74:1683-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 204] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Kouser L, Abdul-Aziz M, Nayak A, Stover CM, Sim RB, Kishore U. Properdin and factor h: opposing players on the alternative complement pathway “see-saw”. Front Immunol. 2013;4:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Ferreira VP, Cortes C, Pangburn MK. Native polymeric forms of properdin selectively bind to targets and promote activation of the alternative pathway of complement. Immunobiology. 2010;215:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Nilsson B, Nilsson Ekdahl K. The tick-over theory revisited: is C3 a contact-activated protein? Immunobiology. 2012;217:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Fearon DT, Austen KF. Properdin: initiation of alternative complement pathway. Proc Natl Acad Sci USA. 1975;72:3220-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Wallis R, Mitchell DA, Schmid R, Schwaeble WJ, Keeble AH. Paths reunited: Initiation of the classical and lectin pathways of complement activation. Immunobiology. 2010;215:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Reid KB, Colomb MG, Loos M. Complement component C1 and the collectins: parallels between routes of acquired and innate immunity. Immunol Today. 1998;19:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kinoshita T, Takata Y, Kozono H, Takeda J, Hong KS, Inoue K. C5 convertase of the alternative complement pathway: covalent linkage between two C3b molecules within the trimolecular complex enzyme. J Immunol. 1988;141:3895-3901. [PubMed] |
| 17. | Takata Y, Kinoshita T, Kozono H, Takeda J, Tanaka E, Hong K, Inoue K. Covalent association of C3b with C4b within C5 convertase of the classical complement pathway. J Exp Med. 1987;165:1494-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Cooper NR, Müller-Eberhard HJ. The reaction mechanism of human C5 in immune hemolysis. J Exp Med. 1970;132:775-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Köhl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753-2766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 554] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 20. | Hugli TE. Structure and function of C3a anaphylatoxin. Curr Top Microbiol Immunol. 1990;153:181-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 776] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 22. | Björk J, Hugli TE, Smedegård G. Microvascular effects of anaphylatoxins C3a and C5a. J Immunol. 1985;134:1115-1119. [PubMed] |
| 23. | Ehrengruber MU, Geiser T, Deranleau DA. Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 1994;346:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Fernandez HN, Henson PM, Otani A, Hugli TE. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978;120:109-115. [PubMed] |
| 25. | DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao P. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J Immunol. 1999;162:1127-1136. [PubMed] |
| 26. | Elsner J, Oppermann M, Czech W, Kapp A. C3a activates the respiratory burst in human polymorphonuclear neutrophilic leukocytes via pertussis toxin-sensitive G-proteins. Blood. 1994;83:3324-3331. [PubMed] |
| 27. | Elsner J, Oppermann M, Czech W, Dobos G, Schöpf E, Norgauer J, Kapp A. C3a activates reactive oxygen radical species production and intracellular calcium transients in human eosinophils. Eur J Immunol. 1994;24:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | McPhail LC, Snyderman R. Activation of the respiratory burst enzyme in human polymorphonuclear leukocytes by chemoattractants and other soluble stimuli. Evidence that the same oxidase is activated by different transductional mechanisms. J Clin Invest. 1983;72:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Norgauer J, Dobos G, Kownatzki E, Dahinden C, Burger R, Kupper R, Gierschik P. Complement fragment C3a stimulates Ca2+ influx in neutrophils via a pertussis-toxin-sensitive G protein. Eur J Biochem. 1993;217:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119-2127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 207] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Zwirner J, Götze O, Moser A, Sieber A, Begemann G, Kapp A, Elsner J, Werfel T. Blood- and skin-derived monocytes/macrophages respond to C3a but not to C3a(desArg) with a transient release of calcium via a pertussis toxin-sensitive signal transduction pathway. Eur J Immunol. 1997;27:2317-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Bokisch VA, Müller-Eberhard HJ. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. J Clin Invest. 1970;49:2427-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 382] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Roglic A, Prossnitz ER, Cavanagh SL, Pan Z, Zou A, Ye RD. cDNA cloning of a novel G protein-coupled receptor with a large extracellular loop structure. Biochim Biophys Acta. 1996;1305:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Ames RS, Li Y, Sarau HM, Nuthulaganti P, Foley JJ, Ellis C, Zeng Z, Su K, Jurewicz AJ, Hertzberg RP. Molecular cloning and characterization of the human anaphylatoxin C3a receptor. J Biol Chem. 1996;271:20231-20234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 184] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614-617. [PubMed] |
| 36. | Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42:9406-9415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 217] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem. 2005;280:39677-39680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol. 2009;46:1149-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, Lee T, Baribault H, Tian H, Yeh WC. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 40. | Li R, Coulthard LG, Wu MC, Taylor SM, Woodruff TM. C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013;27:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 41. | Plummer TH, Hurwitz MY. Human plasma carboxypeptidase N. Isolation and characterization. J Biol Chem. 1978;253:3907-3912. [PubMed] |
| 42. | Levin Y, Skidgel RA, Erdös EG. Isolation and characterization of the subunits of human plasma carboxypeptidase N (kininase i). Proc Natl Acad Sci USA. 1982;79:4618-4622. [PubMed] |
