Published online Aug 26, 2015. doi: 10.4252/wjsc.v7.i7.999
Peer-review started: May 20, 2015
First decision: June 18, 2015
Revised: June 29, 2015
Accepted: July 16, 2015
Article in press: July 18, 2015
Published online: August 26, 2015
Processing time: 100 Days and 5 Hours
Stem cells offer great promise for the treatment of multiple disorders throughout the body. Critical to this premise is the ability to govern stem cell pluripotency, proliferation, and differentiation. The mechanistic target of rapamycin (mTOR), 289-kDa serine/threonine protein kinase, that is a vital component of mTOR Complex 1 and mTOR Complex 2 represents a critical pathway for the oversight of stem cell maintenance. mTOR can control the programmed cell death pathways of autophagy and apoptosis that can yield variable outcomes in stem cell survival and be reliant upon proliferative pathways that include Wnt signaling, Wnt1 inducible signaling pathway protein 1 (WISP1), silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), and trophic factors. mTOR also is a necessary component for the early development and establishment of stem cells as well as having a significant impact in the regulation of the maturation of specific cell phenotypes. Yet, as a proliferative agent, mTOR can not only foster cancer stem cell development and tumorigenesis, but also mediate cell senescence under certain conditions to limit invasive cancer growth. mTOR offers an exciting target for the oversight of stem cell therapies but requires careful consideration of the diverse clinical outcomes that can be fueled by mTOR signaling pathways.
Core tip: Mechanistic target of rapamycin, the mechanistic target of rapamycin, can directly impact stem cell maintenance, proliferation, and differentiation to offer new therapeutic strategies for multiple disease entities.
- Citation: Maiese K. Stem cell guidance through the mechanistic target of rapamycin. World J Stem Cells 2015; 7(7): 999-1009
- URL: https://www.wjgnet.com/1948-0210/full/v7/i7/999.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i7.999
The mechanistic target of rapamycin (mTOR) is a 289-kDa serine/threonine protein kinase that is encoded by a single gene FRAP1[1,2]. mTOR, also known as the mammalian target of rapamycin and the FK506-binding protein 12-rapamycin complex-associated protein 1, oversees a complex array of cellular functions that involve gene transcription, cellular proliferation, senescence, metabolism, survival, and cellular death. The target of rapamycin (TOR) was initially identified in Saccharomyces cerevisiae with the genes TOR1 and TOR2 that encode two isoforms in yeast Tor1 and Tor2 through the use of rapamycin-resistant TOR mutants[3]. Rapamycin is a macrolide antibiotic derived from Streptomyces hygroscopicus that that can inhibit TOR as well as mTOR activity.
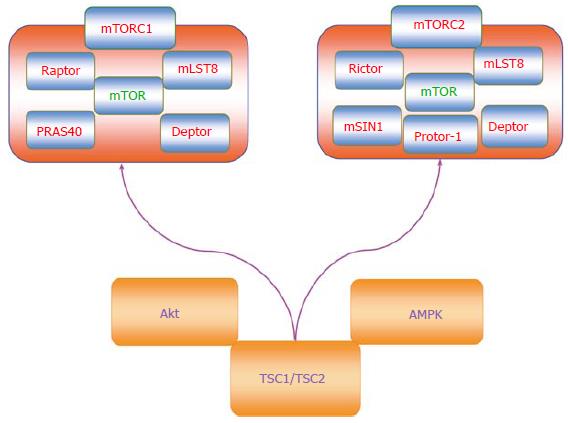
mTOR is a vital component for the function of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) (Figure 1)[4-7]. Rapamycin primarily inhibits mTORC1 by blocking mTOR phosphorylation[8]. However, mTORC2 activity can be limited during chronic administration of rapamycin. mTORC1 is composed of Raptor (Regulatory-Associated Protein of mTOR), the proline rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mLST8/GβL (mammalian lethal with Sec13 protein 8, termed mLST8). Phosphorylation of Raptor through the protein Ras homologue enriched in brain (Rheb) leads to mTORC1 activation. PRAS40 is inhibitory to mTOR activity and can prevent the binding of mTORC1 to Raptor[9]. Phosphorylation of PRAS40 by protein kinase B (Akt) frees PRAS40 from Raptor and allows PRAS40 to be sequestered by the cytoplasmic docking protein 14-3-3 to activate mTORC1[4-7]. Similar to PRAS40, Deptor inhibits mTORC1 activity through the binding of the FAT domain of mTOR (for FKBP associated protein, Ataxia-telangiectasia, and Transactivation/transformation domain-associated protein). In contrast to PRAS40 and Deptor, mLST8 fosters mTOR kinase activity through p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) that bind to Raptor[10]. PRAS40 can block mTORC1 activity by preventing p70S6K and 4EBP1 to associate with Raptor[9,11].
mTOR activity also is controlled by Akt and AMP activated protein kinase (AMPK) through the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex (Figure 1)[12,13]. TSC2 is considered to be a principal site to govern the activity of the TSC1/TSC2 complex that is an inhibitor of mTORC1. As a GTPase-activating protein (GAP) that can convert Ras homologue enriched in brain (Rheb-GTP) to the inactive GDP-bound form (Rheb-GDP), TSC2 prevents the activity of Rheb-GTP and blocks mTORC1 activity by limiting binding of 4EBP1 to mTORC1. Akt can phosphorylate TSC2 to disrupt the TSC1/TSC2 complex, force TSC2 to be sequestered by the cytoplasmic protein 14-3-3, and activate mTORC1[14]. It should be noted that under some cellular protection scenarios, a limited activity of TSC2 as well as AMPK appears necessary since complete knockdown of TSC2 can prevent cellular protection[15].
