Published online Aug 26, 2015. doi: 10.4252/wjsc.v7.i7.1064
Peer-review started: February 22, 2015
First decision: April 27, 2015
Revised: May 27, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: August 26, 2015
Processing time: 187 Days and 4.1 Hours
AIM: To devise a simplified and efficient method for long-term culture and maintenance of embryonic stem cells requiring less frequent passaging.
METHODS: Mouse embryonic stem cells (ESCs) labeled with enhanced yellow fluorescent protein were cultured in three-dimensional (3-D) self-assembling scaffolds and compared with traditional two-dimentional (2-D) culture techniques requiring mouse embryonic fibroblast feeder layers or leukemia inhibitory factor. 3-D scaffolds encapsulating ESCs were prepared by mixing ESCs with polyethylene glycol tetra-acrylate (PEG-4-Acr) and thiol-functionalized dextran (Dex-SH). Distribution of ESCs in 3-D was monitored by confocal microscopy. Viability and proliferation of encapsulated cells during long-term culture were determined by propidium iodide as well as direct cell counts and PrestoBlue (PB) assays. Genetic expression of pluripotency markers (Oct4, Nanog, Klf4, and Sox2) in ESCs grown under 2-D and 3-D culture conditions was examined by quantitative real-time polymerase chain reaction. Protein expression of selected stemness markers was determined by two different methods, immunofluorescence staining (Oct4 and Nanog) and western blot analysis (Oct4, Nanog, and Klf4). Pluripotency of 3-D scaffold grown ESCs was analyzed by in vivo teratoma assay and in vitro differentiation via embryoid bodies into cells of all three germ layers.
RESULTS: Self-assembling scaffolds encapsulating ESCs for 3-D culture without the loss of cell viability were prepared by mixing PEG-4-Acr and Dex-SH (1:1 v/v) to a final concentration of 5% (w/v). Scaffold integrity was dependent on the degree of thiol substitution of Dex-SH and cell concentration. Scaffolds prepared using Dex-SH with 7.5% and 33% thiol substitution and incubated in culture medium maintained their integrity for 11 and 13 d without cells and 22 ± 5 d and 37 ± 5 d with cells, respectively. ESCs formed compact colonies, which progressively increased in size over time due to cell proliferation as determined by confocal microscopy and PB staining. 3-D scaffold cultured ESCs expressed significantly higher levels (P < 0.01) of Oct4, Nanog, and Kl4, showing a 2.8, 3.0 and 1.8 fold increase, respectively, in comparison to 2-D grown cells. A similar increase in the protein expression levels of Oct4, Nanog, and Klf4 was observed in 3-D grown ESCs. However, when 3-D cultured ESCs were subsequently passaged in 2-D culture conditions, the level of these pluripotent markers was reduced to normal levels. 3-D grown ESCs produced teratomas and yielded cells of all three germ layers, expressing brachyury (mesoderm), NCAM (ectoderm), and GATA4 (endoderm) markers. Furthermore, these cells differentiated into osteogenic, chondrogenic, myogenic, and neural lineages expressing Col1, Col2, Myog, and Nestin, respectively.
CONCLUSION: This novel 3-D culture system demonstrated long-term maintenance of mouse ESCs without the routine passaging and manipulation necessary for traditional 2-D cell propagation.
Core tip: The pluripotent nature of embryonic stem cells (ESCs) makes them an ideal source for cell-based therapeutics and regenerative medicine. Efficient and reproducible expansion of ESCs ex vivo is critical for high quality cells for translational applications. However, propagation of ESCs is technically challenging, and often leads to differentiation due to inefficient two-dimensional culture techniques in vitro. To mimic the three-dimensional microenvironment in vivo, self-assembling scaffolds made from thiol-functionalized dextran and polyethylene glycol tetra-acrylate were designed to encapsulate and propagate mouse ESCs. This culture system is simple, robust, efficient and reproducible, permitting long-term maintenance of ESCs without routine passaging and manipulation.
- Citation: McKee C, Perez-Cruet M, Chavez F, Chaudhry GR. Simplified three-dimensional culture system for long-term expansion of embryonic stem cells. World J Stem Cells 2015; 7(7): 1064-1077
- URL: https://www.wjgnet.com/1948-0210/full/v7/i7/1064.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i7.1064
The pluripotent state of embryonic stem cells (ESCs) allows their use in a wide array of translational and clinical applications[1]. Mouse ESCs have been used to investigate developmental and diseases processes, toxicology, cell-based therapeutics and regenerative medicine[2,3]. Research performed using animal models[4,5] and more recently human clinical trials[6,7] have shown promising potential for ESCs in cell therapies, repair of damaged tissues and organs, and in vitro disease modeling. However, these applications require routine and efficient expansion of pluripotent ESCs and controlled differentiation to obtain a homogenous population of cells. The pluripotency of ESCs is controlled by an intrinsic regulatory network[8] and extrinsic factors including the microenvironment, organization and composition of the extracellular matrix (ECM), cell-cell signaling, and the temporal and spatial gradient of soluble factors[9-12]. The complex relationship between stem cell fate and their native microenvironment results in a large discrepancy between in vivo and in vitro culture conditions effecting the quality of cultured cells[13].
Conventionally, ESCs are grown in two-dimensional (2-D) plastic culture plates on mouse embryonic fibroblast (MEF) feeder layers or ECM components (such as gelatin and Matrigel)[14]. Mouse ESCs can be maintained in their pluripotent state by the addition of soluble cytokines, such as leukemia inhibitory factor (LIF), to the culture media[11,15]. However, reliance on MEF feeder layer, cytokines, and/or growth factors complicates maintenance of ESCs due to the potential transmission of xenogeneic pathogens and the fluctuation of lot-to-lot quality[9]. Furthermore, the distribution of soluble factors in 2-D culture lacks the spatial gradient observed in three-dimensional (3-D) microenvironments, which can alter cell growth and fate determination[16]. Studies have shown that the ECM composition and organization send mechanical signals for cell differentiation and the culture of ESCs in 2-D culture can signal differentiation into specific cell lineages[17]. For these reasons, the maintenance of the self-renewing state of pluripotent ESCs and induced-pluripotent stem cells remains a challenge[18]. In addition to strict culture media and growth conditions, ESCs require regular passaging (every 2 to 3 d). Consequently, culturing of ESCs is laborious, expensive and requires a high level of expertise[19].