| 43. | Wilken HC, Götze O, Werfel T, Zwirner J. C3a(desArg) does not bind to and signal through the human C3a receptor. Immunol Lett. 1999;67:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Wetsel RA. Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cells. Immunol Lett. 1995;44:183-187. [PubMed] |
| 45. | Erdei A, Kerekes K, Pecht I. Role of C3a and C5a in the activation of mast cells. Exp Clin Immunogenet. 1997;14:16-18. [PubMed] |
| 46. | Girke G, Kohl B, Busch C, John T, Godkin O, Ertel W, Schulze-Tanzil G. Tenocyte activation and regulation of complement factors in response to in vitro cell injury. Mol Immunol. 2014;60:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Busch C, Girke G, Kohl B, Stoll C, Lemke M, Krasnici S, Ertel W, Silawal S, John T, Schulze-Tanzil G. Complement gene expression is regulated by pro-inflammatory cytokines and the anaphylatoxin C3a in human tenocytes. Mol Immunol. 2013;53:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Schulze-Tanzil G, Kohl B, El Sayed K, Arens S, Ertel W, Stölzel K, John T. Anaphylatoxin receptors and complement regulatory proteins in human articular and non-articular chondrocytes: interrelation with cytokines. Cell Tissue Res. 2012;350:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Onuma H, Masuko-Hongo K, Yuan G, Sakata M, Nakamura H, Kato T, Aoki H, Nishioka K. Expression of the anaphylatoxin receptor C5aR (CD88) by human articular chondrocytes. Rheumatol Int. 2002;22:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Yuan G, Wei J, Zhou J, Hu H, Tang Z, Zhang G. Expression of C5aR (CD88) of synoviocytes isolated from patients with rheumatoid arthritis and osteoarthritis. Chin Med J (Engl). 2003;116:1408-1412. [PubMed] |
| 51. | Drouin SM, Kildsgaard J, Haviland J, Zabner J, Jia HP, McCray PB, Tack BF, Wetsel RA. Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J Immunol. 2001;166:2025-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Schraufstatter IU, Trieu K, Sikora L, Sriramarao P, DiScipio R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002;169:2102-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 54. | Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962-5970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Riedemann NC, Guo RF, Sarma VJ, Laudes IJ, Huber-Lang M, Warner RL, Albrecht EA, Speyer CL, Ward PA. Expression and function of the C5a receptor in rat alveolar epithelial cells. J Immunol. 2002;168:1919-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Wilmer WA, Kaumaya PT, Ember JA, Cosio FG. Receptors for the anaphylatoxin C5a (CD88) on human mesangial cells. J Immunol. 1998;160:5646-5652. [PubMed] |
| 57. | Wan JX, Fukuda N, Endo M, Tahira Y, Yao EH, Matsuda H, Ueno T, Matsumoto K. Complement 3 is involved in changing the phenotype of human glomerular mesangial cells. J Cell Physiol. 2007;213:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Daveau M, Benard M, Scotte M, Schouft MT, Hiron M, Francois A, Salier JP, Fontaine M. Expression of a functional C5a receptor in regenerating hepatocytes and its involvement in a proliferative signaling pathway in rat. J Immunol. 2004;173:3418-3424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, Ratajczak J, Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182:3827-3836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 62. | Chmilewsky F, Jeanneau C, Laurent P, About I. Pulp fibroblasts synthesize functional complement proteins involved in initiating dentin-pulp regeneration. Am J Pathol. 2014;184:1991-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Pettersen HB, Johnson E, Hetland G. Human alveolar macrophages synthesize active complement components C6, C7, and C8 in vitro. Scand J Immunol. 1987;25:567-570. [PubMed] |
| 64. | Høgåsen AK, Würzner R, Abrahamsen TG, Dierich MP. Human polymorphonuclear leukocytes store large amounts of terminal complement components C7 and C6, which may be released on stimulation. J Immunol. 1995;154:4734-4740. [PubMed] |
| 65. | Tu Z, Bu H, Dennis JE, Lin F. Efficient osteoclast differentiation requires local complement activation. Blood. 2010;116:4456-4463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Arend WP, Mehta G, Antonioli AH, Takahashi M, Takahashi K, Stahl GL, Holers VM, Banda NK. Roles of adipocytes and fibroblasts in activation of the alternative pathway of complement in inflammatory arthritis in mice. J Immunol. 2013;190:6423-6433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Di Paolo NC, Baldwin LK, Irons EE, Papayannopoulou T, Tomlinson S, Shayakhmetov DM. IL-1α and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog. 2014;10:e1004035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Rutar M, Valter K, Natoli R, Provis JM. Synthesis and propagation of complement C3 by microglia/monocytes in the aging retina. PLoS One. 2014;9:e93343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | de Vries B, Köhl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol. 2003;170:3883-3889. [PubMed] |
| 70. | Zheng X, Zhang X, Feng B, Sun H, Suzuki M, Ichim T, Kubo N, Wong A, Min LR, Budohn ME. Gene silencing of complement C5a receptor using siRNA for preventing ischemia/reperfusion injury. Am J Pathol. 2008;173:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Ducruet AF, Hassid BG, Mack WJ, Sosunov SA, Otten ML, Fusco DJ, Hickman ZL, Kim GH, Komotar RJ, Mocco J. C3a receptor modulation of granulocyte infiltration after murine focal cerebral ischemia is reperfusion dependent. J Cereb Blood Flow Metab. 2008;28:1048-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 286] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 73. | Hammerschmidt DE, Weaver LJ, Hudson LD, Craddock PR, Jacob HS. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980;1:947-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 344] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 74. | Zilow G, Sturm JA, Rother U, Kirschfink M. Complement activation and the prognostic value of C3a in patients at risk of adult respiratory distress syndrome. Clin Exp Immunol. 1990;79:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Vallota EH, Götze O, Spiegelberg HL, Forristal J, West CD, Müller-Eberhard HJ. A serum factor in chronic hypocomplementemic hephritis distinct from immunoglobulins and activating the alternate pathway of complement. J Exp Med. 1974;139:1249-1261. [PubMed] |