AMPK also provides a mechanism to control the activity of the TSC1/TSC2 complex, but in contrast to Akt serves to promote TSC2 activity and block mTORC1 function. AMPK phosphorylates TSC2 to enhance GAP activity to process Rheb-GTP into Rheb-GDP that can then block mTORC1 activity. Interestingly, AMPK can influence sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae) (SIRT1) activity that can be critical for stem cell survival and proliferation[16]. AMPK increases the cellular NAD+/NADH ratio that results in the deacetylation of the SIRT1 targets peroxisome proliferator-activated receptor-gamma coactivator 1 (PGC-1α) and forkhead transcription factors FoxO1[17] and FoxO3a[18]. AMPK also can increase nicotinamide phosphoribosyltransferase (NAMPT) activity that catalyzes the conversion of nicotinamide to nicotinamide mononucleotide[19], increases nicotinamide adenine dinucleotide (NAD+) levels, decreases levels of the SIRT1 inhibitor nicotinamide, and promotes SIRT1 transcription[20-22]. SIRT1 up-regulation in combination with AMPK activation promotes the induction of autophagy that can protect endothelial cells exposed to oxidized low-density lipoproteins[23]. Similar to AMPK that is an inhibitor of mTOR, SIRT1 appears to exert its effects over cellular proliferation through blockade of mTOR[24]. SIRT1 inhibits mTOR activity to preserve the integrity of embryonic stem cells during oxidant stress[25]. SIRT1 also inhibits mTOR signaling to foster neuronal growth[26] and assist with mesangial cell proliferation during high glucose exposure[27].
In relation to mTORC2, this complex consists of Rictor (Rapamycin-Insensitive Companion of mTOR), mLST8, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), and the protein observed with Rictor-1 (Protor-1)[28-33]. Rictor and mSIN1 through mTORC2 can activate Akt to promote cell survival[11,29,34]. Protor-1 is a Rictor-binding subunit of mTORC2 and is believed to activate serum and glucocorticoid induced protein kinase 1 (SGK1), since loss of Protor-1 reduces the hydrophobic motif phosphorylation of SGK1 and the substrate N-Myc down-regulated gene 1 in the kidney (NRDG1)[35]. mTORC2 is a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family of protein kinases and is activated by growth factors to control ion transport. mTORC2 also controls cytoskeleton remodeling through protein kinase C-α (PKCα) and oversees cell migration through the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and through Rho signaling. In contrast to mTORC1, mTORC2 is activated by the TSC1/TSC2 complex through the N-terminal region of TSC2 and the C-terminal region of Rictor[36].
mTOR is an important component in the control of programmed cell death that involves autophagy and apoptosis for stem cell proliferation and survival (Figure 2). The process of autophagy recycles cytoplasmic components to remove defective organelles that can no longer be used by the cell[37]. Autophagy has three categories of chaperone-mediated autophagy, microautophagy, and macroautophagy[38]. Chaperone-mediated autophagy uses cytosolic chaperones that transport cytoplasmic components across lysosomal membranes[39]. Microautophagy sequesters components of the cytoplasm through invagination of the lysosomal membrane for digestion[40]. The most prevalent of the three categories is macroautophagy that sequesters cytoplasmic proteins and organelles into autophagosomes. These autophagosomes then fuse with lysosomes for degradation and are recycled for future use[24,41].
In yeast and mammalians, TOR and mTOR are associated with genes that control autophagy[4,6,42]. At least 33 autophagic related genes (Atg) have been identified in yeast that can affect multiple disorders including cancer, diabetes, vascular disease, and neurodegenerative disorders[37,38,40,43-48]. In this group, Atg1 and Atg13 (also known as Apg13) are associated with phosphoinositide 3-kinase (PI 3-K), Akt, and the TOR pathways. When Atg13 is dephosphorylated such as during starvation, Atg1 is active to promote autophagy. Phosphorylation of Atg13 through a TOR dependent pathway releases it from Atg1 and lessens Atg1 activity. In mammals, the homologues of Atg1 are UNC-51 like kinase 1 (ULK1) and ULK2[4]. Mammalian Atg13 binds to ULK1, ULK2, and FIP200 (focal adhesion kinase family interacting protein of 200 kDa) to activate ULKs, promote phosphorylation of FIP200 by ULKs, and lead to the induction of autophagy[49]. Activation of mTOR prevents the induction of autophagy by phosphorylating Atg13 and ULKs to inhibit the ULK-Atg13-FIP200 complex.
Autophagy can become a vital determinant for stem cell survival and proliferation. In some stem cell populations, activation of autophagy can lead to stem cell demise. Breast cancer stem cells have been shown to succumb to apoptosis during the activation of autophagy and the inhibition of Wnt signaling[46]. Wnt proteins are cysteine-rich glycosylated proteins that oversee stem cell proliferation and tumor cell growth[50-56]. Reduction in autophagy also may prevent the development of cellular senescence[57]. Endothelial progenitor cells that lead to the regeneration of vascular endothelium become dysfunctional during exposure to elevated glucose as a result of autophagy activity[58].
However, under other conditions, autophagy appears critical for stem cell survival. In endothelial progenitor cells, SIRT1 activity prevents apoptotic cell death during oxidative stress through the induction of autophagy[59]. In human embryonic stem cells challenged with oxidative stress, autophagy was found to be protective and required SIRT1 activity with the down-regulation of mTOR[25]. Furthermore, activation of SIRT1 is necessary to promote autophagy to maintain proteostasis, produce energy during nutrient deprivation, and maintain muscle stem cell activation[60]. In such cases, SIRT1 may have an inverse relationship with mTOR to foster stem cell survival[16,20].