In order to overcome the problems associated with 2-D culture, we hypothesized that 3-D culture may better mimic the in vivo environment supporting the growth and maintenance of ESC pluripotency. 3-D growth of ESCs can be facilitated by hydrogel scaffolds, composed of hydrophilic polymer networks, which emulate the fully hydrated native ECM and natural soft tissue[20]. Hydrogel constructs incorporating drugs, cytokines, and growth factors have been shown to promote proliferation, directed differentiation, and integration of cells to regenerate target tissue[21-24]. Recently, ESCs were cultured in 3-D hydrogel scaffolds but required routine passaging, much like 2-D cultures[19,25].
Studies have utilized dextran-based hydrogels to promote neovascularization and differentiation of ESCs into endothelial cells[24,26]. Whereas, thiol-functionalized dextran (Dex-SH) was combined with PEG functionalized with tetra-acrylate (PEG-4-Acr) to form chemically cross-linked hydrogels by a Michael-type addition for differentiation of chondrogenic progenitors[27]. In this report, we developed a 3-D culture system for propagation and maintenance of mouse ESCs utilizing Dex-SH and PEG-4-Acr. Cells grown in the 3-D scaffolds proliferated for extended periods of time, and exhibited ESC characteristics including self-renewal and pluripotency. Interestingly, ESCs grown in 3-D scaffolds had upregulated expression of pluripotency genes. This novel culture system is efficient, reproducible and less cumbersome for long-term maintenance of ESCs without the routine passaging and manipulations associated with 2-D culture. These studies should help facilitate development of methods for expansion of high quality and homogenous populations of human ESCs, which are critically important for regenerative medicine and therapeutic applications.
The mouse ESC line 7AC5, labeled with enhanced yellow fluorescent protein (EYFP/GFP), (ATCC, Manassas, VA) was cultured on irradiated MEF feeder layer with ESC medium containing high glucose Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Aleken Biologicals, Nashville, TX), 0.1 mmol/L 2-mercaptoethanol (Sigma, St. Louis, MO), 0.1 mmol/L nonessential amino acids (Invitrogen), 1 mM sodium pyruvate (Sigma), and 1000 U/mL of leukemia inhibitory factor (LIF; Chemicon International, Temecula, CA) as previously described[15].
Dextran (25 kDa, MW/MN = 1.30, Sigma) was functionalized with pendant SH groups at differing degrees of thiol substitution ranging from 4% to 34%, using a published method[27]. Dex-SH was characterized by 1HNMR spectroscopy using a 400 MHz Bruker Avance II spectrometer (Bruker, Billerica, MA).
Hydrogel scaffolds were formed by mixing Dex-SH with PEG-4-Acr (20 kDa, Creative PEGWorks Winston Salem, NC) via a Michael addition reaction, as previously described[22]. For this study, scaffolds were prepared with final polymer concentrations of 5% w/v. The molar ratio of thiol to acrylate groups used was 1:1.
To encapsulate cells, Dex-SH and PEG-4-Acr were dissolved separately in culture medium and mixed with various concentrations (1 × 104 to 4 × 106 cells/mL) of ESCs, which were harvested at 70% confluency. Unless otherwise stated, ESCs were encapsulated at a concentration of 2 × 106 cells/mL. The resulting mixture was transferred to either a well of a 96-well plate or 1cc syringe for polymerization to produce fixed or floating scaffolds, respectively. Floating scaffolds were transferred from the syringes to 24-well plates. ESCs encapsulated in 3-D scaffolds were incubated the ESC medium and changed every 3 to 4 d or as needed. Cell growth was monitored by phase-contrast and confocal microscopy and analyzed by NIS Elements AR software (Nikon Instruments Inc., Melville, NY).
The degradation rate of floating scaffolds was determined by swelling tests performed under physiological conditions (37 °C). The initial dry weight (Wi) of the floating scaffolds was measured before incubation in ESC medium. At regular intervals, the scaffolds were removed from the medium to record the swollen weight (Ws) for analysis. The swelling ratio was defined as the difference between Ws and Wi divided by Wi[28,29]. The degradation time was determined by the time required to completely dissolve the hydrogel scaffolds of each condition prepared in triplicate.
Cell viability was determined qualitatively by propidium iodide (PI, 1 mg/mL) (Fisher Scientific, Pittsburgh, PA) staining in triplicate experiments, and was visualized using fluorescent microscopy.
The quantitative analysis of cell growth in the scaffolds was determined by direct microscopic count using hemocytometer and PrestoBlue (PB) assays (Invitrogen), following the manufacturer’s instructions. The scaffolds were incubated in PB solution for 4 h, before measuring the absorption of the solution at 570 nm and normalized to the reference wavelength of 600 nm using the Epoch microplate reader (BioTek, Winooski, VT). PB, a resazurin-based solution, was reduced proportional to the number of metabolically active cells to fluorescent resorufin.
For teratoma formation, ESCs were harvested following trypsin treatment, washed and re-suspended in PBS, and mixed with an equal volume of Matrigel (BD Biosciences, San Jose, CA)[30]. Cells (1 × 106) were subcutaneously injected (20 μL) using a Hamilton syringe into 4-wk-old male immune-compromised SCID (severe combined immunodeficient)-beige mice (Fox Chase SCID Beige, Charles River, Wilmington, MA). Animals were anesthetized by inhalation of isoflurane gas for the injection of cells, and monitored daily. All efforts were made to minimize discomfort. After teratoma formation (3 to 4 wk), the animals were humanely euthanized by CO2 overdose. Teratoma tissue was explanted, and flash frozen in liquid nitrogen for isolation of RNA using the RNeasy Midi kit (Qiagen, Germantown, MD)[31]. Teratoma assays were performed in triplicate. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Oakland University (IACUC protocol number: 14033).
To differentiate ESCs, embryoid bodies (EBs) were prepared by the hanging drop method[32]. EBs were cultured in ESC growth medium for differentiation into a myogenic phenotype. EBs treated with 10-7 mol/L trans-retinoic acid (RA) were cultured in β-glycerol phosphate (10 mmol/L; Sigma), and ascorbic acid (50 μg/mL; Sigma) for osteogenic differentiation[32]; TGF-β (10 ng/mL; Sigma), insulin (10 μg/mL; Sigma) and ascorbic acid (50 μg/mL; Sigma) for chondrogenic differentiation[15]; and neurobasal medium (Invitrogen) supplemented with B-27 (10 μL/mL; Invitrogen), L-glutamine (0.5 mmol/L; Sigma), penicillin/streptomycin (1 μL/mL; Sigma) and bFGF (5 ng/mL) for neural differentiation[33]. Cell morphology was monitored by light microscopy on a daily basis. Osteogenic cells were analyzed for calcium deposition by von Kossa staining[32]. Proteoglycans produced by chondrogenic derivatives were visualized by alcian blue staining[15]. Analysis of lineage specific markers was performed as described below.