| 76. | Moxley G, Ruddy S. Elevated plasma C3 anaphylatoxin levels in rheumatoid arthritis patients. Arthritis Rheum. 1987;30:1097-1104. [PubMed] |
| 77. | Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, Taylor SM, Woodruff TM, Tenner AJ. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J Immunol. 2009;183:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 78. | Sawant-Mane S, Estep A, Koski CL. Antibody of patients with Guillain-Barré syndrome mediates complement-dependent cytolysis of rat Schwann cells: susceptibility to cytolysis reflects Schwann cell phenotype. J Neuroimmunol. 1994;49:145-152. [PubMed] |
| 79. | Mead RJ, Singhrao SK, Neal JW, Lassmann H, Morgan BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol. 2002;168:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 80. | Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103:2328-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 513] [Article Influence: 25.7] [Reference Citation Analysis (8)] |
| 81. | Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010;128:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Bradley DT, Zipfel PF, Hughes AE. Complement in age-related macular degeneration: a focus on function. Eye (Lond). 2011;25:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Garland DL, Fernandez-Godino R, Kaur I, Speicher KD, Harnly JM, Lambris JD, Speicher DW, Pierce EA. Mouse genetics and proteomic analyses demonstrate a critical role for complement in a model of DHRD/ML, an inherited macular degeneration. Hum Mol Genet. 2014;23:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Schoengraf P, Lambris JD, Recknagel S, Kreja L, Liedert A, Brenner RE, Huber-Lang M, Ignatius A. Does complement play a role in bone development and regeneration? Immunobiology. 2013;218:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Leslie JD, Mayor R. Complement in animal development: unexpected roles of a highly conserved pathway. Semin Immunol. 2013;25:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Rutkowski MJ, Sughrue ME, Kane AJ, Ahn BJ, Fang S, Parsa AT. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res. 2010;59:897-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 87. | Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 330] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 88. | Markiewski MM, Mastellos D, Tudoran R, DeAngelis RA, Strey CW, Franchini S, Wetsel RA, Erdei A, Lambris JD. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J Immunol. 2004;173:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Shinjyo N, Ståhlberg A, Dragunow M, Pekny M, Pekna M. Complement-derived anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cells. 2009;27:2824-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 90. | Moll G, Jitschin R, von Bahr L, Rasmusson-Duprez I, Sundberg B, Lönnies L, Elgue G, Nilsson-Ekdahl K, Mougiakakos D, Lambris JD. Mesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responses. PLoS One. 2011;6:e21703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 91. | DiScipio RG, Khaldoyanidi SK, Moya-Castro R, Schraufstatter IU. Complement C3a signaling mediates production of angiogenic factors in mesenchymal stem cells. J Biomed Sci Eng. 2013;6:1-13. [DOI] [Full Text] |
| 92. | Kimura Y, Madhavan M, Call MK, Santiago W, Tsonis PA, Lambris JD, Del Rio-Tsonis K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J Immunol. 2003;170:2331-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 93. | Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, Page KM, Parsons M, Lambris JD, Mayor R. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell. 2011;21:1026-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 94. | Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 95. | Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, Wetsel RA, Lambris JD. The regulation of liver cell survival by complement. J Immunol. 2009;182:5412-5418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | Guo Q, Cheng J, Zhang J, Su B, Bian C, Lin S, Zhong C. Delayed post-injury administration of C5a improves regeneration and functional recovery after spinal cord injury in mice. Clin Exp Immunol. 2013;174:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Ignatius A, Ehrnthaller C, Brenner RE, Kreja L, Schoengraf P, Lisson P, Blakytny R, Recknagel S, Claes L, Gebhard F. The anaphylatoxin receptor C5aR is present during fracture healing in rats and mediates osteoblast migration in vitro. J Trauma. 2011;71:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 98. | Hugli TE, Erickson BW. Synthetic peptides with the biological activities and specificity of human C3a anaphylatoxin. Proc Natl Acad Sci USA. 1977;74:1826-1830. [PubMed] |
| 99. | Emanueli C, Madeddu P. Targeting kinin receptors for the treatment of tissue ischaemia. Trends Pharmacol Sci. 2001;22:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Hartmann K, Henz BM, Krüger-Krasagakes S, Köhl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863-2870. [PubMed] |
| 101. | Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996;157:1693-1698. [PubMed] |
| 102. | Johnson AR, Hugli TE, Müller-Eberhard HJ. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975;28:1067-1080. [PubMed] |
| 103. | Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 104. | Marder SR, Chenoweth DE, Goldstein IM, Perez HD. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985;134:3325-3331. [PubMed] |
| 105. | Nauta AJ, Daha MR, van Kooten C, Roos A. Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 2003;24:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 106. | Flierman R, Daha MR. The clearance of apoptotic cells by complement. Immunobiology. 2007;212:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Franchi N, Ballarin L. Preliminary characterization of complement in a colonial tunicate: C3, Bf and inhibition of C3 opsonic activity by compstatin. Dev Comp Immunol. 2014;46:430-438. [PubMed] [DOI] [Full Text] |