The programmed cell death pathway of apoptosis also has an important role with mTOR signaling and stem cell survival[61]. During the early stages of apoptotic cell injury, the loss of plasma membrane lipid phosphatidylserine (PS) asymmetry occurs[62-64]. If membrane PS externalization is not reversed and allowed to remain, inflammatory cells are activated that seek out membrane PS positive cells to engulf and remove these cells. Under such circumstances, these membrane PS positive cells may remain functional but their ultimate loss leads to tissue injury[65-71]. During the later phase of apoptotic cell injury, cellular DNA is destroyed which is usually not considered a reversible process[72-75]. During most conditions, activation of mTOR and its related pathways of PI 3-K and Akt can block apoptotic cell death in stem cells. Inhibition of mTOR, such as with rapamycin, leads to endothelial progenitor cell apoptotic death that may be related to inhibition of growth factor signaling[76]. Growth factors that include erythropoietin (EPO)[77,78] are cytoprotective against apoptosis through mTOR activity against sepsis-associated encephalopathy[79], oxidative stress[80], cerebral microglial injury[81], and beta-amyloid (Aβ) toxicity[82]. EPO has been shown to protect retinal progenitor cells from apoptotic cell death during oxidative stress through activation of the PI 3-K, Akt, and mTOR pathways (Figure 2)[83]. Interestingly, protection with EPO and mTOR may be lost with high exogenous EPO concentrations, since elevated concentrations of EPO result in decreased phosphorylation and activity of mTOR with subsequent increased apoptotic cell death[84]. Similar to EPO, other growth factors rely upon mTOR to maintain stem cell integrity (Figure 2). In murine experimental models, mTOR is used by the growth factors epidermal growth factor (EGF) and fibroblast growth factor (FGF) that are protective of stem cells and neurons[85-89] to maintain the proliferation of neural stem and progenitor cells[90]. EGF also uses the PI 3-K, Akt, and mTOR pathways to block cell injury such as during metabolic stress[91] and prevent memory impairment[92]. The growth factor brain derived neurotrophic factor (BDNF) relies upon mTOR activation for memory consolidation[93]. However, in some experimental models with growth factors, mTOR blockade with the induction of autophagy may take precedent over the inhibition of apoptosis to prevent cellular injury. During oxygen deprivation, cortical neurons are protected by BDNF through the induction of autophagy and the inhibition of mTOR[94].
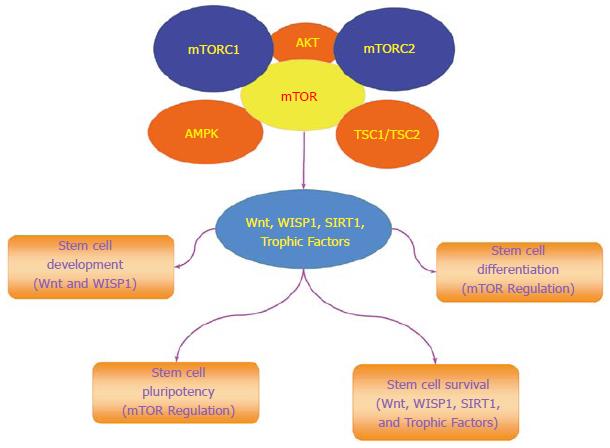
mTOR governs the proliferation and maintenance of stem cell in multiple systems of the body (Figure 2)[4]. The loss of the mTOR gene leads to limited trophoblast growth, faulty implantation, and inability to establish embryonic stem cells[95]. A decrease in proliferation of embryonic stem cells occurs during the deletion of the C-terminal six amino acids of mTOR that blocks the kinase activity of mTOR[96]. mTOR can maintain long-term undifferentiated growth of human embryonic stem cells. Inhibition of mTOR promotes pluripotency, cell proliferation, and blocks mesoderm and endoderm activities in embryonic stem cells[97]. mTOR activity also leads to mesenchymal stem cell senescence[98]. Yet, under some conditions, activation of mTOR signaling components can lead to cell differentiation. In embryonic stem cells, mTOR signaling with p70S6K is limited, but once this signaling is increased, differentiation ensues[99].
In the nervous system, loss of mTORC1 activity in neural stem cells leads to reduced lineage expansion, prevention of differentiation, and blocked neuronal production[100]. Loss of mTOR activity during aging may influence decreased neurogenesis. In the aged brain, mTOR signaling is reduced which leads to a reduction in the proliferation of active neural stem cells[101]. mTOR activity appears important for the timing and control of neurogenesis. Inhibition of mTOR through the RTP801/REDD1 pathway delays neuronal differentiation. However, in newborn and mature neurons, levels of RTP801/REDD1 are diminished with increased mTOR activity to allow for the maturation of neurons[102]. Expression of mTOR is necessary for the neuronal phenotype of post mortem neuronal precursors[103]. Yet, the degree of mTOR activity may independently affect different populations of stem cells since in this model inhibition of mTOR activity leads to cell differentiation into astrocytic cells[90]. Akt and mTORC1 inhibition also has been shown to result in reduced neuronal stem cell self-renewal and earlier neuronal and astroglial differentiation[104]. Neighboring cells also may influence the growth of neuronal stem cells. Endothelial cells can promote mTOR activity and lead to the expansion of long-term glioblastoma stem-like cells[105].
In the cardiovascular system, mTOR is one of several components necessary for the proliferation of human embryonic stem cell-derived cardiomyocytes[106]. The activity of mTOR also controls the proliferation of hematopoietic stem and progenitor cells[107]. Maintenance of hematopoietic stem cells and inhibiting differentiation is tied to mTOR signaling and the reduction in phosphorylation of p70S6K[108]. Failure of endothelial progenitor cell development may be the result of decreased growth factor signaling and loss of mTOR activity[76]. Growth factors such as EPO have been shown to require mTOR activation to regulate bone homeostasis with osteoblastogenesis and osteoclastogenesis[109]. Differentiation of neural precursor cells that may be used for neurodegenerative disorders also is dependent upon EPO and mTOR[103]. mTOR may be necessary to increase angiogenesis from endothelial progenitor cells that may provide neuroprotection during cerebral ischemia[110]. The ability of human amniotic fluid stem cells to influence the differentiation of embryonic kidney cells is dependent upon mTOR activity[111].
During tumorigenesis, mTOR activation may affect neural precursor and oligodendroglial precursor cells to promote high-grade glioma proliferation[112]. Blockade of mTOR can prevent the conversion of astrocytoma cells to oligodendroglioma cells that can lead to glioblastoma multiforme[113]. Inhibition of mTOR signaling may reduce the population of cancer stem cells that can lead to disease recurrence and therapeutic resistance[114].