Gene expression studies were performed using quantitative real time polymerase chain reaction (qRT-PCR). RNA was isolated from cells using the RNeasy Mini kit (Qiagen). ESCs grown in 3-D scaffolds were flash frozen with liquid nitrogen, ground into a fine powder using a mortar and pestle, and homogenized using the QIAshredder column (Qiagen)[34]. RNA was purified by treating with RNase-free DNase (Promega, Madison, WI) and cDNA was synthesized with the iScript kit (BioRad, Hercules, CA). PCR reactions were performed in a 10 μL reaction volume using the BioRad CFX90 Real-Time PCR system and SsoAdvanced SYBR Green Supermix. The specific PCR conditions used were as follows: polymerase activation 3 min at 95 °C, 40 cycles of denaturation, 15 s at 95 °C; annealing, 20 s at 60 °C; and melt curve, 5 s/step at 60 °C-95 °C. The markers used in this study represent pluripotency, all three germ layers, as well as osteogenic, chondral, myogenic, and neural cell lineages. Primers (IDT Technologies, Coralville, IA) are listed in the supplemental material (Table 1). All reactions were prepared in triplicate and normalized to reference genes, Gapdh and β-Actin.
| Target-gene | Forward (5’- 3’) | Reverse (5’-3’) | Product size (bp) |
| Oct4 | TCTGTTCCCGTCACTGCTCT | TGTCTACCTCCCTTGCCTTG | 96 |
| Nanog | GCAAGCGGTGGCAGAAAAAC | GCAATGGATGCTGGGATACTCA | 92 |
| Klf4 | AAGCCAAAGAGGGGAAGAAG | CAGTGGTAAGGTTTCTCGCC | 146 |
| Sox2 | CGAACTGGAGAAGGGGAGAG | AAGCGTTAATTTGGATGGGA | 165 |
| Col1 | GCAGGTTCACCTACTCTGTC | CTTGCCCCATTCATTTGTCT | 62 |
| Col2 | ACCCCCAGGTGCTAATGG | AACACCTTTGGGACCATCTTT | 76 |
| Myog | CCTAAAGTGGAGATCCTGCG | GTGGGAGTTGCATTCACTGG | 147 |
| Nestin | AGACAGTGAGGCAGATGAG | CTCTCAGCTGTGGTGGTGAA | 224 |
| Brachyury (T) | CACACCACTGACGCACAC | GAGGCTATGAGGAGGCTTTG | 132 |
| FGF5 | GCTCAATGATCAGAAGGAGGA | TCAGCTGGTCTTGAATGAGG | 175 |
| GATA4 | GATGGGACGGGACACTACCTG | ACCTGCTGGCGTCTTAGATTT | 309 |
| Gapdh | GCACAGTCAAGGCCGAGAAT | GCCTTCTCCATGGTGGTGAA | 151 |
| β-Actin | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA | 228 |
Cells were fixed in 4% paraformaldehyde for 10 min, washed with PBS, permeabilized with 0.5% Triton X-100 (Sigma) for 10 min, and then blocked with 2% BSA (Sigma) for 1 h at room temperature. Fixed cells were treated with primary antibodies (1:100 diluted in blocking buffer), Oct4 (ab19857, Abcam Inc., Cambridge, MA), Nanog (sc-33760, Santa Cruz Biotechnology, Santa Cruz, CA), brachyury (sc-20109, Santa Cruz), NCAM (sc-10735, Santa Cruz), and GATA4 (sc-25310) overnight at 4 °C. Primary antibody treated cells were washed, and then stained with 1:200 diluted secondary antibodies, anti-rabbit Alexa Fluor 568 (A-11011, Molecular Probes, Eugene, OR) or Cy3-labeled anti-mouse IgG (072-01-18-06, KPL, Gaithersburg, MD, United States). Nuclei were counterstained with 1 mg/mL of 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes). Images were acquired using confocal microscopy.
Cells were lysed in RIPA buffer with protease inhibitors (1 mmol/L PMSF) (Fisher Scientific), centrifuged at 12000 rpm for 20 min at 4 °C, and the supernatants were collected. Protein concentrations were determined by the Pierce 660 nm protein assay (Fisher Scientific,) using the NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE), with BSA as a standard. Equal amounts of protein (10 μg) of 2-D and 3-D scaffold grown cells were resolved on 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (BioRad). Membranes were incubated for 30 min at room temperature in blocking buffer [5% non-fat dry milk in PBS containing 0.1% Tween-20 (PBST)]. The blocked membranes were then probed with 1:200 diluted primary antibodies overnight at 4 °C against Oct4 (Abcam), Nanog (Santa Cruz), Klf4 (ab21949, Abcam) and β-Actin (sc-130656, Santa Cruz). After washing with PBST, membranes were incubated with 1:10000 diluted horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature (Santa Cruz). Proteins on the membranes were detected by using an ECL chemiluminescence kit (BioRad) and by exposing the membranes to X-ray film. Finally, protein bands were analyzed using ImageJ (NIH, Bethesda, MA), normalized to β-Actin and expressed in arbitrary densitometric units.
All quantitative data were expressed as mean ± SE. One-way ANOVA analysis was performed on natural log transformed data, and analyzed for unequal variances. Post hoc tests used for multiple comparisons include Games-Howell analysis for PB analysis as well as Tukey and Dunnett’s test for relative gene expression in qRT-PCR studies. Results with a P value less than 0.05 were considered to be significant (aP < 0.05 and bP < 0.01). All analyses were performed using SPSS version 11.5 (SPSS Inc, United States). The statistical methods of this study were reviewed by Dr. Harvey Qu from the Department of Statistics at Oakland University.