| 108. | Fraser DA, Tenner AJ. Innate immune proteins C1q and mannan-binding lectin enhance clearance of atherogenic lipoproteins by human monocytes and macrophages. J Immunol. 2010;185:3932-3939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 109. | Stienstra R, Dijk W, van Beek L, Jansen H, Heemskerk M, Houtkooper RH, Denis S, van Harmelen V, Willems van Dijk K, Tack CJ. Mannose-binding lectin is required for the effective clearance of apoptotic cells by adipose tissue macrophages during obesity. Diabetes. 2014;63:4143-4153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Nepomuceno RR, Tenner AJ. C1qRP, the C1q receptor that enhances phagocytosis, is detected specifically in human cells of myeloid lineage, endothelial cells, and platelets. J Immunol. 1998;160:1929-1935. [PubMed] |
| 111. | Verneret M, Tacnet-Delorme P, Osman R, Awad R, Grichine A, Kleman JP, Frachet P. Relative contribution of c1q and apoptotic cell-surface calreticulin to macrophage phagocytosis. J Innate Immun. 2014;6:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 112. | Païdassi H, Tacnet-Delorme P, Garlatti V, Darnault C, Ghebrehiwet B, Gaboriaud C, Arlaud GJ, Frachet P. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 113. | Païdassi H, Tacnet-Delorme P, Lunardi T, Arlaud GJ, Thielens NM, Frachet P. The lectin-like activity of human C1q and its implication in DNA and apoptotic cell recognition. FEBS Lett. 2008;582:3111-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Jäkel A, Reid KB, Clark H. Surfactant protein A (SP-A) binds to phosphatidylserine and competes with annexin V binding on late apoptotic cells. Protein Cell. 2010;1:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Duus K, Hansen EW, Tacnet P, Frachet P, Arlaud GJ, Thielens NM, Houen G. Direct interaction between CD91 and C1q. FEBS J. 2010;277:3526-3537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | Tarr J, Eggleton P. Immune function of C1q and its modulators CD91 and CD93. Crit Rev Immunol. 2005;25:305-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Eggleton P, Tenner AJ, Reid KB. C1q receptors. Clin Exp Immunol. 2000;120:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 119. | Sim E, Wood AB, Hsiung LM, Sim RB. Pattern of degradation of human complement fragment, C3b. FEBS Lett. 1981;132:55-60. [PubMed] |
| 120. | Helmy KY, Katschke KJ, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 444] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 121. | Takizawa F, Tsuji S, Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 1996;397:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 512] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 123. | Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004;164:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 124. | Skeie JM, Fingert JH, Russell SR, Stone EM, Mullins RF. Complement component C5a activates ICAM-1 expression on human choroidal endothelial cells. Invest Ophthalmol Vis Sci. 2010;51:5336-5342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 125. | Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 126. | Speidl WS, Kastl SP, Hutter R, Katsaros KM, Kaun C, Bauriedel G, Maurer G, Huber K, Badimon JJ, Wojta J. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J. 2011;25:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 127. | DiScipio RG, Schraufstatter IU, Sikora L, Zuraw BL, Sriramarao P. C5a mediates secretion and activation of matrix metalloproteinase 9 from human eosinophils and neutrophils. Int Immunopharmacol. 2006;6:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | Kurihara R, Yamaoka K, Sawamukai N, Shimajiri S, Oshita K, Yukawa S, Tokunaga M, Iwata S, Saito K, Chiba K. C5a promotes migration, proliferation, and vessel formation in endothelial cells. Inflamm Res. 2010;59:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 129. | Cortright DN, Meade R, Waters SM, Chenard BL, Krause JE. C5a, but not C3a, increases VEGF secretion in ARPE-19 human retinal pigment epithelial cells. Curr Eye Res. 2009;34:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 130. | Nunez-Cruz S, Gimotty PA, Guerra MW, Connolly DC, Wu YQ, DeAngelis RA, Lambris JD, Coukos G, Scholler N. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 2012;14:994-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 131. | Rohrer B, Long Q, Coughlin B, Wilson RB, Huang Y, Qiao F, Tang PH, Kunchithapautham K, Gilkeson GS, Tomlinson S. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:3056-3064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 132. | Langer HF, Chung KJ, Orlova VV, Choi EY, Kaul S, Kruhlak MJ, Alatsatianos M, DeAngelis RA, Roche PA, Magotti P. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116:4395-4403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 133. | Marshall KM, He S, Zhong Z, Atkinson C, Tomlinson S. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J Exp Med. 2014;211:1793-1805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 134. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2126] [Cited by in RCA: 2250] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 135. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1423] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 136. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3303] [Article Influence: 150.1] [Reference Citation Analysis (1)] |
| 137. | Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566-2573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 138. | Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 463] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 139. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 1013] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 140. | Lucchini G, Introna M, Dander E, Rovelli A, Balduzzi A, Bonanomi S, Salvadè A, Capelli C, Belotti D, Gaipa G. Platelet-lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft-versus-host disease in a pediatric population. Biol Blood Marrow Transplant. 2010;16:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 141. | Aaronson SA. Growth factors and cancer. Science. 1991;254:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 879] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 142. | Granero-Moltó F, Weis JA, Miga MI, Landis B, Myers TJ, O’Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 402] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 143. | Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 629] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 144. | Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C, Schlegelberger B, Cornils K, Zustin J, Spiess AN. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One. 2011;6:e14486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 145. | Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1192] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 146. | Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638-14643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 147. | Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 477] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 148. | Bartunek J, Croissant JD, Wijns W, Gofflot S, de Lavareille A, Vanderheyden M, Kaluzhny Y, Mazouz N, Willemsen P, Penicka M. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H1095-H1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 149. | Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 150. | Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Su H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 151. | Schu S, Nosov M, O’Flynn L, Shaw G, Treacy O, Barry F, Murphy M, O’Brien T, Ritter T. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094-2103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 152. | Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010;19:1803-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 153. | Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120:3436-3443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 154. | Lee DS, Yi TG, Lee HJ, Kim SN, Park S, Jeon MS, Song SU. Mesenchymal stem cells infected with Mycoplasma arginini secrete complement C3 to regulate immunoglobulin production in B lymphocytes. Cell Death Dis. 2014;5:e1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 155. | Ignatius A, Schoengraf P, Kreja L, Liedert A, Recknagel S, Kandert S, Brenner RE, Schneider M, Lambris JD, Huber-Lang M. Complement C3a and C5a modulate osteoclast formation and inflammatory response of osteoblasts in synergism with IL-1β. J Cell Biochem. 2011;112:2594-2605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 156. | Soland MA, Bego M, Colletti E, Zanjani ED, St Jeor S, Porada CD, Almeida-Porada G. Mesenchymal stem cells engineered to inhibit complement-mediated damage. PLoS One. 2013;8:e60461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 157. | Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 546] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 158. | Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 159. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1108] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 160. | Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 444] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 161. | Chamberlain G, Wright K, Rot A, Ashton B, Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS One. 2008;3:e2934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 162. | Ciuculescu F, Giesen M, Deak E, Lang V, Seifried E, Henschler R. Variability in chemokine-induced adhesion of human mesenchymal stromal cells. Cytotherapy. 2011;13:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 163. | Hareendran S, Sathishkumar S, Abbas S, Mackay AM, Rajan P. A novel composition for the culture of human adipose stem cells which includes complement C3. Cytotechnology. 2010;62:389-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 164. | Pan ZK. Anaphylatoxins C5a and C3a induce nuclear factor kappaB activation in human peripheral blood monocytes. Biochim Biophys Acta. 1998;1443:90-98. [PubMed] |
| 165. | van den Berk LC, Jansen BJ, Siebers-Vermeulen KG, Roelofs H, Figdor CG, Adema GJ, Torensma R. Mesenchymal stem cells respond to TNF but do not produce TNF. J Leukoc Biol. 2010;87:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 166. | Qiu Y, Marquez-Curtis LA, Janowska-Wieczorek A. Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy. 2012;14:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 167. | Cai K, Wan Y, Wang Z, Wang Y, Zhao X, Bao X. C5a promotes the proliferation of human nasopharyngeal carcinoma cells through PCAF-mediated STAT3 acetylation. Oncol Rep. 2014;32:2260-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 168. | Hawksworth OA, Coulthard LG, Taylor SM, Wolvetang EJ, Woodruff TM. Brief report: complement C5a promotes human embryonic stem cell pluripotency in the absence of FGF2. Stem Cells. 2014;32:3278-3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 169. | Legoedec J, Gasque P, Jeanne JF, Fontaine M. Expression of the complement alternative pathway by human myoblasts in vitro: biosynthesis of C3, factor B, factor H and factor I. Eur J Immunol. 1995;25:3460-3466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 170. | Legoedec J, Gasque P, Jeanne JF, Scotte M, Fontaine M. Complement classical pathway expression by human skeletal myoblasts in vitro. Mol Immunol. 1997;34:735-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 171. | Gasque P, Morgan BP, Legoedec J, Chan P, Fontaine M. Human skeletal myoblasts spontaneously activate allogeneic complement but are resistant to killing. J Immunol. 1996;156:3402-3411. [PubMed] |