Under some conditions, mTOR may be protective against tumor cell growth by inhibiting proliferative pathways of Wnt. Wnt signaling can lead to rapid cell proliferation and cancerous growth, but in epithelial stem cells this process is blocked by mTOR that maintains cell senescence and prevents tumor growth[115]. Wnt may result in malignant melanoma[116], metastatic disease[117-120], and glioma proliferation[121,122]. It should be recognized that Wnt signaling also leads to beneficial and cytoprotective effects[52,54,123-125]. Loss of Wnt can result in human monocyte injury[126], impairment in bone repair[127], spinal cord injury[125], loss of neurogenesis[128], inhibition of wound healing[129], loss of stem cell differentiation[130], programmed cell death[38,66,131], and defects in placental development[132]. Wnt signaling activation can block inflammatory cell loss during neurodegenerative disorders[66,70,82,133], prevent cerebral ischemia[134,135], and protect cells during experimental diabetes[136-138]. Furthermore, trophic factors employ cytoprotective pathways of Wnt to prevent cerebral endothelial cell injury[137], preserve mesenchymal stem cells[139], block apoptosis during forkhead transcription factor activation[136,140], promote the maintenance of immune cells in the nervous system[81], and prevent Aβ toxicity in cerebral microglia[82]. However, prolonged exposure of growth factors such as EPO that rely upon Wnt signaling can have ill effects with the proliferation of cancer[141-143], inflammation, and blood-brain barrier injury[144].
In the Wnt signaling pathway, Wnt1 inducible signaling pathway protein 1 (WISP1), also known as CCN4, is a member of the six secreted extracellular matrix associated CCN family of proteins that are involved in cellular survival and stem cell proliferation[145]. WISP1 can activate the components of the mTOR pathway that determine stem cell survival (Figure 2)[21]. WISP1 increases mTOR activity by blocking the inhibitory actions of the mTOR component PRAS40[146]. WISP1 controls the post-translational phosphorylation of AMP activated protein kinase (AMPK), a pathway involved in stem cell proliferation and cellular metabolism[12,147]. WISP1 differentially decreases phosphorylation of TSC2 at Ser1387, a target of AMPK, and increases phosphorylation of TSC2 at Thr1462, a target of Akt[15].
As a tightly linked pathway to mTOR, WISP1 can significantly influence stem cell survival and proliferation. During stem cell migration, WISP1 expression is increased[148]. WISP1 is differentially regulated during human embryonic stem cell and adipose-derived stem cell differentiation. WISP1 expression is increased during human adipocyte differentiation[149] and is repressed in adipose-derived stem cells during hepatic differentiation[51]. WISP1 can modulate induced pluripotent stem cell reprogramming[150,151]. WISP1 is one of several genes that are over-expressed during pancreatic regeneration[152]. WISP1 also may support vascular regeneration during saphenous vein crush injury[153]. WISP1 oversees cellular senescence[154] and does not appear to foster excessive cellular proliferation under circumstances involving aging vascular cells[155]. However, as a proliferative agent, WISP1 can lead to excessive cell growth. WISP1 may promote distant metastatic disease[156] and WISP1 expression is increased in neurofibromatosis type 1 tumorigenesis[157]. Variants of WISP1 can be extremely aggressive in promoting cell growth[158] in comparison to non-variant WISP1 expression that may be protective under specific scenarios and block tumor cell invasion, motility, and metastases[159].
Stem cells represent an important strategy as well as a vital experimental tool for the treatment of multiple disorders that can affect diverse systems of the body that include the brain, cardiovascular system, metabolism, and tumor cell growth. mTOR, a 289-kDa serine/threonine protein kinase and a critical component for the protein complexes of mTORC1 and mTORC2, oversees cellular development, proliferation, and senescence that can directly impact stem cell maintenance, proliferation, and differentiation.
Although mTOR is a highly attractive target to control stem cell maintenance and differentiation, the complexity of this system raises a number of considerations. How does mTOR interface with programmed cell death pathways that can directly affect stem cell populations? mTOR regulates the programmed cell death pathways of autophagy and apoptosis that have a complex outcome in stem cell survival. Through the modulation of Wnt signaling, activation of autophagy that necessitates inhibition of mTOR can block breast cancer stem cell growth. Yet, activation of autophagy that may work in concert with SIRT1 has been shown to play a vital role to maintain muscle stem cell activation and the protection of endothelial progenitor cells. Apoptosis that consists of both an early stage with membrane PS externalization and a late stage involving the destruction of genomic DNA usually relies upon activation of mTOR and its related pathways of PI 3-K, Akt, and growth factors such as EPO, EGF, FGF, and BDNF to block apoptotic cell death in stem cells. However, during some toxic environments, stem cells that become differentiated may require the induction of autophagy with mTOR inhibition to prevent apoptotic cell death.
Another consideration for mTOR is its variable role in the maintenance of stem cell populations and the eventual differentiation of cells into specific phenotypes. mTOR is necessary for trophoblast growth, implantation, the establishment of embryonic stem cells, and the maintenance of pluripotency. Loss of mTOR, such as in the aged brain, may be a factor that results in the reduction of the proliferation of active neural stem cells. Yet, mTOR signaling that can involve p70S6K also affects the modulation of stem cell genesis and cellular differentiation. The activation of mTOR rather that its inhibition can be necessary for stem cell differentiation as well as the ability to selectively promote the maturation of specific cell phenotypes. The control of stem cell development, migration, and proliferation by mTOR can be dependent upon both Wnt and WISP1 signaling.
Ultimately, consideration also must be given for the role mTOR plays to block tumorigenesis and the ability of mTOR signaling to at times accelerate tumor cell growth. Given its proliferative role, mTOR can foster cancer stem cell development and the conversion of differentiated cells into cells that have invasive growth. The degree of mTOR activity may be critical during tumorigenesis, since mTOR in some cell populations can either maintain cell senescence and prevent tumor growth or conversely promote cancer stem cell development that can lead to disease recurrence and therapeutic resistance. By clearly addressing the challenges that lie ahead, targeting mTOR and its signaling pathways offer an exciting approach to translate the development and utilization of stem cells into new therapeutic strategies for multiple disease entities.