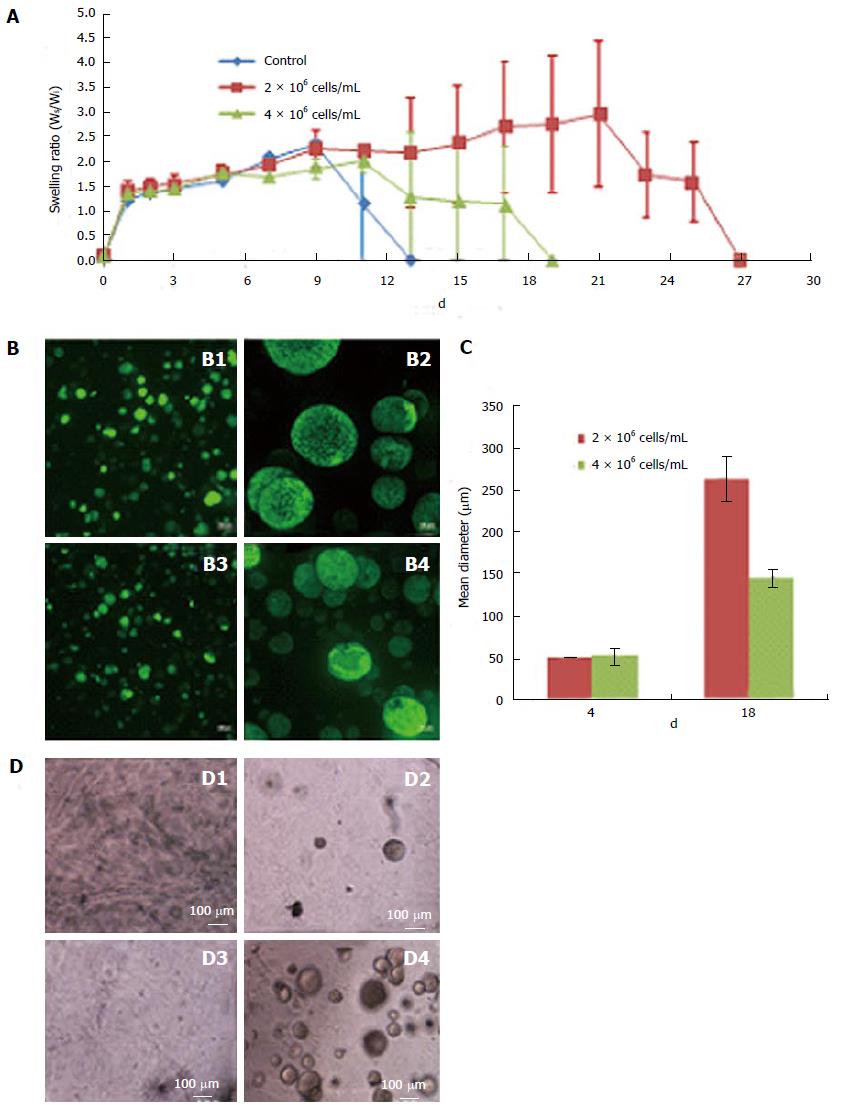
The viability of 3-D grown ESCs was not affected by encapsulation or the process of scaffold self-assembly. Cell growth increased over a period of 3 wk, as evident by the increase in the intensity as well as the number of EYFP/GFP expressing cells in fixed scaffolds (Figure 1A-B). PI staining of the scaffolds 1 d after culturing showed that a majority of the cells were viable cells (green) and contained only a small fraction (< 5%) of dead cells (yellow). Presence of dead cells could be due to damage resulting from trypsinization of ESCs used for encapsulation. When trypsinized ESCs were tested by trypan blue staining, a similar fraction of dead cells (about 10%) was observed, indicating that encapsulation did not cause cell death. However, it is interesting to note that unlike in 2-D culture, encapsulated dead cells did not lyse during 3-D culture. We speculate that the scaffold microenvironment limited proteolytic activity. In our preliminary studies, the colony size of viable cells was restricted due to the limited swelling of fixed scaffolds (Figure 1A and B). We reasoned that if the scaffolds were allowed to swell freely, ESC growth and colony size would increase. Indeed, when ESCs were cultured in the floating scaffolds, cell growth and colony size increased in a time dependent manner (Figure 1C and D). Growth of encapsulated cells in floating scaffolds was confirmed by direct cell counts as shown in Figure 1E. After a lag period, ESCs grew at a generation rate of 36 h. A similar growth pattern was observed using PB staining. The results depicted in Figure 1F showed a continuous and significant increase in proliferation at 2, 3 and 4 wk of culture following a period of acclimatization. The highest increase in cell growth was observed between week 3 and 4, following which, the scaffolds degraded rapidly. Evidently, cell growth and colony size increased concomitant with reduction in scaffold integrity. Conceivably, scaffolds that retain integrity beyond 4 wk would continue to support the undifferentiated growth of ESCs.
The polymer concentration and MW of Dex-SH and PEG-4-Acr, as well as the degree of thiol substitution of Dex-SH influenced the polymerization and degradation rate of scaffolds (Table 2), similar to previous reports[23,27]. After preliminary evaluation, we selected Dex-SH (MW = 25 kDa) with 7.5% and 33% thiol substitution and PEG-4-Acr (MW = 20 kDa) at 5% w/v for further studies for propagating cells for various culture periods; these preparations yielded scaffolds that degraded without cells in 11 and 13 d and degraded with cells in 22 ± 5 d and 37 ± 5 d, respectively.
| Degree of thiol substitution of Dex-SH | Time in degradation (d) |
| 4% | 10 ± 2 |
| 6% | 13 ± 4 |
| 7.5% | 22 ± 5 |
| 12% | 28 ± 6 |
| 30% | 35 ± 5 |
| 33% | 37 ± 5 |
| 34% | 37 ± 5 |
Scaffolds prepared using 7.5% thiol substitution of Dex-SH and various cell concentrations, ranging from 1 × 104 cells/mL to 10 × 106 cells/mL, exhibited different swelling profiles, although the initial swelling capacity of the scaffolds was similar. Scaffolds containing various cell concentrations (0, 2 × 106 cells/mL or 4 × 106 cells/mL) as shown in Figure 2A, exhibited comparable swelling ratios until day 9; after which, control scaffolds without cells degraded rapidly, followed by scaffolds prepared with 4 × 106 cells/mL and finally scaffolds with 2 × 106 cells/mL. Scaffolds with the higher number of cells (4 × 106 cells/mL) swelled to 2-fold of their initial weight and started to degrade on day 19 of culture. Whereas, scaffolds with a lower number of cells (2 × 106 cells/mL) swelled to nearly 3 times their initial weight and started to degrade on day 27 of culture. Furthermore, scaffolds prepared with the lower cell concentration (2 × 106 cells/mL) resulted in rapid clonal growth (Figure 2B; B1, B2) as compared to the higher cell concentration (4 × 106 cells/mL) (Figure 2B; B3, B4). Quantitative analysis of colony size showed a 2-fold increase in the mean diameter of colonies in scaffolds prepared with lower numbers of cells (Figure 2C). The scaffold supported the growth of encapsulated ESCs at concentrations as low as 1 × 104 cells/mL (or 103/cm2), suggesting that low concentration had no effect on the cell viability (Figure 2D). Maintenance of ESCs at low concentrations is more advantageous in 3-D culture compared to 2-D culture (Table 3), since low seeding densities in 2-D culture results in poor growth, decreased viability and differentiation of ESCs.
| Culture conditions | Cell concentration | |
| (cells/mL) | (cells/cm2) | |
| 2-D Culture | --- | 3 × 104 to 5 × 104[33] |
| 3-D Culture | 1 × 104 | 1 × 103 |
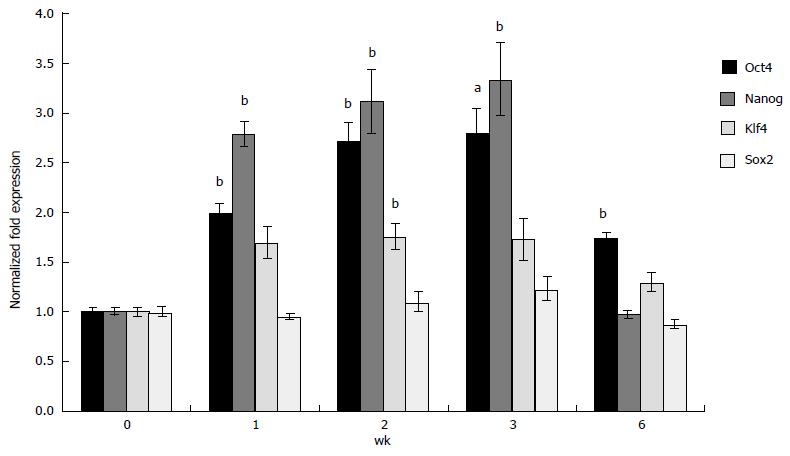
To confirm whether 3-D grown ESCs maintained pluripotency, expression of selected ESC-specific markers was analyzed using qRT-PCR. The results depicted in Figure 3 indicate that expression of Oct4 and Nanog increased 2.8 and 3.0 fold, respectively in 3-D scaffold (prepared using 33% Dex-SH) grown ESCs as compared to cells grown under 2-D culture conditions. The expression of these markers significantly and successively increased in 3-D grown ESCs during 1, 2 and 3 wk. The expression of Klf4 also increased significantly (1.8 fold) above the level of 2-D grown ESCs but only during the second week of cell growth; whereas, the expression of Sox2 was not affected by 3-D culture. Concomitant with the start of degradation of the scaffolds at 6 wk of culture, expression of pluripotency markers gradually decreased to the levels of 2-D grown ESCs.
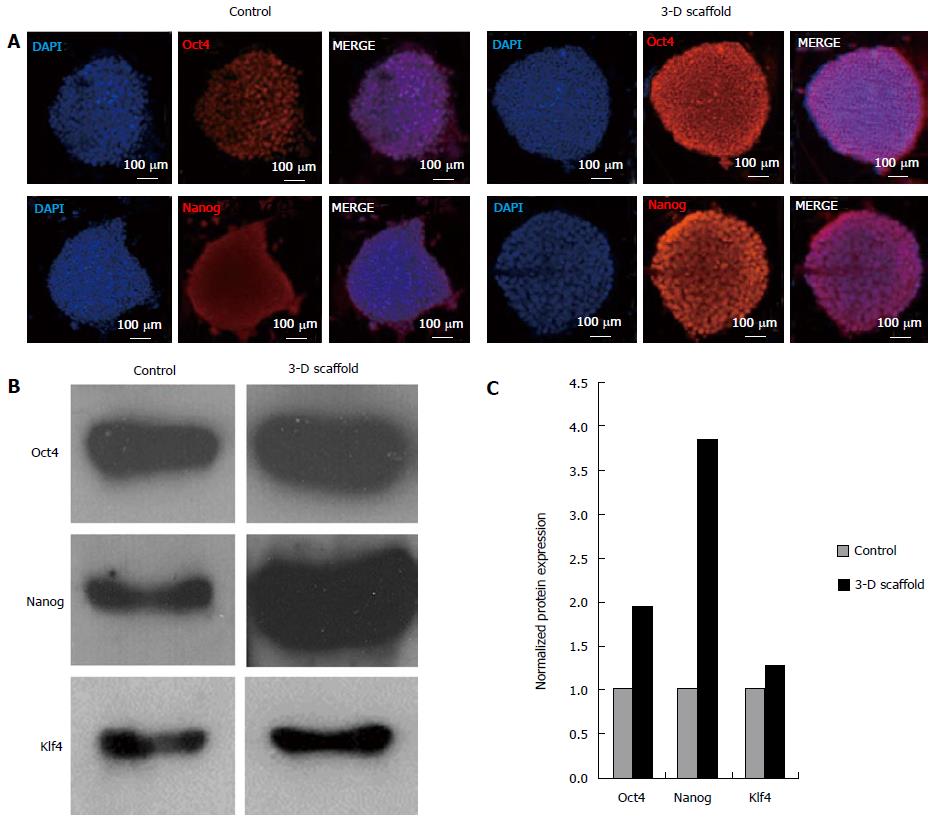
Immunofluorescence analysis of 3-D grown ESCs showed upregulation of protein levels of Oct4 and Nanog as evident by the increase in the fluorescent intensity of cells stained with the respective antibodies (Figure 4A). Furthermore, expression of these proteins was analyzed by western blot and results are shown in Figure 4B. Similarly, levels of Oct4, Nanog and Klf4 were visibly increased in the 3-D grown cells when compared with the control. Quantitative analysis of the western blot confirmed the increase in Oct4, Nanog and Klf4 (1.9, 3.9 and 1.3 fold, respectively) compared to the control (Figure 4C); this was well correlated with the observed transcription levels of these genes.
Maintenance of pluripotency of 3-D grown ESCs was further investigated by subculturing under 2-D culture conditions (Figure 5A, A1-A3). They displayed typical compact colony morphology (even upon passaging five times) in 2-D culture and were indistinguishable from the initial ESCs seeded and grown in 3-D scaffolds. Expression of pluripotent genes in ESCs first grown in 3-D scaffolds for 2 wk and then subcultured under 2-D conditions were analyzed. The results depicted in Figure 5B showed that the expression of Oct4, Nanog, and Klf4 returned to levels comparable to traditionally 2-D propagated ESCs (Figure 5B).
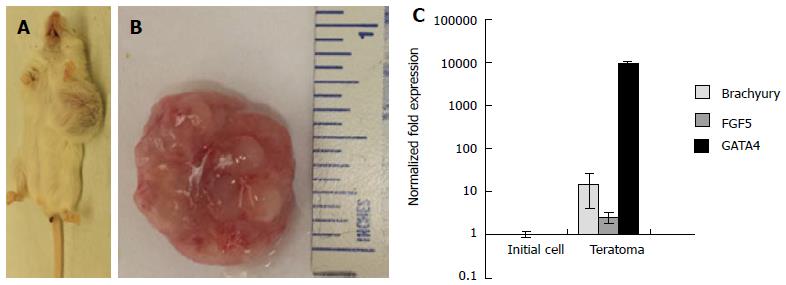
Pluripotency of 3-D scaffold grown ESCs was further validated by in vivo teratoma assays using immune-compromised mice. The animals injected with 1 × 106 cells formed teratomas within 4 wk (Figure 6A and B). Analysis of explanted teratoma tissue showed expression of Brachyury, FGF5, and GATA4, suggesting differentiation of injected ESCs into cell lineages of all three germ layers (Figure 6C). Conceivably, the 3-D scaffold provided a microenvironment for upregulation of pluripotent genes, maintenance of pluripotency and self-renewal of ESCs.
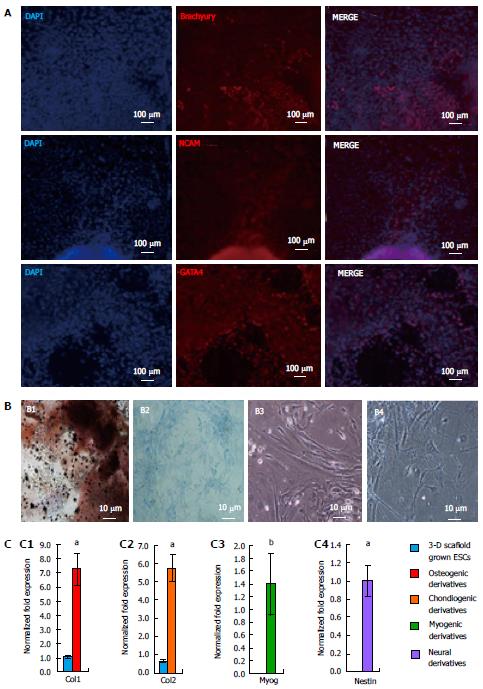
Differentiation potential of the 3-D grown ESCs was also studied via EB formation. The EBs from 3-D grown ESCs were allowed to spontaneously differentiate into all three germ layers, mesoderm, ectoderm, and endoderm and expressed specific protein markers such as Brachyury, NCAM, and GATA4 (Figure 7A). Furthermore, 3-D grown ESCs were induced to differentiate into various cell lineages, including osteogenic, chondrogenic, myogenic, and neural cell types. The results of differentiation depicted osteogenic derivatives that had the cobblestone appearance of osteoblast cells and were positive for calcium deposition as determined by von Kossa staining (Figure 7B, B1). Chondrogenic derivatives of 3-D grown ESCs were analyzed by alcian blue staining showing positive proteoglycan production (Figure 7B, B2). Light micrographs of myogenic derivatives of 3-D grown ESCs had spindle shaped morphology (Figure 7B, B3), while neural derivatives of ESCs displayed neurofilaments (Figure 7B, B4). Further analysis of the 3-D grown ESC derivatives revealed expression of cell-specific markers (Figure 7C) as determined by qRT-PCR; osteogenic, chondrogenic, myogenic, and neural derivatives expressed Collagen type 1 (Col1), Collagen type 2 (Col2), Myogenin (Myog), and Nestin, respectively. The expression of these cell-specific markers signified that 3-D grown ESCs maintained differentiation potential.
In this report, we described an alternative to traditional 2-D culturing of ESCs without the need for a MEF feeder layer and routine passaging. This method was developed utilizing two components, Dex-SH and PEG-4-Acr, for self-assembling 3-D hydrogel scaffolds. Evidently, this hydrogel scaffold better emulated the fully hydrated native 3-D microenvironment and supported the pluripotent growth of ESCs. This method eliminates laborious and time-consuming manipulations, which often results in the loss of ESC lines due to contamination of cultures or differentiation of cells.
The polymerization of Dex-SH and PEG-4-Acr, via a Michael addition reaction, facilitated the self-assembly of the 3-D scaffolds and encapsulation of ESCs. Polymerization was dependent upon several factors including concentration and ratio of polymers, degree of thiol substitution of Dex-SH, and cell concentration. Optimal conditions for scaffold polymerization, cell encapsulation, and cell viability were achieved by varying these parameters. The results showed that higher the degree of thiol substitution of Dex-SH, the faster the polymerization of hydrogel. Consistent with a previous report[27], the ratio and amount of Dex-SH and PEG-4-Acr also affected the formation and swelling properties of the hydrogel scaffolds. In one study, 10% w/v polymer concentrations were used to prepare Dex-SH and PEG-4-Acr scaffolds to promote differentiation of ESCs[23]. However, we determined that 5% w/v polymer concentrations were ideal to provide a scaffold microenvironment with a greater degree of flexibility and suitability for ESC growth. Mixing of cells with PEG-4-Acr prior to scaffold molding yielded homogeneous distribution of cells in both fixed and floating scaffolds. The 3-D scaffold grown ESCs displayed undifferentiated compact round colony morphology, even upon prolonged culturing. Previously, Gerecht et al[26] reported the potential of hyaluronic acid-based hydrogels in maintaining human ESCs with an undifferentiated morphology for 20 d. However, polymerization of hyaluronic acid-based hydrogels was achieved with ultraviolet light, a potent carcinogen. Whereas, self-assembly of the Dex-SH and PEG-4-Acr scaffold does not require a mutagenic catalyst, and ESCs can be maintained for over 6 wk. They also reported that at least 5 × 106 cells/mL to 10 × 106 cells/mL were required for cell growth and colony formation in the hyaluronic acid-based scaffolds. In contrast, we found that use of Dex-SH and PEG-4-Acr scaffolds supported ESC growth at concentrations as low as 1 × 104 cells/mL or 1 × 103/cm2 in 3-D scaffolds. At concentrations below 1 × 104 cells/mL successful encapsulation in the scaffold was difficult to assess due to low cell density. Furthermore, low concentration may also affect the viability of the cells. In traditional 2-D culture, optimal maintenance of ESCs requires a much higher cell density (3 × 104 to 5 × 104 cells/cm2)[33].
