Published online Jan 26, 2015. doi: 10.4252/wjsc.v7.i1.65
Peer-review started: July 31, 2014
First decision: September 16, 2014
Revised: October 6, 2014
Accepted: October 23, 2014
Article in press: December 16, 2014
Published online: January 26, 2015
Processing time: 168 Days and 12.6 Hours
Low back pain is a common clinical problem, which leads to significant social, economic and public health costs. Intervertebral disc (IVD) degeneration is accepted as a common cause of low back pain. Initially, this is characterized by a loss of proteoglycans from the nucleus pulposus resulting in loss of tissue hydration and hydrostatic pressure. Conservative management, including analgesia and physiotherapy often fails and surgical treatment, such as spinal fusion, is required. Stem cells offer an exciting possible regenerative approach to IVD disease. Preclinical research has demonstrated promising biochemical, histological and radiological results in restoring degenerate IVDs. Cell tracking provides an opportunity to develop an in-depth understanding of stem cell survival, differentiation and migration, enabling optimization of stem cell treatment. Magnetic Resonance Imaging (MRI) is a non-invasive, non-ionizing imaging modality with high spatial resolution, ideally suited for stem cell tracking. Furthermore, novel MRI sequences have the potential to quantitatively assess IVD disease, providing an improved method to review response to biological treatment. Superparamagnetic iron oxide nanoparticles have been extensively researched for the purpose of cell tracking. These particles are biocompatible, non-toxic and act as excellent MRI contrast agents. This review will explore recent advances and issues in stem cell tracking and molecular imaging in relation to the IVD.
Core tip: Mesenchymal stem cell (MSC) transplantation shows exciting promise for the future regenerative approach to intervertebral disc (IVD) disease. Extensive preclinical research has demonstrated benefits from MSC treatment in disc degeneration. Cell tracking, with iron oxide nanoparticles and MRI, provides an opportunity to develop an in-depth understanding of stem cell survival, differentiation and migration, enabling optimization of stem cell treatment. This review summarizes the current literature relating to MSC tracking in the IVD, which is limited to short term monitoring. Medium to long-term cell tracking is required to accelerate translation of MSC treatment in the IVD to clinical practice.
- Citation: Handley C, Goldschlager T, Oehme D, Ghosh P, Jenkin G. Mesenchymal stem cell tracking in the intervertebral disc. World J Stem Cells 2015; 7(1): 65-74
- URL: https://www.wjgnet.com/1948-0210/full/v7/i1/65.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i1.65
Low back pain is the leading cause of disability in the developed world[1]. Lifetime prevalence is 75%-80%[2], with the annual cost in the United States alone estimated to be as high as $500 billion[3]. Low back pain is strongly linked to disc degeneration, with a two-fold increase in chronic lower back pain in patients with radiological evidence of degeneration[4,5].
Current treatments, including analgesia, physiotherapy and spinal fusion only address symptoms, not the underlying disease. Regenerative strategies, such as stem cell therapy, provide an exciting future in the treatment of intervertebral disc (IVD) disease. Tracking and long term monitoring of these cells is essential to develop an understanding of their survival, migration, proliferation and differentiation in vivo, which will enable optimization of this promising therapy. Magnetic resonance imaging (MRI) combined with contrast agents is the modality of choice for cell tracking. This review will summarize recent advances in stem cell tracking, current problems and their application to the treatment of IVD disease.
The intervertebral disc is composed of 3 main regions: the tough annulus fibrosis (AF), peripherally, the amorphous nucleus pulposus (NP), centrally, and cartilaginous endplates which bind the disc to the adjacent superior and inferior vertebral bodies[6]. With ageing and degeneration, significant cellular and matrix changes occur within the IVD. An early hallmark of disc degeneration is the loss of proteoglycans (PG), and associated water molecules, from the NP and AF, accompanied by structural changes to the lamellae of the AF[7-11]. With the loss of the water binding PGs from the disc, its functional capacity as a hydroelastic cushion is diminished, leading to additional mechanical stresses acting on the fibrocartilagenous AF. With time, these events can result in the presence of concentric and radiating tears in the lamellae of the AF that may eventually extend into the NP[7,8,11-13]. In addition, there is emerging evidence that damage to the cartilaginous endplate plays a role in the pathophysiology of degenerative disc disease, up-regulating matrix degrading enzymes and inflammatory cytokines in the NP[14]. Structural deficiencies in the NP are considered to provoke neovascularisation and growth of nerve fibers, normally confined to the periphery of the AF, to the deeper regions of the disc[13,15]. The establishment of these extended nerve fibers has been cited as a major cause of chronic lower back pain in degenerate discs[15-17].
Current strategies to treat low back pain fail to regenerate the intervertebral disc or even reverse the degenerative process. Analgesics, non-steroidal anti-inflammatory drugs, physical therapies and other multimodal palliative modalities represent the mainstay of conservative therapy for low back pain[18-20]. There is no evidence, however, that any of these therapies provide long-term benefit by improving the underlying pathobiology of disc degeneration. In fact, recent research has demonstrated simple analgesia does not improve recovery time from acute low back pain[21]. When non-operative treatments fail, surgical interventions such as spinal fusion or total disc arthroplasty are commonly undertaken. These interventions aim to remove the pain generator, however, many patients remain with chronic pain and disability. Furthermore, spinal fusion has biomechanical consequences, which accelerates degeneration at adjacent levels[22-24].