| 1. | Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Neasta J, Barak S, Hamida SB, Ron D. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem. 2014;130:172-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905-909. [PubMed] |
| 4. | Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99:128-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246-3256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 473] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 6. | Maiese K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014;46:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21-35. [PubMed] |
| 8. | Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159-168. [PubMed] |
| 9. | Wang H, Zhang Q, Wen Q, Zheng Y, Lazarovici P, Jiang H, Lin J, Zheng W. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895-904. [PubMed] |
| 11. | Maiese K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res. 2014;9:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Chong ZZ, Maiese K. Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc Diabetol. 2012;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Martínez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, Dieguez C, Lopez M. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med. 2014;14:3-21. [PubMed] |
| 14. | Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081-32089. [PubMed] |
| 15. | Shang YC, Chong ZZ, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013;10:29-38. [PubMed] |
| 16. | Maiese K. SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7:235-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond). 2009;116:191-203. [PubMed] |
| 18. | Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325-331. [PubMed] |
| 19. | Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446-3485. [PubMed] |
| 20. | Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Maies K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2015;2015:875961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011;585:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Jin X, Chen M, Yi L, Chang H, Zhang T, Wang L, Ma W, Peng X, Zhou Y, Mi M. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Mol Nutr Food Res. 2014;58:1941-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert Opin Drug Discov. 2013;8:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 26. | Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011;89:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Zhang S, Cai G, Fu B, Feng Z, Ding R, Bai X, Liu W, Zhuo L, Sun L, Liu F. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev. 2012;133:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374-391. [PubMed] |
| 29. | Glidden EJ, Gray LG, Vemuru S, Li D, Harris TE, Mayo MW. Multiple site acetylation of Rictor stimulates mammalian target of rapamycin complex 2 (mTORC2)-dependent phosphorylation of Akt protein. J Biol Chem. 2012;287:581-588. [PubMed] |
| 30. | James MF, Stivison E, Beauchamp R, Han S, Li H, Wallace MR, Gusella JF, Stemmer-Rachamimov AO, Ramesh V. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012;10:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Kamarudin MN, Mohd Raflee NA, Hussein SS, Lo JY, Supriady H, Abdul Kadir H. (R)-(+)-α-lipoic acid protected NG108-15 cells against H₂O₂-induced cell death through PI3K-Akt/GSK-3β pathway and suppression of NF-κβ-cytokines. Drug Des Devel Ther. 2014;8:1765-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Maiese K. Cutting through the complexities of mTOR for the treatment of stroke. Curr Neurovasc Res. 2014;11:177-186. [PubMed] |
| 33. | Tang Z, Baykal AT, Gao H, Quezada HC, Zhang H, Bereczki E, Serhatli M, Baykal B, Acioglu C, Wang S. mTor is a signaling hub in cell survival: a mass-spectrometry-based proteomics investigation. J Proteome Res. 2014;13:2433-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865-1870. [PubMed] |
| 35. | Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011;436:169-179. [PubMed] |
| 36. | Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104-4115. [PubMed] |
| 37. | Vakifahmetoglu-Norberg H, Xia HG, Yuan J. Pharmacologic agents targeting autophagy. J Clin Invest. 2015;125:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 38. | Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 39. | Nakka VP, Prakash-Babu P, Vemuganti R. Crosstalk Between Endoplasmic Reticulum Stress, Oxidative Stress, and Autophagy: Potential Therapeutic Targets for Acute CNS Injuries. Mol Neurobiol. 2014;Dec 9; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 40. | Chen W, Sun Y, Liu K, Sun X. Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res. 2014;9:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Dello Russo C, Lisi L, Feinstein DL, Navarra P. mTOR kinase, a key player in the regulation of glial functions: relevance for the therapy of multiple sclerosis. Glia. 2013;61:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Peng N, Meng N, Wang S, Zhao F, Zhao J, Su L, Zhang S, Zhang Y, Zhao B, Miao J. An activator of mTOR inhibits oxLDL-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein E⁻/⁻ mice. Sci Rep. 2014;4:5519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 43. | Chen Y, Liu XR, Yin YQ, Lee CJ, Wang FT, Liu HQ, Wu XT, Liu J. Unravelling the multifaceted roles of Atg proteins to improve cancer therapy. Cell Prolif. 2014;47:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | François A, Rioux Bilan A, Quellard N, Fernandez B, Janet T, Chassaing D, Paccalin M, Terro F, Page G. Longitudinal follow-up of autophagy and inflammation in brain of APPswePS1dE9 transgenic mice. J Neuroinflammation. 2014;11:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | François A, Terro F, Quellard N, Fernandez B, Chassaing D, Janet T, Rioux Bilan A, Paccalin M, Page G. Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Mol Brain. 2014;7:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y, Zhu J, Mi M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS One. 2014;9:e102535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 47. | Geng Y, Ju Y, Ren F, Qiu Y, Tomita Y, Tomoeda M, Kishida M, Wang Y, Jin L, Su F. Insulin receptor substrate 1/2 (IRS1/2) regulates Wnt/β-catenin signaling through blocking autophagic degradation of dishevelled2. J Biol Chem. 2014;289:11230-11241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Wu H, Lu MH, Wang W, Zhang MY, Zhu QQ, Xia YY, Xu RX, Yang Y, Chen LH, Ma QH. Lamotrigine Reduces β-Site AβPP-Cleaving Enzyme 1 Protein Levels Through Induction of Autophagy. J Alzheimers Dis. 2015;Apr 8; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992-2003. [PubMed] |
| 50. | Berwick DC, Harvey K. The regulation and deregulation of Wnt signaling by PARK genes in health and disease. J Mol Cell Biol. 2014;6:3-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Heo J, Ahn EK, Jeong HG, Kim YH, Leem SH, Lee SJ, Park EK, Yang M. Transcriptional characterization of Wnt pathway during sequential hepatic differentiation of human embryonic stem cells and adipose tissue-derived stem cells. Biochem Biophys Res Commun. 2013;434:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006;21:103-124. [PubMed] |
| 53. | Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58-81. [PubMed] |
| 54. | Thorfve A, Lindahl C, Xia W, Igawa K, Lindahl A, Thomsen P, Palmquist A, Tengvall P. Hydroxyapatite coating affects the Wnt signaling pathway during peri-implant healing in vivo. Acta Biomater. 2014;10:1451-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Wexler EM, Rosen E, Lu D, Osborn GE, Martin E, Raybould H, Geschwind DH. Genome-wide analysis of a Wnt1-regulated transcriptional network implicates neurodegenerative pathways. Sci Signal. 2011;4:ra65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Zeljko M, Pecina-Slaus N, Martic TN, Kusec V, Beros V, Tomas D. Molecular alterations of E-cadherin and beta-catenin in brain metastases. Front Biosci (Elite Ed). 2011;3:616-624. [PubMed] |
| 57. | Chun P. Role of sirtuins in chronic obstructive pulmonary disease. Arch Pharm Res. 2015;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Kim KA, Shin YJ, Akram M, Kim ES, Choi KW, Suh H, Lee CH, Bae ON. High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biol Pharm Bull. 2014;37:1248-1252. [PubMed] |
| 59. | Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, Špes A, Turk B, Goligorsky MS. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol. 2012;180:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 2014;33:2782-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 61. | Maiese K. mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Maiese K. Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr Neurovasc Res. 2015;12:173-188. [PubMed] |
| 63. | Weinberg E, Maymon T, Weinreb M. AGEs induce caspase-mediated apoptosis of rat BMSCs via TNFα production and oxidative stress. J Mol Endocrinol. 2014;52:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | Yang Y, Li H, Hou S, Hu B, Liu J, Wang J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One. 2013;8:e65309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 65. | Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Müller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91:601-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150-1162. [PubMed] |
| 67. | Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7:95-112. [PubMed] |
| 68. | Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262-275. [PubMed] |
| 69. | Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116:993-1001. [PubMed] |
| 70. | Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22:1317-1329. [PubMed] |
| 71. | Viola G, Bortolozzi R, Hamel E, Moro S, Brun P, Castagliuolo I, Ferlin MG, Basso G. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem Pharmacol. 2012;83:16-26. [PubMed] |
| 72. | Kim S, Kang IH, Nam JB, Cho Y, Chung DY, Kim SH, Kim JS, Cho YD, Hong EK, Sohn NW. Ameliorating the effect of astragaloside IV on learning and memory deficit after chronic cerebral hypoperfusion in rats. Molecules. 2015;20:1904-1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Maiese K. Novel applications of trophic factors, wnt and wisp for neuronal repair and regeneration in metabolic disease. Neural Regenn Res. 2015;10:518-528. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 74. | Xin YJ, Yuan B, Yu B, Wang YQ, Wu JJ, Zhou WH, Qiu Z. Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Sci Rep. 2015;5:7645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 75. | Yu T, Li L, Chen T, Liu Z, Liu H, Li Z. Erythropoietin attenuates advanced glycation endproducts-induced toxicity of Schwann cells in vitro. Neurochem Res. 2015;40:698-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Miriuka SG, Rao V, Peterson M, Tumiati L, Delgado DH, Mohan R, Ramzy D, Stewart D, Ross HJ, Waddell TK. mTOR inhibition induces endothelial progenitor cell death. Am J Transplant. 2006;6:2069-2079. [PubMed] |
| 77. | Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217-234. [PubMed] |
| 78. | Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90-95. [PubMed] |
| 79. | Wang GB, Ni YL, Zhou XP, Zhang WF. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem. 2014;385:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 is an integral regulatory component of erythropoietin mTOR signaling and cytoprotection. PLoS One. 2012;7:e45456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8:270-285. [PubMed] |
| 82. | Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of β-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY). 2012;4:187-201. [PubMed] |
| 83. | Sanghera KP, Mathalone N, Baigi R, Panov E, Wang D, Zhao X, Hsu H, Wang H, Tropepe V, Ward M. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci. 2011;47:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Andreucci M, Fuiano G, Presta P, Lucisano G, Leone F, Fuiano L, Bisesti V, Esposito P, Russo D, Memoli B. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009;42:554-561. [PubMed] |
| 85. | Chang ZY, Yeh MK, Chiang CH, Chen YH, Lu DW. Erythropoietin protects adult retinal ganglion cells against NMDA-, trophic factor withdrawal-, and TNF-α-induced damage. PLoS One. 2013;8:e55291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells’ generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014;2014:628591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 87. | Maiese K. Protein kinase C modulates the protective ability of peptide growth factors during anoxia. J Auton Nerv Syst. 1994;49 Suppl:S187-S193. [PubMed] |
| 88. | Maiese K, Boccone L. Neuroprotection by peptide growth factors against anoxia and nitric oxide toxicity requires modulation of protein kinase C. J Cereb Blood Flow Metab. 1995;15:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Maiese K, Boniece I, DeMeo D, Wagner JA. Peptide growth factors protect against ischemia in culture by preventing nitric oxide toxicity. J Neurosci. 1993;13:3034-3040. [PubMed] |
| 90. | Sato A, Sunayama J, Matsuda K, Tachibana K, Sakurada K, Tomiyama A, Kayama T, Kitanaka C. Regulation of neural stem/progenitor cell maintenance by PI3K and mTOR. Neurosci Lett. 2010;470:115-120. [PubMed] |
| 91. | Kimura R, Okouchi M, Kato T, Imaeda K, Okayama N, Asai K, Joh T. Epidermal growth factor receptor transactivation is necessary for glucagon-like peptide-1 to protect PC12 cells from apoptosis. Neuroendocrinology. 2013;97:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Ramanan VK, Nho K, Shen L, Risacher SL, Kim S, McDonald BC, Farlow MR, Foroud TM, Gao S, Soininen H. Shaw LM, Trojanowski JQ, Weiner MW, Green RC, Toga AW, De Jager PL, Yu L, Bennett DA, Saykin AJ. FASTKD2 is associated with memory and hippocampal structure in older adults. Mol Psychiatry. 2014;Nov 11; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. [PubMed] |