| 172. | Andrades JA, Nimni ME, Becerra J, Eisenstein R, Davis M, Sorgente N. Complement proteins are present in developing endochondral bone and may mediate cartilage cell death and vascularization. Exp Cell Res. 1996;227:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 173. | Billiard J, Moran RA, Whitley MZ, Chatterjee-Kishore M, Gillis K, Brown EL, Komm BS, Bodine PV. Transcriptional profiling of human osteoblast differentiation. J Cell Biochem. 2003;89:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 174. | Ehrnthaller C, Huber-Lang M, Nilsson P, Bindl R, Redeker S, Recknagel S, Rapp A, Mollnes T, Amling M, Gebhard F. Complement C3 and C5 deficiency affects fracture healing. PLoS One. 2013;8:e81341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 175. | Kalbasi Anaraki P, Patecki M, Larmann J, Tkachuk S, Jurk K, Haller H, Theilmeier G, Dumler I. Urokinase receptor mediates osteogenic differentiation of mesenchymal stem cells and vascular calcification via the complement C5a receptor. Stem Cells Dev. 2014;23:352-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 176. | Distelmaier K, Adlbrecht C, Jakowitsch J, Winkler S, Dunkler D, Gerner C, Wagner O, Lang IM, Kubicek M. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thromb Haemost. 2009;102:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 177. | Ivey CL, Williams FM, Collins PD, Jose PJ, Williams TJ. Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role for C5a and interleukin-8. J Clin Invest. 1995;95:2720-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 178. | De Hoog VC, Timmers L, Van Duijvenvoorde A, De Jager SC, Van Middelaar BJ, Smeets MB, Woodruff TM, Doevendans PA, Pasterkamp G, Hack CE. Leucocyte expression of complement C5a receptors exacerbates infarct size after myocardial reperfusion injury. Cardiovasc Res. 2014;103:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 179. | Syriga M, Mavroidis M. Complement system activation in cardiac and skeletal muscle pathology: friend or foe? Adv Exp Med Biol. 2013;735:207-218. [PubMed] |
| 180. | Mullick A, Tremblay J, Leon Z, Gros P. A novel role for the fifth component of complement (C5) in cardiac physiology. PLoS One. 2011;6:e22919. [PubMed] |
| 181. | Wysoczynski M, Solanki M, Borkowska S, van Hoose P, Brittian KR, Prabhu SD, Ratajczak MZ, Rokosh G. Complement component 3 is necessary to preserve myocardium and myocardial function in chronic myocardial infarction. Stem Cells. 2014;32:2502-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 182. | Lara-Astiaso D, Izarra A, Estrada JC, Albo C, Moscoso I, Samper E, Moncayo J, Solano A, Bernad A, Díez-Juan A. Complement anaphylatoxins C3a and C5a induce a failing regenerative program in cardiac resident cells. Evidence of a role for cardiac resident stem cells other than cardiomyocyte renewal. Springerplus. 2012;1:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 183. | Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 371] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 184. | Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 185. | Reca R, Wysoczynski M, Yan J, Lambris JD, Ratajczak MZ. The role of third complement component (C3) in homing of hematopoietic stem/progenitor cells into bone marrow. Adv Exp Med Biol. 2006;586:35-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 186. | Ratajczak MZ, Borkowska S, Ratajczak J. An emerging link in stem cell mobilization between activation of the complement cascade and the chemotactic gradient of sphingosine-1-phosphate. Prostaglandins Other Lipid Mediat. 2013;104-105:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 187. | Wysoczynski M, Reca R, Lee H, Wu W, Ratajczak J, Ratajczak MZ. Defective engraftment of C3aR-/- hematopoietic stem progenitor cells shows a novel role of the C3a-C3aR axis in bone marrow homing. Leukemia. 2009;23:1455-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 188. | Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, Janowska-Wieczorek A, Wetsel RA, Ross GD, Ratajczak MZ. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 189. | Wu MC, Brennan FH, Lynch JP, Mantovani S, Phipps S, Wetsel RA, Ruitenberg MJ, Taylor SM, Woodruff TM. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc Natl Acad Sci USA. 2013;110:9439-9444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 190. | Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol. 2000;165:5406-5409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 191. | Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J, Ratajczak MZ. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 192. | Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052-2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 193. | Bénard M, Gonzalez BJ, Schouft MT, Falluel-Morel A, Vaudry D, Chan P, Vaudry H, Fontaine M. Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation. Neuroprotective effect of C5a against apoptotic cell death. J Biol Chem. 2004;279:43487-43496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 194. | O’Barr SA, Caguioa J, Gruol D, Perkins G, Ember JA, Hugli T, Cooper NR. Neuronal expression of a functional receptor for the C5a complement activation fragment. J Immunol. 2001;166:4154-4162. [PubMed] |
| 195. | Mukherjee P, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through mitogen-activated protein kinase-dependent inhibition of caspase 3. J Neurochem. 2001;77:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 196. | Bogestål YR, Barnum SR, Smith PL, Mattisson V, Pekny M, Pekna M. Signaling through C5aR is not involved in basal neurogenesis. J Neurosci Res. 2007;85:2892-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 197. | Järlestedt K, Rousset CI, Ståhlberg A, Sourkova H, Atkins AL, Thornton C, Barnum SR, Wetsel RA, Dragunow M, Pekny M. Receptor for complement peptide C3a: a therapeutic target for neonatal hypoxic-ischemic brain injury. FASEB J. 2013;27:3797-3804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 198. | van Beek J, Nicole O, Ali C, Ischenko A, MacKenzie ET, Buisson A, Fontaine M. Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. Neuroreport. 2001;12:289-293. [PubMed] |
| 199. | Ischenko A, Sayah S, Patte C, Andreev S, Gasque P, Schouft MT, Vaudry H, Fontaine M. Expression of a functional anaphylatoxin C3a receptor by astrocytes. J Neurochem. 1998;71:2487-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 200. | Jauneau AC, Ischenko A, Chatagner A, Benard M, Chan P, Schouft MT, Patte C, Vaudry H, Fontaine M. Interleukin-1beta and anaphylatoxins exert a synergistic effect on NGF expression by astrocytes. J Neuroinflammation. 2006;3:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 201. | Ducruet AF, Zacharia BE, Sosunov SA, Gigante PR, Yeh ML, Gorski JW, Otten ML, Hwang RY, DeRosa PA, Hickman ZL. Complement inhibition promotes endogenous neurogenesis and sustained anti-inflammatory neuroprotection following reperfused stroke. PLoS One. 2012;7:e38664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 202. | Benoit ME, Tenner AJ. Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression. J Neurosci. 2011;31:3459-3469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 203. | Benoit ME, Hernandez MX, Dinh ML, Benavente F, Vasquez O, Tenner AJ. C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid-β neurotoxicity. J Biol Chem. 2013;288:654-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 204. | Bialas AR, Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773-1782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 431] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 205. | Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1901] [Cited by in RCA: 2496] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 206. | Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci USA. 2010;107:7975-7980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 207. | Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci USA. 2003;100:13350-13355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 503] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 208. | Abeyta MJ, Clark AT, Rodriguez RT, Bodnar MS, Pera RA, Firpo MT. Unique gene expression signatures of independently-derived human embryonic stem cell lines. Hum Mol Genet. 2004;13:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 209. | Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 375] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 210. | Koch CA, Jordan CE, Platt JL. Complement-dependent control of teratoma formation by embryonic stem cells. J Immunol. 2006;177:4803-4809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 211. | Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165-2175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 409] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 212. | Haeger M, Bengtson A, Karlsson K, Heideman M. Complement activation and anaphylatoxin (C3a and C5a) formation in preeclampsia and by amniotic fluid. Obstet Gynecol. 1989;73:551-556. [PubMed] |
| 213. | Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198:385.e1-385.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 214. | Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, Branch DW, Goodship T, Fremeaux-Bacchi V, Atkinson JP. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8:e1001013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 215. | Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FH, Bruijn JA, Bloemenkamp KW, Baelde HJ. Preeclampsia is characterized by placental complement dysregulation. Hypertension. 2012;60:1332-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 216. | McLin VA, Hu CH, Shah R, Jamrich M. Expression of complement components coincides with early patterning and organogenesis in Xenopus laevis. Int J Dev Biol. 2008;52:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 217. | Guéguinou N, Huin-Schohn C, Ouzren-Zarhloul N, Ghislin S, Frippiat JP. Molecular cloning and expression analysis of Pleurodeles waltl complement component C3 under normal physiological conditions and environmental stresses. Dev Comp Immunol. 2014;46:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 218. | Usami M, Mitsunaga K, Miyajima A, Sunouchi M, Doi O. Complement component C3 functions as an embryotrophic factor in early postimplantation rat embryos. Int J Dev Biol. 2010;54:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 219. | Haynes T, Luz-Madrigal A, Reis ES, Echeverri Ruiz NP, Grajales-Esquivel E, Tzekou A, Tsonis PA, Lambris JD, Del Rio-Tsonis K. Complement anaphylatoxin C3a is a potent inducer of embryonic chick retina regeneration. Nat Commun. 2013;4:2312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 220. | Denny KJ, Coulthard LG, Jeanes A, Lisgo S, Simmons DG, Callaway LK, Wlodarczyk B, Finnell RH, Woodruff TM, Taylor SM. C5a receptor signaling prevents folate deficiency-induced neural tube defects in mice. J Immunol. 2013;190:3493-3499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 221. | Tschopp J. Ultrastructure of the membrane attack complex of complement. Heterogeneity of the complex caused by different degree of C9 polymerization. J Biol Chem. 1984;259:7857-7863. [PubMed] |
| 222. | Podack ER, Esser AF, Biesecker G, Müller-Eberhard HJ. Membrane attack complex of complement: a structural analysis of its assembly. J Exp Med. 1980;151:301-313. [PubMed] |
| 223. | Kolb WP, Muller-Eberhard HJ. The membrane attack mechanism of complement. Isolation and subunit composition of the C5b-9 complex. J Exp Med. 1975;141:724-735. [PubMed] |
| 224. | Murphy BF, Kirszbaum L, Walker ID, d’Apice AJ. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988;81:1858-1864. [PubMed] |
| 225. | de Silva HV, Stuart WD, Duvic CR, Wetterau JR, Ray MJ, Ferguson DG, Albers HW, Smith WR, Harmony JA. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990;265:13240-13247. [PubMed] |
| 226. | Seiffert D, Smith JW. The cell adhesion domain in plasma vitronectin is cryptic. J Biol Chem. 1997;272:13705-13710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 227. | Høgåsen K, Mollnes TE, Harboe M. Heparin-binding properties of vitronectin are linked to complex formation as illustrated by in vitro polymerization and binding to the terminal complement complex. J Biol Chem. 1992;267:23076-23082. [PubMed] |
| 228. | Plow EF. Vitronectin: back into the spotlight. J Thromb Haemost. 2005;3:873-874. [PubMed] |
| 229. | Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol. 1999;31:539-544. [PubMed] |
| 230. | Preissner KT, Reuning U. Vitronectin in vascular context: facets of a multitalented matricellular protein. Semin Thromb Hemost. 2011;37:408-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 231. | Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864-868. [PubMed] |
| 232. | Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697-715. [PubMed] |
| 233. | Upton Z, Webb H, Hale K, Yandell CA, McMurtry JP, Francis GL, Ballard FJ. Identification of vitronectin as a novel insulin-like growth factor-II binding protein. Endocrinology. 1999;140:2928-2931. [PubMed] |
| 234. | Kirszbaum L, Sharpe JA, Murphy B, d’Apice AJ, Classon B, Hudson P, Walker ID. Molecular cloning and characterization of the novel, human complement-associated protein, SP-40,40: a link between the complement and reproductive systems. EMBO J. 1989;8:711-718. [PubMed] |