Recent research suggests novel biological therapies can provide restorative treatment of disc degeneration. Cell types investigated include NP cells[25-29], chondrocytes[30-33] and mesenchymal stem cells (MSC)[29,30,34-43]. MSCs, initially isolated from bone marrow, can also be derived from a range of tissues, including adipose and synovial[44,45]. In addition, MSCs are reported to be non-immunogenic and, in contrast to embryonic stem cells, cannot under-go malignant transformation[46,47]. Moreover, MSCs have the capacity for self-renewal, enabling maintenance of an undifferentiated phenotype in multiple subcultures[48]. However, in the appropriate environment, MSCs are capable of differentiating into multiple cell types including chondrocytes, osteocytes, tenocytes and adipose cells[44,45].
There are numerous preclinical studies investigating the use of MSCs in rat, rabbit, goat and porcine models[29,30,34-43]. MSC implantation in the rabbit model has resulted in an increase in PG content, partial restoration of disc height and disc hydration[42,43]. In the ovine model, intra-discal MSCs treatment has been shown to restore disc extracellular matrix, increase disc height and reduce radiological and histological grading scores 6 mo following injection[49,50]. How stem cells produce these effects remains unclear from current preclinical studies.
Early clinical research has been promising. Yoshikawa et al[51] reported two cases of autologous MSC implantation in markedly degenerate intervertebral discs. Symptomatic and radiological improvement was demonstrated without significant adverse effects[51]. A larger series of 10 patients treated with autologous MSCs demonstrated improvement in pain, disability and disc hydration[52]. Prior to translation to clinical practice, greater understanding of the mechanism of action of these cells is required.
Longitudinal tracking of MSCs is necessary to ensure survival, evaluate distribution and assess if cells migrate to affected pathological sites in the IVD. Previous methods, based on histological analysis provide a snapshot view of the cells at a set time point. This is useful at the end of a scientific study but these methods cannot be translated to the clinical setting. Cellular labeling provides a non-invasive technique to assess the cells in vivo at any given time point. Thus, it can assess cell viability, track cell migration patterns and provide some information on efficacy. It may provide an understanding on mechanism of action, for example, potentially being able to determine whether cells differentiate into chondrocytic cells or act to modulate the resident native cell population through paracrine actions. In addition, cell tracking is required to ensure MSCs retention, as leakage of transplanted cells outside the disc has been reported to induce osteophyte formation[53].
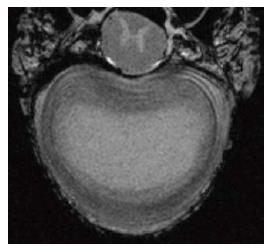
In vivo, non-invasive techniques are required for cell tracking in the research and clinical setting. Multiple imaging modalities have been utilized, including MRI[54-58], positron emission tomography (PET)[59-62], single photon emission computed tomography[63], bioluminescence imaging[64,65], fluorescence imaging[66-72] and computed tomography (CT)[73]. MRI has been accepted as the best modality given its high spatial resolution (< 100 microns), prolonged effective imaging window, lack of ionizing radiation and ability to provide detailed anatomical information[74,75]. There are now very high resolution MRI machines available such as 9.4 Tesla which provides high quality images (Figure 1).
The ideal contrast agent would be highly sensitive, biocompatible, non-toxic, easily taken up by the targeted cells and provide clear contrast between the labeled cells and surrounding tissue. Due to their superior sensitivity and excellent biocompatibility, iron oxide nanoparticles are the preferred contrast agent for cell labeling[76]. Superparamagnetic iron oxide nanoparticles (SPIONs) consist of an iron oxide core, usually magnetite (Fe3O4) or maghemite (γFe2O3) and a biocompatible coating. Coating substrates include dextran, carboxydextran, polyethylene glycol (PEG), polystyrene and silica[77]. Iron oxide nanoparticles can be subdivided into standard SPION, 60 to 150 nm in size, and ultrasmall superparamagnetic iron oxide nanoparticles (USPION) which measure 10 to 20 nm[78,79]. The type of coating, size and method of synthesis affect the SPIONs biocompatibility and magnetic properties[80].
MRI generates images by utilizing the differences in proton density and the local magnetic environment of hydrogen atoms[75]. There are two MR relaxation time constants, T1 and T2. Commonly used contrast agents, paramagnetic gadolinium analogues, alter longitudinal (T1) relaxation time of hydrogen protons and appear hyperintense. The abovementioned, superparamagnetic nanoparticles, affect the transverse (T2) relaxation time of hydrogen protons and appear hypointense. Due to their size, USPION demonstrate additional T1 effects[75]. Iron oxide nanoparticles can be detected at micromolar concentrations and offer sufficient sensitivity to be identified on T2* weighted imaging[81].