| 94. | Chen A, Xiong LJ, Tong Y, Mao M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep. 2013;8:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 95. | Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508-9516. [PubMed] |
| 96. | Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710-6718. [PubMed] |
| 97. | Zhou J, Su P, Wang L, Chen J, Zimmermann M, Genbacev O, Afonja O, Horne MC, Tanaka T, Duan E. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci USA. 2009;106:7840-7845. [PubMed] |
| 98. | Zhang D, Yan B, Yu S, Zhang C, Wang B, Wang Y, Wang J, Yuan Z, Zhang L, Pan J. Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxid Med Cell Longev. 2015;2015:867293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 99. | Easley CA, Ben-Yehudah A, Redinger CJ, Oliver SL, Varum ST, Eisinger VM, Carlisle DL, Donovan PJ, Schatten GP. mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells. Cell Reprogram. 2010;12:263-273. [PubMed] |
| 100. | Hartman NW, Lin TV, Zhang L, Paquelet GE, Feliciano DM, Bordey A. mTORC1 targets the translational repressor 4E-BP2, but not S6 kinase 1/2, to regulate neural stem cell self-renewal in vivo. Cell Rep. 2013;5:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 101. | Romine J, Gao X, Xu XM, So KF, Chen J. The proliferation of amplifying neural progenitor cells is impaired in the aging brain and restored by the mTOR pathway activation. Neurobiol Aging. 2015;36:1716-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 102. | Malagelada C, López-Toledano MA, Willett RT, Jin ZH, Shelanski ML, Greene LA. RTP801/REDD1 regulates the timing of cortical neurogenesis and neuron migration. J Neurosci. 2011;31:3186-3196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 103. | Marfia G, Madaschi L, Marra F, Menarini M, Bottai D, Formenti A, Bellardita C, Di Giulio AM, Carelli S, Gorio A. Adult neural precursors isolated from post mortem brain yield mostly neurons: an erythropoietin-dependent process. Neurobiol Dis. 2011;43:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Magri L, Cambiaghi M, Cominelli M, Alfaro-Cervello C, Cursi M, Pala M, Bulfone A, Garcìa-Verdugo JM, Leocani L, Minicucci F. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell. 2011;9:447-462. [PubMed] |
| 105. | Galan-Moya EM, Le Guelte A, Lima Fernandes E, Thirant C, Dwyer J, Bidere N, Couraud PO, Scott MG, Junier MP, Chneiweiss H. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep. 2011;12:470-476. [PubMed] |
| 106. | Földes G, Mioulane M, Wright JS, Liu AQ, Novak P, Merkely B, Gorelik J, Schneider MD, Ali NN, Harding SE. Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy? J Mol Cell Cardiol. 2011;50:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 107. | Benyoucef A, Calvo J, Renou L, Arcangeli ML, van den Heuvel A, Amsellem S, Mehrpour M, Larghero J, Soler E, Naguibneva I. The SCL/TAL1 Transcription Factor Represses the Stress Protein DDiT4/REDD1 in Human Hematopoietic Stem/Progenitor Cells. Stem Cells. 2015;33:2268-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Iriuchishima H, Takubo K, Matsuoka S, Onoyama I, Nakayama KI, Nojima Y, Suda T. Ex vivo maintenance of hematopoietic stem cells by quiescence induction through Fbxw7& amp; alpha; overexpression. Blood. 2011;117:2373-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, Wang J, Krebsbach PH, Taichman RS. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 110. | Moon HE, Byun K, Park HW, Kim JH, Hur J, Park JS, Jun JK, Kim HS, Paek SL, Kim IK. COMP-Ang1 Potentiates EPC Treatment of Ischemic Brain Injury by Enhancing Angiogenesis Through Activating AKT-mTOR Pathway and Promoting Vascular Migration Through Activating Tie2-FAK Pathway. Exp Neurobiol. 2015;24:55-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Siegel N, Rosner M, Unbekandt M, Fuchs C, Slabina N, Dolznig H, Davies JA, Lubec G, Hengstschläger M. Contribution of human amniotic fluid stem cells to renal tissue formation depends on mTOR. Hum Mol Genet. 2010;19:3320-3331. [PubMed] |
| 112. | Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 2015;161:803-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 678] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 113. | Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7:356-368. [PubMed] |
| 114. | Kolev VN, Wright QG, Vidal CM, Ring JE, Shapiro IM, Ricono J, Weaver DT, Padval MV, Pachter JA, Xu Q. PI3K/mTOR dual inhibitor VS-5584 preferentially targets cancer stem cells. Cancer Res. 2015;75:446-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 115. | Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 315] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 116. | Uzdensky AB, Demyanenko SV, Bibov MY. Signal transduction in human cutaneous melanoma and target drugs. Curr Cancer Drug Targets. 2013;13:843-866. [PubMed] |
| 117. | James RG, Davidson KC, Bosch KA, Biechele TL, Robin NC, Taylor RJ, Major MB, Camp ND, Fowler K, Martins TJ. WIKI4, a novel inhibitor of tankyrase and Wnt/ß-catenin signaling. PLoS One. 2012;7:e50457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 118. | Kafka A, Bašić-Kinda S, Pećina-Šlaus N. The cellular story of dishevelleds. Croat Med J. 2014;55:459-467. [PubMed] |
| 119. | Klinke DJ. Induction of Wnt-inducible signaling protein-1 correlates with invasive breast cancer oncogenesis and reduced type 1 cell-mediated cytotoxic immunity: a retrospective study. PLoS Comput Biol. 2014;10:e1003409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 120. | Knoblich K, Wang HX, Sharma C, Fletcher AL, Turley SJ, Hemler ME. Tetraspanin TSPAN12 regulates tumor growth and metastasis and inhibits β-catenin degradation. Cell Mol Life Sci. 2014;71:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 121. | Liu Y, Yan W, Zhang W, Chen L, You G, Bao Z, Wang Y, Wang H, Kang C, Jiang T. MiR-218 reverses high invasiveness of glioblastoma cells by targeting the oncogenic transcription factor LEF1. Oncol Rep. 2012;28:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 122. | Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin W, Zhang Y. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1. Cancer Res. 2013;73:6046-6055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 123. | Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert Opin Ther Targets. 2008;12:905-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 124. | Wang S, Sun Z, Zhang X, Li Z, Wu M, Zhao W, Wang H, Chen T, Yan H, Zhu J. Wnt1 positively regulates CD36 expression via TCF4 and PPAR-γ in macrophages. Cell Physiol Biochem. 2015;35:1289-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | Xu D, Zhao W, Pan G, Qian M, Zhu X, Liu W, Cai G, Cui Z. Expression of Nemo-like kinase after spinal cord injury in rats. J Mol Neurosci. 