ESC self-renewal was not affected by the duration of growth in 3-D scaffolds as long as the integrity of the scaffold was maintained. Scaffold integrity also influenced the cell proliferation rate and colony size. ESCs grew at a slower rate with smaller colony size in fixed scaffolds. However, cell growth rate and colony size was less restricted in floating scaffolds. In agreement with previous reports[35,36], we found that mouse ESCs doubled nearly every 12 h in 2-D culture, whereas the generation time for ESCs in the 3-D culture system was approximately 36 h. It has been reported that the growth rate of stem cells in vivo vary from a few hours to months depending upon the niche[37]. In 2-D culture, ESCs reached near confluency in 3 d and started to differentiate, whereas the growth rate in 3-D was slower; the cells did not differentiate and continued to grow for up to 6 wk, limited only by scaffold degradation. Using the exponential cell growth formula N = N0× 2g, where N is the final cell number, N0 is the initial cell number (2 × 106), and g is the number generations (25), the expected total number of cells after 6 wk of growth would be 134 × 1012 cells. The fact that ESCs grew for an extended period without the loss of cell viability and their self-renewal potential suggested that the 3-D culture system described here was superior to 2-D culture, which requires routine passaging.
In addition to the concentration of the scaffold components and degree of thiol substitution, cell concentration significantly affected the rate of scaffold degradation. Consistent with previous studies[23], scaffolds without cells degraded earlier compared to seeded scaffolds (13 d vs 27 d, respectively) suggesting that the addition of cells improved the stability of the scaffolds. Moreover, scaffolds encapsulating a higher concentration of ESCs degraded faster and swelled less than scaffolds with lower concentration of ESCs. Consequently, ESC colony size was smaller in scaffolds with a higher concentration of cells as compared to scaffolds prepared with a lower cell concentration. In addition, encapsulated ESCs in floating scaffolds grew rapidly with larger colony sizes as compared to fixed scaffolds of similar composition. These results suggested that the swelling plasticity of the self-assembling scaffolds favorably promoted the growth and maintenance of ESC colonies. Previous studies also indicated that cell encapsulation increased integrity of the scaffold as compared to scaffolds without cells[23]. However, in this report the effect of cell concentrations on scaffold integrity was not reported. Our observations indicated that higher cell concentrations inversely effected scaffold integrity suggesting that the cells may be producing factors that de-stabilize the scaffolds. The nature of these factors remains unknown.
ESCs grown in the 3-D scaffolds for 6 wk maintained their pluripotency and differentiation potential, as determined by their ability to spontaneously differentiate into cell types comprising all three germ layers. The EBs differentiated into osteogenic, chondrogenic, myogenic, and neural cell types, expressing cell-specific markers. These results indicated that the self-assembling scaffolds support the self-renewal and pluripotency of ESCs, even after prolonged periods of culturing. The pluripotency of 3-D grown ESCs was further confirmed by teratoma formation in vivo. Teratomas explanted from immune-compromised mice following injection of 3-D grown ESCs showed specific gene expression representing all three germ layers.
Interestingly, the expression of three pluripotency markers, Oct4, Nanog and Klf4, was higher in 3-D cultured ESCs as compared to 2-D grown ESCs both at a transcriptional and translational level. The expression of these genes remained at higher levels throughout the extended growth period (up to 6 wk) until the onset of scaffold degradation. In contrast, Sox2 expression levels in 3-D grown ESCs was found to be similar to the control (2-D grown ESCs). Although high expression of Nanog and Klf4 has been reported to enhance self-renewal and pluripotency[38], overexpression of Oct4 has been implicated in spontaneous ESC differentiation[39]. Contrary to this, we did not observe differentiation of ESCs grown in 3-D scaffolds. When 3-D grown ESCs were subcultured under 2-D culture conditions, expression of pluripotent markers returned to levels similar to 2-D grown cells. Previous reports[40] also described differential regulation of stemness genes, with an upregulation of Oct4 but downregulation of Sox2 in ESCs cultured in three different scaffolds prepared using PGLA, collagen and chitosan; whereas Nanog was only highly upregulated in chitosan scaffolds. Taken together with our findings, it can be argued that the scaffold microenvironments play an important role in influencing cell-matrix communication, thus effecting gene expression. It is also possible that cellular proteins may have interacted with thiol groups during the encapsulation step of self-assembly of the scaffolds resulting in alteration of their activities. Nevertheless, the underlying molecular mechanism responsible for the upregulation of pluripotent genes in ESCs grown in 3-D Dex-SH and PEG-4-Acr scaffolds remain unknown and warrants further investigation.
The findings reported here demonstrate a novel, efficient, reproducible, and simple approach to ESC cultivation. However, these results were obtained using mouse ESCs as a model, further studies are required to expand these results to human pluripotent stem cells. Previous attempts at 3-D cultivation of ESCs have required routine passaging[19,25,26]. To our knowledge this is the first report that described a robust system for 3-D culturing of ESCs for extended periods without passaging or manipulation. These improvements in the maintenance of ESCs will help their use in translational research, disease modeling, stem cell therapies, and regenerative medicine.
Oakland University Provost Graduate Student Research Award provided support for C. McKee. We would like to thank Dr. Bashir Kaskar for help with the synthesis and analysis of Dex-SH, Naimisha Reddy Beeravolu for her assistance with the western blot analysis and Dr. Harvey Qu for his evaluation of the statistical analysis.
Embryonic stem cells (ESCs) are capable of differentiating into any of the over 200 cell types found in the body, which makes them an ideal source for cell-based therapies and regenerative medicine. These applications require efficient and reproducible expansion of ESCs ex vivo for high quality cells, which then can be uniformly differentiated for clinical use. However, propagation of ESCs is technically challenging, laborious and expensive. Traditional ESC culture techniques are heavily dependent on cell adherence to two-dimensional (2-D) plastic culture plates, regular passaging (every two to three days) and xenogeneic feeder layers. This often results in differentiation and loss of cell lines.
In order to overcome the problems associated with 2-D cell culture, few studies have reported the use of three-dimensional (3-D) scaffolds to propagate ESCs. However, these methods are not efficient and require the use of carcinogenic catalysts for polymerization of the scaffolds. Moreover, these techniques required routine passaging in order to maintain the pluripotency of ESCs.
A novel 3-D culture system was developed for propagation of ESCs for an extended period of time. In this system, ESCs were encapsulated upon self-assembly of 3-D scaffolds prepared with thiol-functionalized dextran and polyethylene glycol tetra-acrylate. The 3-D scaffold microenvironment supported the growth of ESCs while maintaining their pluripotency and self–renewal potential for extended periods without passaging and extensive manipulation, which is required for 2-D culture of ESCs.
This new culture system should help develop methods of expansion for high quality and homogenous populations of ESCs, which are critically important for regenerative medicine and therapeutic applications.
A scaffold is an engineered matrix formed from biomaterials mimicking the microenvironment and capable of supporting cell growth. In this study, self-assembling scaffolds were formed upon combination of two polymers, polyethylene glycol tetra-acrylate and thiol-functionalized dextran.
The authors designed a simplified and efficient system, which mimics the microenvironment in vivo for long-term proliferation and maintenance of ESCs. Furthermore, the culture system is efficient and reproducible and is expected to lead to further innovations.
| 1. | Stojkovic M, Lako M, Strachan T, Murdoch A. Derivation, growth and applications of human embryonic stem cells. Reproduction. 2004;128:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 469] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Zhang S, Zhang Y, Chen L, Liu T, Li Y, Wang Y, Geng Y. Efficient large-scale generation of functional hepatocytes from mouse embryonic stem cells grown in a rotating bioreactor with exogenous growth factors and hormones. Stem Cell Res Ther. 2013;4:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 837] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 5. | Trounson A. New perspectives in human stem cell therapeutic research. BMC Med. 2009;7:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Balami JS, Fricker RA, Chen R. Stem cell therapy for ischaemic stroke: translation from preclinical studies to clinical treatment. CNS Neurol Disord Drug Targets. 2013;12:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Carr AJ, Smart MJ, Ramsden CM, Powner MB, da Cruz L, Coffey PJ. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci. 2013;36:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136:2311-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 329] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15:205-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Lund AW, Yener B, Stegemann JP, Plopper GE. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev. 2009;15:371-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1061] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 12. | Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11:381-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Geckil H, Xu F, Zhang X, Moon S, Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine (Lond). 2010;5:469-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 786] [Cited by in RCA: 673] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 14. | Jozefczuk J, Drews K, Adjaye J. Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. J Vis Exp. 2012;pii: 3854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Fecek C, Yao D, Kaçorri A, Vasquez A, Iqbal S, Sheikh H, Svinarich DM, Perez-Cruet M, Chaudhry GR. Chondrogenic derivatives of embryonic stem cells seeded into 3D polycaprolactone scaffolds generated cartilage tissue in vivo. Tissue Eng Part A. 2008;14:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14:61-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 727] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 17. | Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1597] [Cited by in RCA: 1405] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Charles LF, Zarembinski TI, Johnson KI, Atzet SK, Wesselschmidt RL, Wight ME, Kuhn LT. Modified hyaluronan hydrogels support the maintenance of mouse embryonic stem cells and human induced pluripotent stem cells. Macromol Biosci. 2012;12:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Fisher OZ, Khademhosseini A, Langer R, Peppas NA. Bioinspired materials for controlling stem cell fate. Acc Chem Res. 2010;43:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Benoit DS, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12:1663-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (6)] |
| 22. | Hiemstra C, Zhong Z, van Steenbergen MJ, Hennink WE, Feijen J. Release of model proteins and basic fibroblast growth factor from in situ forming degradable dextran hydrogels. J Control Release. 2007;122:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Jukes JM, van der Aa LJ, Hiemstra C, van Veen T, Dijkstra PJ, Zhong Z, Feijen J, van Blitterswijk CA, de Boer J. A newly developed chemically crosslinked dextran-poly(ethylene glycol) hydrogel for cartilage tissue engineering. Tissue Eng Part A. 2010;16:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 425] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 25. | Lei Y, Schaffer DV. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci USA. 2013;110:E5039-E5048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 26. | Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298-11303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 483] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 27. | Hiemstra C, Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Rapidly in situ-forming degradable hydrogels from dextran thiols through Michael addition. Biomacromolecules. 2007;8:1548-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Zawko SA, Suri S, Truong Q, Schmidt CE. Photopatterned anisotropic swelling of dual-crosslinked hyaluronic acid hydrogels. Acta Biomater. 2009;5:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Hiemstra C, Zhou W, Zhong Z, Wouters M, Feijen J. Rapidly in situ forming biodegradable robust hydrogels by combining stereocomplexation and photopolymerization. J Am Chem Soc. 2007;129:9918-9926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Zhang WY, de Almeida PE, Wu JC. Teratoma formation: A tool for monitoring pluripotency in stem cell research. StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute, 2008- 2012; Jun 10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Gertow K, Przyborski S, Loring JF, Auerbach JM, Epifano O, Otonkoski T, Damjanov I, Ahrlund-Richter L. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol. 2007;Chapter 1:Unit1B.4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Chaudhry GR, Yao D, Smith A, Hussain A. Osteogenic Cells Derived From Embryonic Stem Cells Produced Bone Nodules in Three-Dimensional Scaffolds. J Biomed Biotechnol. 2004;2004:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Chaudhry GR, Fecek C, Lai MM, Wu WC, Chang M, Vasquez A, Pasierb M, Trese MT. Fate of embryonic stem cell derivatives implanted into the vitreous of a slow retinal degenerative mouse model. Stem Cells Dev. 2009;18:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Wang C, Hao J, Zhang F, Su K, Wang DA. RNA extraction from polysaccharide-based cell-laden hydrogel scaffolds. Anal Biochem. 2008;380:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Kidder BL, Oseth L, Miller S, Hirsch B, Verfaillie C, Coucouvanis E. Embryonic stem cells contribute to mouse chimeras in the absence of detectable cell fusion. Cloning Stem Cells. 2008;10:231-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Tamm C, Pijuan Galitó S, Annerén C. A comparative study of protocols for mouse embryonic stem cell culturing. PLoS One. 2013;8:e81156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 439] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 38. | Nur-E-Kamal A, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Radzisheuskaya A, Silva JC. Do all roads lead to Oct4? the emerging concepts of induced pluripotency. Trends Cell Biol. 2014;24:275-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Wei J, Han J, Zhao Y, Cui Y, Wang B, Xiao Z, Chen B, Dai J. The importance of three-dimensional scaffold structure on stemness maintenance of mouse embryonic stem cells. Biomaterials. 2014;35:7724-7733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chen CP, Liu L S- Editor: Tian YL L- Editor: A E- Editor: Wu HL