MR cell labeling requires the transfer of particles from extracellular to intracellular. The simplest method is spontaneous uptake of particles by phagocytic cells such as macrophages. While enhanced with an appropriate biocompatible coating, iron oxide nanoparticles are not efficiently taken up by stem cells[82,83]. However, labeling can be facilitated by incubation with cationic transfection agents, including poly-L-lysine and protamine sulfate[82,84-86]. If extended incubation time is not appropriate, other rapid techniques, magnetoelectroporation or magnetosonoporation, can be employed[87,88].
A number of contrast agents have been approved for clinical use in medical imaging. Previously, the most commonly used iron oxide nanoparticle for cell labeling was Feridex®, which contains an iron oxide core and dextran coating[77]. Dextran coated SPIONs have been shown to be biocompatible and biodegradable via iron metabolism through Kupffer cells, located in the liver[89]. Another widely used SPION, Resovist®, has a carboxydextran coating[90,91]. Both these products have been discontinued from production by the pharmaceutical companies[77,92]. Other commercial products continue to be utilized, such as SiMAG®, an SPIO with an unmodified silica surface. For example, Markides et al[93] labeled MSCs with SiMAG® in a rheumatoid arthritis mouse model.
Extensive research has been devoted to designing novel iron oxide nanoparticles for the purpose of stem cell labeling[92]. van Buul et al[94] demonstrated ferumoxides (Endorem®) complexed with protamine sulfate are superior to ferucarbotran particles for cell labeling. Subsequently, this group demonstrated safety and efficacy of the ferumoxide-protamine sulfate complex for MSC labeling in articular cartilage repair[95]. USPION have also been investigated recently. Coated with dextran and PEG and combined with protamine sulfate, USPIONs have been cultured with human Adipose Derived Stem Cells (hADSCs) within a three dimensional scaffold[96]. In vitro, no effect on cell viablilty or osteogenic differentiation was seen from cell labeling. The USPIONs were effectively internalized by the hADSC and demonstrated T2* signal change. Hypointense regions, representing labeled cells, were seen in vivo 28 d following implantation[96]. Further research is required to optimize SPIONs for cell tracking.
Transfection agents are potentially toxic and, furthermore, there is capacity for iron oxide nanoparticles in vivo to cause toxicity to other organs, including liver and spleen[97,98]. Small polyhedral SPIONs with a silica coating have shown efficient MSC labeling without the need for a transfection agent and may offer a solution[99].
T2 signal change is due to the overall effect of magnetic nanoparticles rather than total number of cells[100]. Typically, a few hundred cells are required for detection with conventional MRI sequences[77]. Stem cells are known to proliferate following transplantation, leading to dilution of the iron oxide label and loss of MR signal over time[77]. If cells divide asymmetrically, with one daughter cell receiving the majority of nanoparticles, rapid dilution of signal can occur to an undetectable level[101]. Labeled cells could also become undetectable if they migrate in small rather than large groups. Sensitivity may be improved with post acquisition software analysis or a higher magnetic field strength.
A number of endogenous substances produce negative (or hypointense) MR signal, such as blood products containing haemosiderin or methaemoglobin. This leads to challenges differentiating blood product from labeled cells in an injured IVD. Novel MRI methodology has been adopted to help differentiate the labeled cells from endogenous substances, such as Inversion-Recovery With ON-Resonant Water Suppression, which delineates SPION labeled cells as positive contrast[102]. Further novel sequences are being developed to provide an exciting possibility to enhance non-invasive cell tracking.
Iron oxide nanoparticles fail to differentiate between live and dead cells. SPION signal has been demonstrated in the CNS long after cell death[103]. Multimodal imaging may be required to ensure cellular function, such as combining MRI with PET imaging. A study investigating iron oxide labeled stem cells in hemi-Parkinsonian rats used this multi modal technique. MRI visualized stem cells in the striatum and PET confirmed cellular viability[104].
To date, there is limited published research tracking MSCs in the IVD and this is summarized in Table 1. Saldanha et al[105] demonstrated feasibility by imaging MSCs labeled with SPION (Feridex®) in vitro to quantitatively characterize signal intensity loss using T1, T2 and T2* relaxation parameters. T2* weighted gradient echo (GRE) images demonstrated the most significant loss of signal intensity from labeled cells. Conversely, SPION labeled cells were indistinguishable from unlabeled cells on T1 weighted imaging[105]. This group also demonstrated SPION labeled cells, loaded in a fibrin gel and injected via fluoroscopic guidance, could be identified ex vivo within the IVD of excised rat tails[105]. Further research by Prologo et al[62] imaged MSCs labeled with a radioactive marker (iodine-124 2’fluoro-2’-deoxy-1β-D-arabinofuranosyl-5-iodouracil) using CT and PET. Four female pigs had approximately 100000 labeled MSCs injected under fluoroscopic guidance to the NP of discs which had degeneration induced 10 d prior. CT and PET were performed immediately following and three days after cell delivery. One animal was inaccurately injected. Results from the other three demonstrated accurate delivery and maintenance of labeled cells at three days[62]. While demonstrating useful preclinical results, using radioactive cell labeling in conjunction with CT and PET requires a significant amount of ionizing radiation, a consideration if used in clinical practice.