| 235. | O’Bryan MK, Baker HW, Saunders JR, Kirszbaum L, Walker ID, Hudson P, Liu DY, Glew MD, d’Apice AJ, Murphy BF. Human seminal clusterin (SP-40,40). Isolation and characterization. J Clin Invest. 1990;85:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 236. | Choi-Miura NH, Sakamoto T, Tobe T, Nakano Y, Tomita M. The role of HDL consisting of SP-40,40, apo A-I, and lipids in the formation of SMAC of complement. J Biochem. 1993;113:484-487. [PubMed] |
| 237. | Jenne DE, Lowin B, Peitsch MC, Böttcher A, Schmitz G, Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem. 1991;266:11030-11036. [PubMed] |
| 238. | Vickers KC, Remaley AT. HDL and cholesterol: life after the divorce? J Lipid Res. 2014;55:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 239. | Kaji H. High-density lipoproteins and the immune system. J Lipids. 2013;2013:684903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 240. | Zhu X, Parks JS. New roles of HDL in inflammation and hematopoiesis. Annu Rev Nutr. 2012;32:161-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 241. | Heinecke JW. The protein cargo of HDL: implications for vascular wall biology and therapeutics. J Clin Lipidol. 2010;4:371-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 242. | Xu J, Qian J, Xie X, Lin L, Ma J, Huang Z, Fu M, Zou Y, Ge J. High density lipoprotein cholesterol promotes the proliferation of bone-derived mesenchymal stem cells via binding scavenger receptor-B type I and activation of PI3K/Akt, MAPK/ERK1/2 pathways. Mol Cell Biochem. 2012;371:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 243. | Zhang Q, Yin H, Liu P, Zhang H, She M. Essential role of HDL on endothelial progenitor cell proliferation with PI3K/Akt/cyclin D1 as the signal pathway. Exp Biol Med (Maywood). 2010;235:1082-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 244. | Klos A, Bank S, Gietz C, Bautsch W, Köhl J, Burg M, Kretzschmar T. C3a receptor on dibutyryl-cAMP-differentiated U937 cells and human neutrophils: the human C3a receptor characterized by functional responses and 125I-C3a binding. Biochemistry. 1992;31:11274-11282. [PubMed] |
| 245. | Huey R, Hugli TE. Characterization of a C5a receptor on human polymorphonuclear leukocytes (PMN). J Immunol. 1985;135:2063-2068. [PubMed] |
| 246. | Gerard NP, Hodges MK, Drazen JM, Weller PF, Gerard C. Characterization of a receptor for C5a anaphylatoxin on human eosinophils. J Biol Chem. 1989;264:1760-1766. [PubMed] |
| 247. | Zwirner J, Werfel T, Wilken HC, Theile E, Götze O. Anaphylatoxin C3a but not C3a(desArg) is a chemotaxin for the mouse macrophage cell line J774. Eur J Immunol. 1998;28:1570-1577. [PubMed] |
| 248. | Soruri A, Riggert J, Schlott T, Kiafard Z, Dettmer C, Zwirner J. Anaphylatoxin C5a induces monocyte recruitment and differentiation into dendritic cells by TNF-alpha and prostaglandin E2-dependent mechanisms. J Immunol. 2003;171:2631-2636. [PubMed] |
| 249. | Ali H, Ahamed J, Hernandez-Munain C, Baron JL, Krangel MS, Patel DD. Chemokine production by G protein-coupled receptor activation in a human mast cell line: roles of extracellular signal-regulated kinase and NFAT. J Immunol. 2000;165:7215-7223. [PubMed] |
| 250. | Werfel T, Kirchhoff K, Wittmann M, Begemann G, Kapp A, Heidenreich F, Götze O, Zwirner J. Activated human T lymphocytes express a functional C3a receptor. J Immunol. 2000;165:6599-6605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 251. | Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 252. | Kawamoto S, Yalcindag A, Laouini D, Brodeur S, Bryce P, Lu B, Humbles AA, Oettgen H, Gerard C, Geha RS. The anaphylatoxin C3a downregulates the Th2 response to epicutaneously introduced antigen. J Clin Invest. 2004;114:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 253. | Zachariae CO, Kaltoft K, Thestrup-Pedersen K. Human T lymphocytes and T-cell lines as target cells for lymphocyte chemotaxis. Arch Dermatol Res. 1992;284:77-81. [PubMed] |
| 254. | Matsuoka K, Park KA, Ito M, Ikeda K, Takeshita S. Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J Bone Miner Res. 2014;29:1522-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 255. | Thangam EB, Venkatesha RT, Zaidi AK, Jordan-Sciutto KL, Goncharov DA, Krymskaya VP, Amrani Y, Panettieri RA, Ali H. Airway smooth muscle cells enhance C3a-induced mast cell degranulation following cell-cell contact. FASEB J. 2005;19:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 256. | Braun MC, Reins RY, Li TB, Hollmann TJ, Dutta R, Rick WA, Teng BB, Ke B. Renal expression of the C3a receptor and functional responses of primary human proximal tubular epithelial cells. J Immunol. 2004;173:4190-4196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 257. | Thurman JM, Lenderink AM, Royer PA, Coleman KE, Zhou J, Lambris JD, Nemenoff RA, Quigg RJ, Holers VM. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178:1819-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 258. | Wan J, Zhou X, Cui J, Zou Z, Xu Y, You D. Role of complement 3 in TNF-α-induced mesenchymal transition of renal tubular epithelial cells in vitro. Mol Biotechnol. 2013;54:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 259. | Boor P, Konieczny A, Villa L, Schult AL, Bücher E, Rong S, Kunter U, van Roeyen CR, Polakowski T, Hawlisch H. Complement C5 mediates experimental tubulointerstitial fibrosis. J Am Soc Nephrol. 2007;18:1508-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 260. | Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol. 1998;160:3543-3554. [PubMed] |
| 261. | Lacy M, Jones J, Whittemore SR, Haviland DL, Wetsel RA, Barnum SR. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes and microglia. J Neuroimmunol. 1995;61:71-78. [PubMed] |
| 262. | Sayah S, Ischenko AM, Zhakhov A, Bonnard AS, Fontaine M. Expression of cytokines by human astrocytomas following stimulation by C3a and C5a anaphylatoxins: specific increase in interleukin-6 mRNA expression. J Neurochem. 1999;72:2426-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 263. | Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)--implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34:986-995. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gao ZL, Guo ZK, Kan L S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK