Published online Jan 26, 2015. doi: 10.4252/wjsc.v7.i1.174
Peer-review started: July 28, 2014
First decision: September 4, 2014
Revised: September 16, 2014
Accepted: September 18, 2014
Article in press: December 16, 2014
Published online: January 26, 2015
Processing time: 170 Days and 3.7 Hours
Pluripotent stem cells (PSCs) are able to differentiate into several cell types, including pancreatic β cells. Differentiation of pancreatic β cells depends on certain transcription factors, which function in a coordinated way during pancreas development. The existing protocols for in vitro differentiation produce pancreatic β cells, which are not highly responsive to glucose stimulation except after their transplantation into immune-compromised mice and allowing several weeks for further differentiation to ensure the maturation of these cells in vivo. Thus, although the substantial improvement that has been made for the differentiation of induced PSCs and embryonic stem cells toward pancreatic β cells, several challenges still hindering their full generation. Here, we summarize recent advances in the differentiation of PSCs into pancreatic β cells and discuss the challenges facing their differentiation as well as the different applications of these potential PSC-derived β cells.
Core tip: Pluripotent stem cells (PSCs) including induced PSCs and embryonic stem cells are valuable sources for cell replacement therapies and disease modeling of diabetes. Although several studies reported the differentiation of PSCs into pancreatic β cells in vivo, still their response to glucose is very limited in vitro due to their immature nature. In this review, we summarize the current knowledge about the differentiation of PSCs into pancreatic β cells and discuss the challenges facing the differentiation and application of PSC-derived β cells.
- Citation: Abdelalim EM, Emara MM. Advances and challenges in the differentiation of pluripotent stem cells into pancreatic β cells. World J Stem Cells 2015; 7(1): 174-181
- URL: https://www.wjgnet.com/1948-0210/full/v7/i1/174.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i1.174
Diabetes mellitus (DM) is a common disease, characterized by hyperglycemia caused by insufficient insulin production and/or resistance to insulin. Two different kinds of DM are well characterized, type 1 (T1D) and type 2 (T2D). T1D is an autoimmune disease in which insulin-secreting β cells in pancreatic islets are permanently damaged by autoimmune attack, resulting in a lack of insulin production[1]. T2D occurs when the pancreas produces insufficient amounts of insulin and/or the tissues of the body become resistant to normal or even high levels of insulin[2]. Thus, T1D and T2D patients suffer from insulin insufficiency and damage of insulin-secreting β cells by distinct mechanisms[3,4].
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced PSC (iPSCs) have the ability to differentiate into all cell types of the body[5,6], which make them valuable sources for cell replacement therapies and other applications. Several reports have showed the generation of iPSCs from patients with different forms of diabetes[7-12]. Also, the generation of patient-specific ESCs has been recently generated from somatic cells of diabetic patients[13]. Thus, generation of functional β cells from human PSCs represents the most promising approach to treat diabetes. In addition to their high potential in cell therapy, β cells derived from patient-specific PSCs can be used as a disease model, where they provide a new approach to culture cells with a disease genotype and re-establishing pathogenesis in vitro. Disease modeling with such cells has the potential to give insights into the molecular mechanisms underlying diabetes and enable new cell-based drug discovery as well as autologous transplantation[13,14].
Several studies reported the generation of pancreatic β cells from ESCs[15-18] and iPSCs[7,8,12,15,19]. However, a number of obstacles have arisen that render the generation of fully functional pancreatic β cells from PSCs[14]. Therefore, in this review we summarize the current knowledge about the differentiation of ESCs and iPSCs into pancreatic β cells and discuss the challenges facing the differentiation and application of PSC-derived β cells.
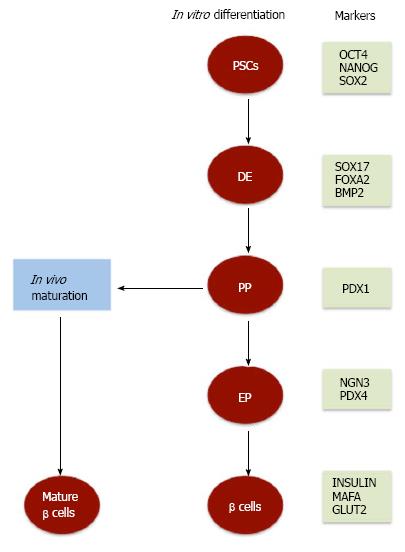
It has been well documented that both human ESCs (hESCs) and human iPSCs (hiPSCs) share the same characteristics and properties and are able to differentiate into various types of cells under certain cultural conditions[6,20,21]. Among these cells, insulin-secreting β cells differentiation from both hESCs and hiPSCs has been studied using similar differentiation protocols (Figure 1)[8,15-17,19]. However, variation in the efficiencies of differentiation has been reported between different hPSC lines[22,23]. These variations might occur due to the use of different protocols and different cell lines. The process of β cells differentiation is controlled by a complex network that depends on transcriptional regulation of genes involved in the development of the pancreas, the type and number of the differentiation factors, and the type and conditions of stem cell culture. Although many factors are important for successful generation of pancreatic insulin-secreting β cells from PSCs, successful manipulation of culture conditions stands out as one of the more critical factors. Several initial studies have used the step-wise approach to differentiate insulin-secreting cells from hESCs. Under these cultural conditions, hESCs generate up to 12% pancreatic β cells; however, the ability of these differentiated cells to produce insulin in response to glucose was very limited[16,18,24]. Therefore, a number of modifications in culture conditions have been done to improve the efficiency of β cell differentiation[16,18,25,26]. These modifications include, but are not limited to, monolayer cultures[16-18,25,27,28] or embryoid body (EB) technique[29,30]. Also, several studies have used xeno-free culture system to generate pancreatic β cells from PSCs in vitro[31-34]. Furthermore, in vitro modifications of cultural conditions to regulate signaling pathways demonstrated the vital role of specific pathways in the enhancement of β cell differentiation (Nostro et al[29], 2011). In hESCs, the regulation of transforming growth factor β (TGFβ) signaling produces high yields of insulin-secreting cells that reaches up to 25%[29]. Importantly, it has been proven that such critical refining in pancreatic differentiation protocols efficiently enhances the differentiation and maturation of hESC-insulin-secreting cells in vivo. These generated insulin-secreting cells showed several properties of functional β cells and were capable to respond efficiently to glucose stimulation in engrafted mice[25].
Similar to embryonic pancreatic development, PSCs differentiation into pancreatic lineage is excised in several steps that start with the differentiation into definitive endoderm (DE) (Figure 1), which is recognized by the expression of specific markers. Previous studies showed a high percentage (60%-80%) of hESC-differentiated cells express a panel of specific DE endodermal markers such as SOX17, FOXA2, CXCR4, and GSC, but not the visceral endodermal marker, SOX7[15,16,25,35-37]. The initiation of DE differentiation is properly induced in hESCs and hiPSCs by NODAL and WNT signals[15,18,35,38]. NODAL signals have been previously reported to be the main inducer of endogenous endoderm[39] and is activated by one of the members of TGFβ family, activin A. Notably, the dose of activin A appears to be crucial for Nodal signaling activation and in-turn DE differentiation. A previous study showed that the use of activin A in a concentration of (50-100 ng/mL) leads to an efficient DE differentiation as compared to lower concentrations[30,40]. In association with activin A, other factors have been shown to play an important role in DE differentiation. A recent study showed that treatment of hESCs with a combination of activin A, wortmannin (PI3K inhibitor), and CHIR99021 improves the percentage (90%) of the generated SOX17-positive cells[41]. Furthermore, it has been previously shown that the combination of activin A with sodium butyrate[16], PI3K pathway antagonists[15,38], or Wnt signaling activators (WNT3A or CHIR9902) enhances the efficiency of DE differentiation in PSCs. It is worth to note that CHIR99021 has been found to be more potent in promoting SOX17-and FOXA2-positive endodermal cells than Wnt3A[28]. Like wise, treating hESCs with GSK3β inhibitor instead of WNT3A increases DE generation[42]. Also, another TGFβ family member, GDF8 (myostatin), has been found to be effective for stimulating DE[43]. However, the efficiency of DE differentiation not only depends on GDF8, or activin A and its associated factors but also is enhanced by small molecules such as IDE1 and IDE2, which has been found to significantly induce the differentiation of approximately 80% of ESCs into SOX17-expressing DE cells[44].
It is well known that DE eventually generates both pancreatic and hepatic tissues. To direct DE cells towards pancreatic differentiation in vitro, the alternative hepatic lineage differentiation is inhibited by treating the cells with different types of growth factors inhibitors, such as SU5402 (FGF receptor antagonist) and Noggin (BMP antagonist)[36]. It has been shown that BMP signaling exerts two opposite effects during pancreatic differentiation[45]. In the dorsal endoderm, BMP signaling inhibition has been found to be required for specific differentiation into pancreatic lineage, whereas it is presence after the formation of pancreatic cells is essential to maintain pancreatic and duodenal homeobox 1 gene (PDX1) expression[45].
In the dorsal endoderm, BMP signaling inhibition has been found to be required for specific differentiation into pancreatic lineage, whereas its presence after the formation of pancreatic cells is essential to maintain pancreatic and PDX1 expression[45]. PDX1 is a transcription factor that is expressed on all pancreatic precursor cells and has been shown to be essential for early pancreatic development[46]. It has been found that the expression of PDX1 is correlated with the pancreas developmental stages. During the early stages of endocrine specification, PDX1 expression becomes restricted, whereas at later stages during β cells development its expression is upregulated as the protein enhances β cell function and is involved in insulin secretion[46]. The differentiation of PDX1-expressing cells in vitro is regulated by several factors that range from signaling pathways inhibitors to protein kinase activators. For example, in hESCs, the differentiation of pancreatic progenitors expressing PDX1 is induced by a small molecule, Indolactam V, that activates protein kinase C[17], and enhanced by retinoic acid and dorsomorphin (a BMP type 1 receptor inhibitor) treatments[28], whereas its proliferation is increased by inducing epidermal growth factor signaling[15]. Two other signaling pathways, NOTCH and HEDGEHOG, have been shown to be involved in the DE differentiation into pancreatic endocrine cells[16] (Figure 1). The inhibition of HEDGEHOG-signaling by Cyclopamine or KAAD cyclopamine induces the generation of PDX1-expressing cells[17,18,25,27,29], whereas Fibroblast growth factor 10 activates NOTCH signaling, which is involved in the proliferation of PDX1-expressing pancreatic progenitors.
Another transcription factor to consider as a marker for late pancreatic cell development is neurogenin 3 (NGN3). NGN3 expression peaks during endocrine differentiation stage, which is subsequent to the generation of PDX1 pancreatic progenitors. It has been previously shown that the capacity of PSCs to differentiate into pancreatic endocrine cells is highly affected by the seeding density of initial cultures. For example, Gage et al[47] demonstrated that high density hESCs led to remarkable increase in that rate of cell differentiation into PDX1- and NGN3-positive cells as compared to low density cultured cells. In contrast to PDX1, NGN3 expression is stimulated by a reduction in NOTCH signaling. It has been previously shown that the blockage of NOTCH signaling at this stage may be driven by DAPT (the gamma secretase inhibitor) treatment and thus drive NGN3-expressing cells formation[17,18,27]. Interestingly, these cells have been found to be differentiated from PDX1-expressing pancreatic progenitors after specific treatments. A recent study used a xeno-free culture system showed that a high NOGGIN concentration is crucial for inducing the differentiation of iPSCs into pancreatic progenitors (PDX1-positive) and then pancreatic endocrine progenitors (NGN3-positive cells)[34]. A previous study showed that TGFβ type 1 receptor inhibitor (SB431542) stimulates the differentiation of hESC-derived PDX1-positive pancreatic cells into NGN3-positive pancreatic endocrine progenitors[28,48]. Another recent study reported the generation of NGN3-positive endocrine precursors after the inhibition of vesicular monoamine transporter 2 by reserpine and tetrabenazine in PDX1-positive cells[49].
Differentiation into pancreaticβcells
The generation of mature pancreatic β cells from hESCs and hiPSCs is characterized by the expression of a panel of different factors, such as PDX1, MAFA, NKX6.1, NEUROD, ISL-1, and GLUT2, C-peptide, and INS (insulin)[15]. Of these factors, NKX6.1 expression has been shown to be essential for the production of functional mature β cells. This significant role of NKX6.1 was remarkably clear in the in vivo studies done by Rezania et al[50], where they showed that hyperglycemia significantly reduced in diabetic mice engrafted with cells expressing substantial amounts of NKX6.1. On the other hand, diabetic mice transplanted with cells expressing low levels of NKX6.1 remains hyperglycemic. In another study, it has been demonstrated that mice lacking NKX6.1 does not develop pancreatic β cells. However, re-expression of Nkx6.1 in pancreatic progenitors (PDX1-positive) restores the development of pancreatic β cells[51]. It has been also reported that overexpression of some pancreatic development-transcription factors enhance pancreatic β cell differentiation[52-54]. In human ESCs, enforced expression of the transcription factor PAX4 significantly promotes cell differentiation into pancreatic β cells[55]. Also, overexpression of human PAX4 in hESC-derived pancreatic progenitors increases the number of insulin-secreting β cells, which produce only one hormone (insulin) by suppressing the expression of ARX and glucagon[56]. In contrast, overexpression of PDX1 induces pancreatic endocrine cell differentiation, but not in vitroβ-cell formation during the differentiation of hESCs into EBs[57].
Several treatments, forskolin (an adenylate cyclase activator) and dexamethasone (a synthetic adrenocortical steroid)[28], hepatocyte growth factor, insulin growth factor 1 and glucagon-like peptide 1[9], have been used to enhance pancreatic β cell maturation in vitro. Several studies have previously reported the successful production of insulin-secreting cells in vitro from either hESCs or hiPSCs[15,16,18,19,24,25,29,30,36,58-60]. However these differentiated β cells showed little ability to respond to glucose, which is considered one of the well-known and important parameters that recognize functional and mature β cells. These differentiated cells also lack the expression of specific mature pancreatic β cell markers such as NKX6.1 and MAFA[19], that categorize them as immature non-functional β cells. Moreover, hPSC-derived β cells co-express multiple hormones such as INS, GCG (glucagon), and C-peptide instead of secreting only insulin hormone, which is secreted only by mature β cells. Therefore, controversy exists as to the ability of PSCs to differentiate into fully functional pancreatic β cells[61]. A previous study showed that endocrine precursors could be differentiated into glucose responsive β cells[49], which is considered one of the major challenges during β cell differentiation process.
In vivo maturation of pluripotent stem cell-derived pancreaticβcells
Despite these contentious in vitro evidences, mentioned above, in vivo microenvironment is likely to be important for pancreatic β cells maturation. Several transplantation studies have demonstrated the differentiation of immature pancreatic β cells or pancreatic progenitors into mature β cells in vivo. In either healthy[25,30] or streptozotocin (STZ)-induced diabetes mice[62], functional and mature pancreatic β cells are successfully developed from hESC-derived pancreatic progenitors transplanted either under the mouse kidney capsule or fat pad. Interestingly, another study showed that pancreatic progenitor cells are still capable to differentiate into mature pancreatic β cells that efficiently secrete insulin within macroencapsulation devices that have been transplanted into diabetic mice[63]. Like wise, in two different mouse models that mimic human T1D and T2D[64,65] in vivo, transplanted iPSC-derived β cells efficiently differentiate into glucose-responsive pancreatic β cells. In these studies as well as another study that has used monkey iPSC-derived β cells[48], the generated functional pancreatic β cells were found to improve hyperglycemia in the transplanted mouse models. Other studies reported that transplantation of pancreatic progenitors derived from PSCs into mice induces the differentiation of polyhormonal cells into glucose responsive pancreatic β cells (Figure 1). Interestingly, it has been found that such transition into mature β cells is associated with dynamic chromatin remodeling[58,66-68].
Moreover, it has been found that urocortin 3 (Ucn3), which is a corticotropin-releasing factor, is highly expressed in pancreatic β cells and regulates glucose-stimulated insulin secretion[69]. Although Ucn3 expression is detected in pancreatic β cells after in vivo maturation, it has not been detected after in vitro differentiation, suggesting the difference between in vitro and in vivo differentiation[70]. Interestingly, the level of Ucn3 expression increases more than sevenfold between immature and mature pancreatic β cells[70]. Thus in vivo maturation is critical for both the functionality and maturity of PSC-derived pancreatic β cells, indicating that there may be specific signals at the transplantation sites that induce and enhance β cell differentiation and maturation.
Several challenges need to be addressed before using PSCs in clinical applications and disease modeling. Differentiation of PSCs into mature, glucose responsive β cells is required for proper cellular therapies as well as for re-establishing the disease phenotypes in vitro to understand the mechanisms underlying different forms of diabetes. Pancreatic β cell maturation is defined based on glucose-stimulated insulin secretion (GSIS). To date, although several reports showed an improvement in generating glucose-responsive insulin-secreting β cells in vivo, still their response to glucose is very limited in vitro due to their immature nature[8,15-17,19]. A recent study has reported that insulin-secreting β cells differentiated from hPSCs are highly similar to human fetal pancreatic β cells than adult β cells[71]. Also, it has been suggested that the inability of pancreatic β cells to produce adequate insulin is due to the gradual loss of a specific group of transcription factors, which is important for insulin secretion and glucose responsiveness. Previous studies reported that dysfunctional pancreatic β cells derived from hESCs fail to secrete PDX1, NKX6.1, and MAFA[18,25,72], indicating that restoring the transcription factors is essential to recover β cell functions. Another study found that some genes related to early embryonic development (DPPA4, LIN28A, and LIN28B) continue to be expressed in the cells differentiated from PSCs, suggesting that the cells differentiated from PSCs are similar to the cells found in the very early stage of human development[73]. Notwithstanding the above findings, transplantation of pancreatic progenitors derived from PSCs into immune-compromised mice induces their differentiation into glucose responsive mature β cells[7,50,58,66-68]. Thus, future studies should focus on the signaling pathways regulating the maturation of pancreatic β cells in vitro.
Another problem hindering cell therapies of PSCs in diabetes is the low efficiency in producing insulin-secreting β cells. The current protocols are still lacking critical signals required for efficient generation of insulin-secreting β cells. Therefore, future studies should focus on studying the molecular mechanisms, guiding normal pancreatic development and to develop highly efficient differentiation protocols to obtain large number of insulin-secreting β cells.
Furthermore, one of the problems facing the stem cell therapies is the potential for unwanted cell growth. Undifferentiated PSCs are characterized by their ability to produce teratoma in vivo due to their high pluripotential capabilities. Previous studies suggested that encapsulation of the transplanted cells prevents overgrowth[74,75]. In addition, encapsulation was found to be crucial to differentiate PSCs into pure culture of insulin-secreting β cells to avoid their contamination with undifferentiated cells. To use the functional pancreatic β cells to cure T1D, the repair of self-tolerance or eliminate the influences of autoimmunity must be considered, thus the immune system cannot destroy the newly transplanted insulin-secreting β cells. Therefore, several studies reported the use of encapsulated devices to protect pancreatic β cells from immunological attack. Glucose responsive insulin-secreting grafts have been generated from encapsulated CyT49-luc hESC-derived pancreatic epithelium and found that maturation occurs without an increase in cell biomass[75]. Thus, encapsulation is considered as a possible tool to overcome some of the grand challenges in diabetes treatment by stem cell therapy.
Another challenge is related to the use of ESCs in cell therapy due to ethical concerns and immune rejection problem. The use of patient-specific ESCs, which are immunologically compatible to the patient, would overcome the immune rejection issue since ESCs can be generated from the somatic cells of the patients using SCNT[13,76].
In conclusion, there is a lot of work need to be done to obtain fully functional pancreatic β cells in vitro. For example, a genome-wide transcriptional analysis should be performed in each stage during pancreatic β cell differentiation to recognize the defects in the transcription factors. Also, comparing in vivo pancreatic development with in vitro pancreatic differentiation is important to identify the difference in gene expression, which may account for the functional differences. Finally, different differentiation protocols should be applied to understand the signaling pathways controlling the differentiation process in vitro.
| 1. | Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2006;175:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Joost HG. Pathogenesis, risk assessment and prevention of type 2 diabetes mellitus. Obes Facts. 2008;1:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 676] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11399] [Cited by in RCA: 10569] [Article Influence: 377.5] [Reference Citation Analysis (0)] |
| 6. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14587] [Article Influence: 810.4] [Reference Citation Analysis (0)] |
| 7. | Hua H, Shang L, Martinez H, Freeby M, Gallagher MP, Ludwig T, Deng L, Greenberg E, Leduc C, Chung WK. iPSC-derived β cells model diabetes due to glucokinase deficiency. J Clin Invest. 2013;123:3146-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768-15773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 408] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 9. | Ohmine S, Squillace KA, Hartjes KA, Deeds MC, Armstrong AS, Thatava T, Sakuma T, Terzic A, Kudva Y, Ikeda Y. Reprogrammed keratinocytes from elderly type 2 diabetes patients suppress senescence genes to acquire induced pluripotency. Aging (Albany NY). 2012;4:60-73. [PubMed] |
| 10. | Teo AK, Windmueller R, Johansson BB, Dirice E, Njolstad PR, Tjora E, Raeder H, Kulkarni RN. Derivation of human induced pluripotent stem cells from patients with maturity onset diabetes of the young. J Biol Chem. 2013;288:5353-5356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Kudva YC, Ohmine S, Greder LV, Dutton JR, Armstrong A, De Lamo JG, Khan YK, Thatava T, Hasegawa M, Fusaki N. Transgene-free disease-specific induced pluripotent stem cells from patients with type 1 and type 2 diabetes. Stem Cells Transl Med. 2012;1:451-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Thatava T, Kudva YC, Edukulla R, Squillace K, De Lamo JG, Khan YK, Sakuma T, Ohmine S, Terzic A, Ikeda Y. Intrapatient variations in type 1 diabetes-specific iPS cell differentiation into insulin-producing cells. Mol Ther. 2013;21:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, Paull D, Nestor MW, Freeby M, Greenberg E. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Abdelalim EM, Bonnefond A, Bennaceur-Griscelli A, Froguel P. Pluripotent stem cells as a potential tool for disease modelling and cell therapy in diabetes. Stem Cell Rev. 2014;10:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 418] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 16. | Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 360] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 17. | Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 362] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 18. | D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1447] [Cited by in RCA: 1431] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 19. | Tateishi K, He J, Taranova O, Liang G, D’Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601-31607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18623] [Article Influence: 931.2] [Reference Citation Analysis (1)] |
| 21. | Evans M. Discovering pluripotency: 30 years of mouse embryonic stem cells. Nat Rev Mol Cell Biol. 2011;12:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 636] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 23. | Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 795] [Cited by in RCA: 736] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 24. | Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1318] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 26. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 974] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 27. | Thatava T, Nelson TJ, Edukulla R, Sakuma T, Ohmine S, Tonne JM, Yamada S, Kudva Y, Terzic A, Ikeda Y. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther. 2011;18:283-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R, Kim JH. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefanty AG, Stanley EG. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Schulz TC, Young HY, Agulnick AD, Babin MJ, Baetge EE, Bang AG, Bhoumik A, Cepa I, Cesario RM, Haakmeester C. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7:e37004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 33. | Micallef SJ, Li X, Schiesser JV, Hirst CE, Yu QC, Lim SM, Nostro MC, Elliott DA, Sarangi F, Harrison LC. INS(GFP/w) human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia. 2012;55:694-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Shahjalal HM, Shiraki N, Sakano D, Kikawa K, Ogaki S, Baba H, Kume K, Kume S. Generation of insulin-producing β-like cells from human iPS cells in a defined and completely xeno-free culture system. J Mol Cell Biol. 2014;6:394-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1306] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 36. | Mfopou JK, Chen B, Mateizel I, Sermon K, Bouwens L. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology. 2010;138:2233-2245, 2245.e1-14. [PubMed] |
| 37. | Johannesson M, Ståhlberg A, Ameri J, Sand FW, Norrman K, Semb H. FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner. PLoS One. 2009;4:e4794. [PubMed] |
| 38. | McLean AB, D’Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 39. | Tian T, Meng AM. Nodal signals pattern vertebrate embryos. Cell Mol Life Sci. 2006;63:672-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 634] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 41. | Takeuchi H, Nakatsuji N, Suemori H. Endodermal differentiation of human pluripotent stem cells to insulin-producing cells in 3D culture. Sci Rep. 2014;4:4488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Bone HK, Nelson AS, Goldring CE, Tosh D, Welham MJ. A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J Cell Sci. 2011;124:1992-2000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Bruin JE, Erener S, Vela J, Hu X, Johnson JD, Kurata HT, Lynn FC, Piret JM, Asadi A, Rezania A. Characterization of polyhormonal insulin-producing cells derived in vitro from human embryonic stem cells. Stem Cell Res. 2014;12:194-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 327] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 45. | Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 46. | Bernardo AS, Cho CH, Mason S, Docherty HM, Pedersen RA, Vallier L, Docherty K. Biphasic induction of Pdx1 in mouse and human embryonic stem cells can mimic development of pancreatic beta-cells. Stem Cells. 2009;27:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Gage BK, Webber TD, Kieffer TJ. Initial cell seeding density influences pancreatic endocrine development during in vitro differentiation of human embryonic stem cells. PLoS One. 2013;8:e82076. [PubMed] |
| 48. | Zhu FF, Zhang PB, Zhang DH, Sui X, Yin M, Xiang TT, Shi Y, Ding MX, Deng H. Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells. Diabetologia. 2011;54:2325-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Sakano D, Shiraki N, Kikawa K, Yamazoe T, Kataoka M, Umeda K, Araki K, Mao D, Matsumoto S, Nakagata N. VMAT2 identified as a regulator of late-stage β-cell differentiation. Nat Chem Biol. 2014;10:141-148. [PubMed] |
| 50. | Rezania A, Bruin JE, Xu J, Narayan K, Fox JK, O’Neil JJ, Kieffer TJ. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432-2442. [PubMed] |
| 51. | Nelson SB, Schaffer AE, Sander M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development. 2007;134:2491-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 299] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 53. | Kwon YD, Oh SK, Kim HS, Ku SY, Kim SH, Choi YM, Moon SY. Cellular manipulation of human embryonic stem cells by TAT-PDX1 protein transduction. Mol Ther. 2005;12:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 572] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 55. | Liew CG, Shah NN, Briston SJ, Shepherd RM, Khoo CP, Dunne MJ, Moore HD, Cosgrove KE, Andrews PW. PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS One. 2008;3:e1783. [PubMed] |
| 56. | Gage BK, Baker RK, Kieffer TJ. Overexpression of PAX4 reduces glucagon expression in differentiating hESCs. Islets. 2014;Jun 17; Epub ahead of print. [PubMed] |
| 57. | Lavon N, Yanuka O, Benvenisty N. The effect of overexpression of Pdx1 and Foxa2 on the differentiation of human embryonic stem cells into pancreatic cells. Stem Cells. 2006;24:1923-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D’Amour KA, Kroon E. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 59. | Cai J, Yu C, Liu Y, Chen S, Guo Y, Yong J, Lu W, Ding M, Deng H. Generation of homogeneous PDX1(+) pancreatic progenitors from human ES cell-derived endoderm cells. J Mol Cell Biol. 2010;2:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | Xu X, Browning VL, Odorico JS. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech Dev. 2011;128:412-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 61. | Teo AK, Wagers AJ, Kulkarni RN. New opportunities: harnessing induced pluripotency for discovery in diabetes and metabolism. Cell Metab. 2013;18:775-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O’Neil JJ. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016-2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 439] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 63. | Bruin JE, Rezania A, Xu J, Narayan K, Fox JK, O’Neil JJ, Kieffer TJ. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56:1987-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 64. | Alipio Z, Liao W, Roemer EJ, Waner M, Fink LM, Ward DC, Ma Y. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci USA. 2010;107:13426-13431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 65. | Jeon K, Lim H, Kim JH, Thuan NV, Park SH, Lim YM, Choi HY, Lee ER, Kim JH, Lee MS. Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem Cells Dev. 2012;21:2642-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao Z, Warnock GL, Kieffer TJ. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60:239-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 67. | Basford CL, Prentice KJ, Hardy AB, Sarangi F, Micallef SJ, Li X, Guo Q, Elefanty AG, Stanley EG, Keller G. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Xie R, Everett LJ, Lim HW, Patel NA, Schug J, Kroon E, Kelly OG, Wang A, D’Amour KA, Robins AJ. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 69. | Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci USA. 2007;104:4206-4211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Blum B, Hrvatin SS, Schuetz C, Bonal C, Rezania A, Melton DA. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol. 2012;30:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 306] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 71. | Hrvatin S, O’Donnell CW, Deng F, Millman JR, Pagliuca FW, DiIorio P, Rezania A, Gifford DK, Melton DA. Differentiated human stem cells resemble fetal, not adult, β cells. Proc Natl Acad Sci USA. 2014;111:3038-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 72. | Schaffer AE, Taylor BL, Benthuysen JR, Liu J, Thorel F, Yuan W, Jiao Y, Kaestner KH, Herrera PL, Magnuson MA. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet. 2013;9:e1003274. [PubMed] |
| 73. | Patterson M, Chan DN, Ha I, Case D, Cui Y, Van Handel B, Mikkola HK, Lowry WE. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2012;22:178-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 74. | Yakhnenko I, Wong WK, Katkov II, Itkin-Ansari P. Cryopreservation of human insulin expressing cells macro-encapsulated in a durable therapeutic immunoisolating device theracyte. Cryo Letters. 2014;33:518-531. [PubMed] |
| 75. | Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res. 2014;12:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 497] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
P- Reviewer: Das UN, Peng SM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/