| Ref. | Animal model | Cell type | Label | Imaging | Results |
| Saldanha et al[105], 2008 | Rat tail (ex vivo) | Human MSCs (Loaded in fibrin gel-Tisseel) | FE-Pro (Feridex and Proatmine Sulphate) | 3T MRI | No significant effect of labeling on MSC viability Hypointensity (signal loss) seen in discs injected with labeled cell |
| Prologo et al[62], 2012 | Porcine n = 4 | Human MSCs | Iodine-124 2’fluoro-2’-deoxy-1b-D-arabinofuranosyl-5-iodouracil | PET/CT | Inaccurate delivery in 1 animal Three animals showed persistence and containment of labeled cells 3 d following injection |
| Barczewska et al[106], 2013 | Porcine n = 1 (ex vivo) n = 3 (in vivo) | Porcine MSCs | Molday ION USPION | 3T MRI | Ex vivo-Injected labeled cells clearly visualized In vivo-hypointense regions identified immediately following injection of labeled cells. Histopathology performed 2 wk later confirmed the presence of MSCs in the disc |
Recently, Barczewska et al[106] developed a technique for real time non-invasive monitoring of minimally invasive MSC delivery. IVD degeneration was induced in porcine discs of 3 animals via a fluoroscopic guided IVD vaporization procedure. Subsequently, the animals were placed in a 3T MRI scanner and 3 × 106 SPIO labeled MSCs were injected into the IVD through a plastic catheter in two divided injections. T2 weighted imaging was performed prior to, and following, each injection. Results demonstrated a statistically significant difference in signal intensity between both SPIO labeled MSCs compared with unlabeled MSCs and SPIO compared with control discs[106].
Although limited, current research has demonstrated MRI is a suitable modality for detecting SPION labeled MSCs in the IVD. Current data, utilizing both MRI and CT/PET, provides only short term tracking of labeled cells, demonstrating adequate initial placement of transplanted cells. Further research is required to monitor long-term retention of MSCs in the IVD. Moreover, longitudinal tracking of injected cells is required to improve understanding of the therapeutic mechanism of action of MSCs in IVD disease.
In addition to monitoring labeled MSCs, MRI has the potential to non-invasively measure response to biological therapies in IVD disease. Several techniques have been developed to classify disc degeneration by MR image criteria[107-110]. All are based on conventional T2 weighted sagittal imaging characteristics, which correspond to later morphological changes in IVD degeneration. The Pfirrmann classification system is the most widely used in the literature, utilizing an algorithm to generate five morphological grades[107]. Research has demonstrated acceptable intra and inter-observer variability for grading systems[107,109]. Ultimately, however, each is based on qualitative assessment and prone to inter-observer variability. In addition, conventional MRI sequences lack sufficient sensitivity to identify early disc biochemical changes[111].
Novel MRI sequences have the potential to quantitatively assess early IVD degeneration. T1ρ MRI uses long duration, low power radiofrequency, or spin-lock (SL) pulses, applied on-resonance to lock magnetization in the transverse plane. Spin-locked magnetization relaxes with the time constant T1ρ, spin lattice relaxation in the rotating frame[112,113]. Post acquisition computer software analysis can be utilized to generate a quantitative T1ρ measurement for a region of interest, such as the NP. T1ρ has been shown to be sensitive to PG content in articular cartilage and the IVD[114,115]. Furthermore, Borthakur et al[112] demonstrated T1ρ values in IVDs correlate with low back pain. Another quantitative method recently developed is the NP voxel count, which has been shown in vivo to quantitatively assess disc degeneration. This assesses nuclear size and hydration with T2-relaxation time measurements[116]. These imaging techniques have the potential to non-invasively, quantitatively, monitor response to biological treatments, as well as diagnose early IVD degeneration.
MSC transplantation shows exciting promise for the future regenerative approach to IVD disease. Cell labeling techniques are integral to ongoing research, to ensure accurate delivery and long-term retention of cells. Current research is limited to only short term monitoring. A greater understanding of the mechanism of action is required, with longitudinal tracking of implanted MSCs. Further research is required to discover if cells remain in situ long term, if they remain viable and if they track to sites of pathology in the IVD. MRI, with SPIONs as contrast agents, provides an excellent imaging modality for cell tracking. Some issues remain, such as dilution of signal through cell division or migration and differentiating labeled cells from intrinsic negative signal. These may be addressed with stronger magnetic fields, post-processing analysis and novel MRI techniques. Furthermore, novel MRI sequences can non-invasively quantify early biochemical changes in disc degeneration. In conjunction with conventional MRI, this provides a mechanism for measuring response to biological therapy, in both the research and clinical setting. Ongoing effective research will facilitate the translation of MSC treatment for IVD disease to clinical practice.
P- Reviewer: Pei M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5305] [Cited by in RCA: 5905] [Article Influence: 421.8] [Reference Citation Analysis (0)] |
| 2. | Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2033] [Cited by in RCA: 2019] [Article Influence: 74.8] [Reference Citation Analysis (2)] |
| 3. | Dagenais S, Tricco AC, Haldeman S. Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. Spine J. 2010;10:514-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Hicks GE, Morone N, Weiner DK. Degenerative lumbar disc and facet disease in older adults: prevalence and clinical correlates. Spine (Phila Pa 1976). 2009;34:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Takatalo J, Karppinen J, Niinimäki J, Taimela S, Näyhä S, Mutanen P, Sequeiros RB, Kyllönen E, Tervonen O. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults? Spine (Phila Pa 1976). 2011;36:2180-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Basu MK, Moss N. Warty dyskeratoma. A note concerning its occurrence on the oral mucosa, and its possible pathogenesis. Br J Oral Surg. 1979;17:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 379] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Vernon-Roberts B, Pirie CJ. Degenerative changes in the intervertebral discs of the lumbar spine and their sequelae. Rheumatol Rehabil. 1977;16:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 189] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Hukins D. Disc structure and function in Biology of the intervertebral disc. Boca Raton, FL: CRC Press 1988; . |
| 9. | Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120-130. [PubMed] |
| 10. | Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88 Suppl 2:10-14. [PubMed] |
| 11. | Gruber HE, Hanley EN. Observations on morphologic changes in the aging and degenerating human disc: secondary collagen alterations. BMC Musculoskelet Disord. 2002;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine (Phila Pa 1976). 1995;20:2690-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Melrose J, Smith SM, Little CB, Moore RJ, Vernon-Roberts B, Fraser RD. Recent advances in annular pathobiology provide insights into rim-lesion mediated intervertebral disc degeneration and potential new approaches to annular repair strategies. Eur Spine J. 2008;17:1131-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Neidlinger-Wilke C, Boldt A, Brochhausen C, Galbusera F, Carstens C, Copf F, Schultheiss M, Lazary A, Brayda-Bruno M, Ignatius A. Molecular interactions between human cartilaginous endplates and nucleus pulposus cells: a preliminary investigation. Spine (Phila Pa 1976). 2014;39:1355-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Brisby H. Pathology and possible mechanisms of nervous system response to disc degeneration. J Bone Joint Surg Am. 2006;88 Suppl 2:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976). 2000;25:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 852] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Liang C, Li H, Tao Y, Shen C, Li F, Shi Z, Han B, Chen Q. New hypothesis of chronic back pain: low pH promotes nerve ingrowth into damaged intervertebral disks. Acta Anaesthesiol Scand. 2013;57:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kuijpers T, van Middelkoop M, Rubinstein SM, Ostelo R, Verhagen A, Koes BW, van Tulder MW. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur Spine J. 2011;20:40-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | van Middelkoop M, Rubinstein SM, Kuijpers T, Verhagen AP, Ostelo R, Koes BW, van Tulder MW. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J. 2011;20:19-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 468] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 20. | White AP, Arnold PM, Norvell DC, Ecker E, Fehlings MG. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Williams CM, Maher CG, Latimer J, McLachlan AJ, Hancock MJ, Day RO, Lin CW. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384:1586-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 22. | Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003;16:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Hambly MF, Wiltse LL, Raghavan N, Schneiderman G, Koenig C. The transition zone above a lumbosacral fusion. Spine (Phila Pa 1976). 1998;23:1785-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 129] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine (Phila Pa 1976). 1996;21:970-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 284] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine (Phila Pa 1976). 1998;23:1531-158; discussion 1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18:988-997. [PubMed] |
| 27. | Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001;94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Huang B, Zhuang Y, Li CQ, Liu LT, Zhou Y. Regeneration of the intervertebral disc with nucleus pulposus cell-seeded collagen II/hyaluronan/chondroitin-6-sulfate tri-copolymer constructs in a rabbit disc degeneration model. Spine (Phila Pa 1976). 2011;36:2252-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Feng G, Zhao X, Liu H, Zhang H, Chen X, Shi R, Liu X, Zhao X, Zhang W, Wang B. Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: a comparison of 2 cell types as potential candidates for disc regeneration. J Neurosurg Spine. 2011;14:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Acosta FL, Metz L, Adkisson HD, Liu J, Carruthers-Liebenberg E, Milliman C, Maloney M, Lotz JC. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011;17:3045-3055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Meisel HJ, Ganey T, Hutton WC, Libera J, Minkus Y, Alasevic O. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15 Suppl 3:S397-S405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5-21. [PubMed] |
| 33. | Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine (Phila Pa 1976). 2003;28:2609-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Allon AA, Aurouer N, Yoo BB, Liebenberg EC, Buser Z, Lotz JC. Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model. Spine J. 2010;10:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Jeong JH, Jin ES, Min JK, Jeon SR, Park CS, Kim HS, Choi KH. Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat. Cytotechnology. 2009;59:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Hee HT, Ismail HD, Lim CT, Goh JC, Wong HK. Effects of implantation of bone marrow mesenchymal stem cells, disc distraction and combined therapy on reversing degeneration of the intervertebral disc. J Bone Joint Surg Br. 2010;92:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Henriksson HB, Svanvik T, Jonsson M, Hagman M, Horn M, Lindahl A, Brisby H. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976). 2009;34:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells. Acta Neurochir (Wien). 2010;152:1771-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel HJ. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: experiments in a canine model. Spine (Phila Pa 1976). 2009;34:2297-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Zhang Y, Drapeau S, Howard SA, Thonar EJ, Anderson DG. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine (Phila Pa 1976). 2011;36:372-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Zhang YG, Guo X, Xu P, Kang LL, Li J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res. 2005;219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976). 2005;30:2379-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Dennis J, Caplan A. Bone Marrow Mesenchymal Stem Cells. In Stem Cell Handbook. Albany, New York: Humana Press 2003; . [DOI] [Full Text] |
| 45. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5053] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 46. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2045] [Article Influence: 113.6] [Reference Citation Analysis (5)] |
| 47. | Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142-9149. [PubMed] |
| 48. | Oehme D, Ghosh P, Shimmon S, Wu J, McDonald C, Troupis JM, Goldschlager T, Rosenfeld JV, Jenkin G. Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model. J Neurosurg Spine. 2014;20:657-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Ghosh P, Moore R, Vernon-Roberts B, Goldschlager T, Pascoe D, Zannettino A, Gronthos S, Itescu S. Immunoselected STRO-3+ mesenchymal precursor cells and restoration of the extracellular matrix of degenerate intervertebral discs. J Neurosurg Spine. 2012;16:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976). 2010;35:E475-E480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 52. | Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 53. | Vadalà G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 54. | Zhang XL, Wang G, Dong FR, Wand ZM. Application of magnetic resonance imaging for monitoring stem cell transplantation for the treatment of cerebral ischaemia. Neural Regen Res. 2012;7:1264-1271. |
| 55. | Rümenapp C, Gleich B, Haase A. Magnetic nanoparticles in magnetic resonance imaging and diagnostics. Pharm Res. 2012;29:1165-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 56. | Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol. 2009;193:314-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 315] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 57. | Hu SL, Lu PG, Zhang LJ, Li F, Chen Z, Wu N, Meng H, Lin JK, Feng H. In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J Cell Biochem. 2012;113:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Bhirde A, Xie J, Swierczewska M, Chen X. Nanoparticles for cell labeling. Nanoscale. 2011;3:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Ma B, Hankenson KD, Dennis JE, Caplan AI, Goldstein SA, Kilbourn MR. A simple method for stem cell labeling with fluorine 18. Nucl Med Biol. 2005;32:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Elhami E, Goertzen AL, Xiang B, Deng J, Stillwell C, Mzengeza S, Arora RC, Freed D, Tian G. Viability and proliferation potential of adipose-derived stem cells following labeling with a positron-emitting radiotracer. Eur J Nucl Med Mol Imaging. 2011;38:1323-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Patel D, Kell A, Simard B, Xiang B, Lin HY, Tian G. The cell labeling efficacy, cytotoxicity and relaxivity of copper-activated MRI/PET imaging contrast agents. Biomaterials. 2011;32:1167-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Prologo JD, Pirasteh A, Tenley N, Yuan L, Corn D, Hart D, Love Z, Lazarus HM, Lee Z. Percutaneous image-guided delivery for the transplantation of mesenchymal stem cells in the setting of degenerated intervertebral discs. J Vasc Interv Radiol. 2012;23:1084-1088.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Bindslev L, Haack-Sørensen M, Bisgaard K, Kragh L, Mortensen S, Hesse B, Kjaer A, Kastrup J. Labelling of human mesenchymal stem cells with indium-111 for SPECT imaging: effect on cell proliferation and differentiation. Eur J Nucl Med Mol Imaging. 2006;33:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Sheyn D, Kallai I, Tawackoli W, Cohn Yakubovich D, Oh A, Su S, Da X, Lavi A, Kimelman-Bleich N, Zilberman Y. Gene-modified adult stem cells regenerate vertebral bone defect in a rat model. Mol Pharm. 2011;8:1592-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Wolbank S, Peterbauer A, Wassermann E, Hennerbichler S, Voglauer R, van Griensven M, Duba HC, Gabriel C, Redl H. Labelling of human adipose-derived stem cells for non-invasive in vivo cell tracking. Cell Tissue Bank. 2007;8:163-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Baba K, Nishida K. Single-molecule tracking in living cells using single quantum dot applications. Theranostics. 