2014;52:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Borrell-Pagès M, Romero JC, Badimon L. LRP5 negatively regulates differentiation of monocytes through abrogation of Wnt signalling. J Cell Mol Med. 2014;18:314-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Macsai CE, Georgiou KR, Foster BK, Zannettino AC, Xian CJ. Microarray expression analysis of genes and pathways involved in growth plate cartilage injury responses and bony repair. Bone. 2012;50:1081-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 128. | L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Deleidi M, Serapide MF, Pluchino S, Marchetti B. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves cross talk between inflammatory and Wnt/β-catenin signaling pathways: functional consequences for neuroprotection and repair. J Neurosci. 2012;32:2062-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 129. | Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han J, Zhu X, Tang C, Hu D. Wnt/β-catenin pathway forms a negative feedback loop during TGF-β1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci. 2012;65:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 130. | Shah N, Morsi Y, Manasseh R. From mechanical stimulation to biological pathways in the regulation of stem cell fate. Cell Biochem Funct. 2014;32:309-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 131. | He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054-3058. [PubMed] |
| 132. | Kohan-Ghadr HR, Smith LC, Arnold DR, Murphy BD, Lefebvre RC. Aberrant expression of E-cadherin and β-catenin proteins in placenta of bovine embryos derived from somatic cell nuclear transfer. Reprod Fertil Dev. 2012;24:588-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Marchetti B, Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol Med. 2013;19:144-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 134. | Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3:153-165. [PubMed] |
| 135. | Xing Y, Zhang X, Zhao K, Cui L, Wang L, Dong L, Li Y, Liu Z, Wang C, Zhang X. Beneficial effects of sulindac in focal cerebral ischemia: a positive role in Wnt/β-catenin pathway. Brain Res. 2012;1482:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3β, and β-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103-120. [PubMed] |
| 137. | Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194-204. [PubMed] |
| 138. | Pandey S. Targeting Wnt-Frizzled signaling in cardiovascular diseases. Mol Biol Rep. 2013;40:6011-6018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 139. | Danielyan L, Schäfer R, Schulz A, Ladewig T, Lourhmati A, Buadze M, Schmitt AL, Verleysdonk S, Kabisch D, Koeppen K. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: the critical role of erythropoietin. Cell Death Differ. 2009;16:1599-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 140. | Maiese K. Foxo proteins in the nervous system. Anal Cell Pathol (Amst). 2015;2015:Article ID 569392. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 141. | Hedley BD, Allan AL, Xenocostas A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin Cancer Res. 2011;17:6373-6380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 143. | Zhang C, Li Z, Cao Q, Qin C, Cai H, Zhou H, Qian J, Tao L, Ju X, Yin C. Association of erythropoietin gene rs576236 polymorphism and risk of adrenal tumors in a Chinese population. J Biomed Res. 2014;28:456-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 144. | Ogunshola OO, Moransard M, Gassmann M. Constitutive excessive erythrocytosis causes inflammation and increased vascular permeability in aged mouse brain. Brain Res. 2013;1531:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Maiese K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr Neurovasc Res. 2014;11:378-389. [PubMed] |
| 146. | Shang YC, Chong ZZ, Wang S, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern β-amyloid apoptotic injury of microglia. Curr Neurovasc Res. 2012;9:239-249. [PubMed] |
| 147. | Xia W, Zhang F, Xie C, Jiang M, Hou M. Macrophage migration inhibitory factor confers resistance to senescence through CD74-dependent AMPK-FOXO3a signaling in mesenchymal stem cells. Stem Cell Res Ther. 2015;6:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 148. | Lough D, Dai H, Yang M, Reichensperger J, Cox L, Harrison C, Neumeister MW. Stimulation of the follicular bulge LGR5+ and LGR6+ stem cells with the gut-derived human alpha defensin 5 results in decreased bacterial presence, enhanced wound healing, and hair growth from tissues devoid of adnexal structures. Plast Reconstr Surg. 2013;132:1159-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 149. | Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Döcke S, Keyhani-Nejad F, Gögebakan Ö, Osterhoff M, Kemper M, Hornemann S. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64:856-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 150. | Jung DW, Kim WH, Williams DR. Reprogram or reboot: small molecule approaches for the production of induced pluripotent stem cells and direct cell reprogramming. ACS Chem Biol. 2014;9:80-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 151. | Yang CS, Lopez CG, Rana TM. Discovery of nonsteroidal anti-inflammatory drug and anticancer drug enhancing reprogramming and induced pluripotent stem cell generation. Stem Cells. 2011;29:1528-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 152. | Lim HW, Lee JE, Shin SJ, Lee YE, Oh SH, Park JY, Seong JK, Park JS. Identification of differentially expressed mRNA during pancreas regeneration of rat by mRNA differential display. Biochem Biophys Res Commun. 2002;299:806-812. [PubMed] |
| 153. | Price RM, Tulsyan N, Dermody JJ, Schwalb M, Soteropoulos P, Castronuovo JJ. Gene expression after crush injury of human saphenous vein: using microarrays to define the transcriptional profile. J Am Coll Surg. 2004;199:411-418. [PubMed] |
| 154. | Du J, Klein JD, Hassounah F, Zhang J, Zhang C, Wang XH. Aging increases CCN1 expression leading to muscle senescence. Am J Physiol Cell Physiol. 2014;306:C28-C36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 155. | Marchand A, Atassi F, Gaaya A, Leprince P, Le Feuvre C, Soubrier F, Lompré AM, Nadaud S. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011;10:220-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 156. | Ono M, Inkson CA, Sonn R, Kilts TM, de Castro LF, Maeda A, Fisher LW, Robey PG, Berendsen AD, Li L. WISP1/CCN4: a potential target for inhibiting prostate cancer growth and spread to bone. PLoS One. 2013;8:e71709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 157. | Pasmant E, Ortonne N, Rittié L, Laurendeau I, Lévy P, Lazar V, Parfait B, Leroy K, Dessen P, Valeyrie-Allanore L. Differential expression of CCN1/CYR61, CCN3/NOV, CCN4/WISP1, and CCN5/WISP2 in neurofibromatosis type 1 tumorigenesis. J Neuropathol Exp Neurol. 2010;69:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 158. | Tanaka S, Sugimachi K, Kameyama T, Maehara S, Shirabe K, Shimada M, Wands JR, Maehara Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 159. | Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278:11465-11470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hopfner M, Hung LY S- Editor: Ji FF L- Editor: A E- Editor: Wu HL