2012;2:655-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Higuchi Y, Wu C, Chang KL, Irie K, Kawakami S, Yamashita F, Hashida M. Polyamidoamine dendrimer-conjugated quantum dots for efficient labeling of primary cultured mesenchymal stem cells. Biomaterials. 2011;32:6676-6682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Zhang Y, Wang TH. Quantum dot enabled molecular sensing and diagnostics. Theranostics. 2012;2:631-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 69. | Hsieh SC, Wang FF, Lin CS, Chen YJ, Hung SC, Wang YJ. The inhibition of osteogenesis with human bone marrow mesenchymal stem cells by CdSe/ZnS quantum dot labels. Biomaterials. 2006;27:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Liu G, Ye X, Zhu Y, Li Y, Sun J, Cui L, Cao Y. Osteogenic differentiation of GFP-labeled human umbilical cord blood derived mesenchymal stem cells after cryopreservation. Cryobiology. 2011;63:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Wang L, Neoh KG, Kang ET, Shuter B, Wang SC. Biodegradable magnetic-fluorescent magnetite/poly(dl-lactic acid-co-alpha,beta-malic acid) composite nanoparticles for stem cell labeling. Biomaterials. 2010;31:3502-3511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Boddington SE, Henning TD, Jha P, Schlieve CR, Mandrussow L, DeNardo D, Bernstein HS, Ritner C, Golovko D, Lu Y. Labeling human embryonic stem cell-derived cardiomyocytes with indocyanine green for noninvasive tracking with optical imaging: an FDA-compatible alternative to firefly luciferase. Cell Transplant. 2010;19:55-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 73. | Gildehaus FJ, Haasters F, Drosse I, Wagner E, Zach C, Mutschler W, Cumming P, Bartenstein P, Schieker M. Impact of indium-111 oxine labelling on viability of human mesenchymal stem cells in vitro, and 3D cell-tracking using SPECT/CT in vivo. Mol Imaging Biol. 2011;13:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Akbarzadeh A, Samiei M, Davaran S. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett. 2012;7:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 518] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 75. | Bhakoo K. In vivo stem cell tracking in neurodegenerative therapies. Expert Opin Biol Ther. 2011;11:911-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Yang CY, Tai MF, Chen ST, Wang YT, Chen YF, Hsiao JK, Wang JL, Liu HM. Labeling of human mesenchymal stem cell: Comparison between paramagnetic and superparamagnetic agents. J Appl Phy. 2009;105:07B314. [DOI] [Full Text] |
| 77. | Cromer Berman SM, Walczak P, Bulte JW. Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:343-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 78. | Zhao X, Zhao H, Chen Z, Lan M. Ultrasmall superparamagnetic iron oxide nanoparticles for magnetic resonance imaging contrast agent. J Nanosci Nanotechnol. 2014;14:210-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Peng XH, Qian X, Mao H, Wang AY, Chen ZG, Nie S, Shin DM. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int J Nanomedicine. 2008;3:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Bull E, Madani SY, Sheth R, Seifalian A, Green M, Seifalian AM. Stem cell tracking using iron oxide nanoparticles. Int J Nanomedicine. 2014;9:1641-1653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 81. | Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1039] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 82. | Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH, Bulte JW. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480-487. [PubMed] |
| 83. | Kalish H, Arbab AS, Miller BR, Lewis BK, Zywicke HA, Bulte JW, Bryant LH, Frank JA. Combination of transfection agents and magnetic resonance contrast agents for cellular imaging: relationship between relaxivities, electrostatic forces, and chemical composition. Magn Reson Med. 2003;50:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 84. | Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, Read EJ, Frank JA. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 410] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 85. | Bulte JW, Arbab AS, Douglas T, Frank JA. Preparation of magnetically labeled cells for cell tracking by magnetic resonance imaging. Methods Enzymol. 2004;386:275-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 86. | Babic M, Horák D, Trchová M, Jendelová P, Glogarová K, Lesný P, Herynek V, Hájek M, Syková E. Poly(L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug Chem. 2008;19:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 87. | Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW. Instant MR labeling of stem cells using magnetoelectroporation. Magn Reson Med. 2005;54:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 88. | Qiu B, Xie D, Walczak P, Li X, Ruiz-Cabello J, Minoshima S, Bulte JW, Yang X. Magnetosonoporation: instant magnetic labeling of stem cells. Magn Reson Med. 2010;63:1437-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Ferrucci JT, Stark DD. Iron oxide-enhanced MR imaging of the liver and spleen: review of the first 5 years. AJR Am J Roentgenol. 1990;155:943-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 205] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 90. | Krejcí J, Pacherník J, Hampl A, Dvorák P. In vitro labelling of mouse embryonic stem cells with SPIO nanoparticles. Gen Physiol Biophys. 2008;27:164-173. [PubMed] |
| 91. | Niemeyer M, Oostendorp RA, Kremer M, Hippauf S, Jacobs VR, Baurecht H, Ludwig G, Piontek G, Bekker-Ruz V, Timmer S. Non-invasive tracking of human haemopoietic CD34(+) stem cells in vivo in immunodeficient mice by using magnetic resonance imaging. Eur Radiol. 2010;20:2184-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, Gu Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3:595-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 93. | Markides H, Kehoe O, Morris RH, El Haj AJ. Whole body tracking of superparamagnetic iron oxide nanoparticle-labelled cells--a rheumatoid arthritis mouse model. Stem Cell Res Ther. 2013;4:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 94. | van Buul GM, Farrell E, Kops N, van Tiel ST, Bos PK, Weinans H, Krestin GP, van Osch GJ, Bernsen MR. Ferumoxides-protamine sulfate is more effective than ferucarbotran for cell labeling: implications for clinically applicable cell tracking using MRI. Contrast Media Mol Imaging. 2009;4:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | van Buul GM, Kotek G, Wielopolski PA, Farrell E, Bos PK, Weinans H, Grohnert AU, Jahr H, Verhaar JA, Krestin GP. Clinically translatable cell tracking and quantification by MRI in cartilage repair using superparamagnetic iron oxides. PLoS One. 2011;6:e17001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Lalande C, Miraux S, Derkaoui SM, Mornet S, Bareille R, Fricain JC, Franconi JM, Le Visage C, Letourneur D, Amédée J. Magnetic resonance imaging tracking of human adipose derived stromal cells within three-dimensional scaffolds for bone tissue engineering. Eur Cell Mater. 2011;21:341-354. [PubMed] |
| 97. | Xu C, Zhao W. Nanoparticle-based Monitoring of Stem Cell Therapy. Theranostics. 2013;3:616-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Arbab AS, Yocum GT, Wilson LB, Parwana A, Jordan EK, Kalish H, Frank JA. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 2004;3:24-32. [PubMed] |
| 99. | Wang HH, Wang YX, Leung KC, Au DW, Xuan S, Chak CP, Lee SK, Sheng H, Zhang G, Qin L. Durable mesenchymal stem cell labelling by using polyhedral superparamagnetic iron oxide nanoparticles. Chemistry. 2009;15:12417-12425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Liu W, Frank JA. Detection and quantification of magnetically labeled cells by cellular MRI. Eur J Radiol. 2009;70:258-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 101. | Walczak P, Kedziorek DA, Gilad AA, Barnett BP, Bulte JW. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: the case of the shiverer dysmyelinated mouse brain. Magn Reson Med. 2007;58:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 102. | Stuber M, Gilson WD, Schär M, Kedziorek DA, Hofmann LV, Shah S, Vonken EJ, Bulte JW, Kraitchman DL. Positive contrast visualization of iron oxide-labeled stem cells using inversion-recovery with ON-resonant water suppression (IRON). Magn Reson Med. 2007;58:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 103. | Cianciaruso C, Pagani A, Martelli C, Bacigaluppi M, Squadrito ML, Lo Dico A, De Palma M, Furlan R, Lucignani G, Falini A. Cellular magnetic resonance with iron oxide nanoparticles: long-term persistence of SPIO signal in the CNS after transplanted cell death. Nanomedicine (Lond). 2014;9:1457-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Jackson J, Chapon C, Jones W, Hirani E, Qassim A, Bhakoo K. In vivo multimodal imaging of stem cell transplantation in a rodent model of Parkinson's disease. J Neurosci Methods. 2009;183:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Saldanha KJ, Piper SL, Ainslie KM, Kim HT, Majumdar S. Magnetic resonance imaging of iron oxide labelled stem cells: applications to tissue engineering based regeneration of the intervertebral disc. Eur Cell Mater. 2008;16:17-25. [PubMed] |
| 106. | Barczewska M, Wojtkiewicz J, Habich A, Janowski M, Adamiak Z, Holak P, Matyjasik H, Bulte JW, Maksymowicz W, Walczak P. MR monitoring of minimally invasive delivery of mesenchymal stem cells into the porcine intervertebral disc. PLoS One. 2013;8:e74658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26:1873-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2447] [Cited by in RCA: 2976] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 108. | Tertti M, Paajanen H, Laato M, Aho H, Komu M, Kormano M. Disc degeneration in magnetic resonance imaging. A comparative biochemical, histologic, and radiologic study in cadaver spines. Spine (Phila Pa 1976). 1991;16:629-634. [PubMed] |
| 109. | Raininko R, Manninen H, Battié MC, Gibbons LE, Gill K, Fisher LD. Observer variability in the assessment of disc degeneration on magnetic resonance images of the lumbar and thoracic spine. Spine (Phila Pa 1976). 1995;20:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 110. | Schiebler ML, Camerino VJ, Fallon MD, Zlatkin MB, Grenier N, Kressel HY. In vivo and ex vivo magnetic resonance imaging evaluation of early disc degeneration with histopathologic correlation. Spine (Phila Pa 1976). 1991;16:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Benneker LM, Heini PF, Anderson SE, Alini M, Ito K. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 112. | Borthakur A, Maurer PM, Fenty M, Wang C, Berger R, Yoder J, Balderston RA, Elliott DM. T1ρ magnetic resonance imaging and discography pressure as novel biomarkers for disc degeneration and low back pain. Spine (Phila Pa 1976). 2011;36:2190-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 113. | Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19:781-821. [PubMed] |
| 114. | Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, Elliott DM. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine (Phila Pa 1976). 2006;31:1253-1257. [PubMed] |
| 115. | Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, Leigh JS, Reddy R. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419-423. [PubMed] |
| 116. | Grunert P, Hudson KD, Macielak MR, Aronowitz E, Borde BH, Alimi M, Njoku I, Ballon D, Tsiouris AJ, Bonassar LJ. Assessment of intervertebral disc degeneration based on quantitative magnetic resonance imaging analysis: an in vivo study. Spine (Phila Pa 1976). 2014;39:E369-E378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |