Published online Sep 26, 2014. doi: 10.4252/wjsc.v6.i4.441
Revised: July 22, 2014
Accepted: August 30, 2014
Published online: September 26, 2014
Processing time: 113 Days and 10.8 Hours
Despite significant effort and research funds, epithelial ovarian cancer remains a very deadly disease. There are no effective screening methods that discover early stage disease; the majority of patients are diagnosed with advanced disease. Treatment modalities consist primarily of radical debulking surgery followed by taxane and platinum-based chemotherapy. Newer therapies including limited targeted agents and intraperitoneal delivery of chemotherapeutic drugs have improved disease-free intervals, but failed to yield long-lasting cures in most patients. Chemotherapeutic resistance, particularly in the recurrent setting, plagues the disease. Targeting the pathways and mechanisms behind the development of chemoresistance in ovarian cancer could lead to significant improvement in patient outcomes. In many malignancies, including blood and other solid tumors, there is a subgroup of tumor cells, separate from the bulk population, called cancer stem cells (CSCs). These CSCs are thought to be the cause of metastasis, recurrence and resistance. However, to date, ovarian CSCs have been difficult to identify, isolate, and target. It is felt by many investigators that finding a putative ovarian CSC and a chemotherapeutic agent to target it could be the key to a cure for this deadly disease. This review will focus on recent advances in this arena and discuss some of the controversies surrounding the concept.
Core tip: Ovarian cancer stem cells (CSCs) are difficult to isolate, identify, and target. However, they are often thought to be the source of development of chemoresistance. Finding a therapeutic target in ovarian CSCs and identifying the mechanisms associated with the development of chemoresistance may lead to a long-lasting cure for patients with epithelial ovarian cancer.
- Citation: Haygood CLW, Arend RC, Straughn JM, Buchsbaum DJ. Ovarian cancer stem cells: Can targeted therapy lead to improved progression-free survival? World J Stem Cells 2014; 6(4): 441-447
- URL: https://www.wjgnet.com/1948-0210/full/v6/i4/441.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i4.441
It is estimated that over 14000 women in the United States will die with ovarian cancer and more than 22000 women will be newly diagnosed with the disease in 2013[1]. Women with early stage disease often have vague symptoms such as bloating, back pain, and fatigue leaving most women undiagnosed until later stages of the disease. Standard treatment of ovarian cancer consists of surgical resection of disease followed by taxane and platinum-based chemotherapy which yields a partial response rate of greater than 80% and a complete response rate of 40%-60% in patients with advanced disease[2]. Although initial response rates are promising, the recurrence rate is approximately 70% and five-year survival is 45% in patients with advanced disease[3]. While it appears that the majority of ovarian cancer cells are initially chemosensitive as evidenced by the high initial chemotherapy response rates, the high recurrence rates suggest development of chemoresistance. Some believe that a population of cells are not killed by chemotherapy, or they repopulate after exposure to chemotherapeutic agents. These cells have been called ovarian cancer stem cells (CSCs).
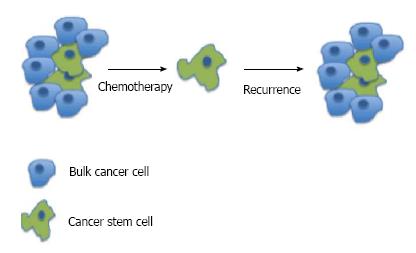
It has been theorized that CSCs exist in certain malignancies, particularly the blood cancers and basal-like breast cancer. For the blood cancers, identifying CSCs has been in progress since the first stem cells were identified[4]. In acute myeloid leukemia, CSCs have been proven to be an immature abnormally differentiated cells that have the ability to self-renew[5] It is felt by some investigators that these CSCs exist to promote tumor growth and metastasize to other organs. They have an increased tumorigenicity and differentiating capacity compared to other cells. The majority of solid tumor cells, may not have a differentiation capacity or the ability to develop chemoresistance but offer support to angiogenesis or signaling pathways. The CSCs (progenitor cells) are typically a small portion of the tumor and give rise to differentiated progeny that comprise the bulk of tumors (Figure 1), and are capable of unlimited growth[6,7]. CSC markers have been shown to be upregulated in cells growing in tumorspheres compared to single cells suggesting that CSCs are enriched in this population. In ovarian cancer, this spheroid form of tumor cells is thought to be involved in the dissemination of cancer in the peritoneal cavity. This suggests that CSCs are involved in metastasis intra-abdominally. CSCs are generally thought to have the ability to self-renew, differentiate, and metastasize to form secondary and tertiary tumors[8]. It has been shown that primary treatment with chemotherapeutic agents results in increased drug-resistant CSCs and this leads to recurrence[9]. Unlike some of the blood cancers which have known normal stem cells, there is no known normal ovarian stem cell[6]. This obviously complicates the identification of specific ovarian CSCs. The majority of evidence in favor of ovarian CSCs exists from the identification of markers of “stemness” as identified in other malignancies. Still, many researchers are investigating the existence of specific ovarian CSCs.
The isolation of ovarian CSCs is fraught with difficulty, like that of many other solid tumors. For isolation to occur, a single-cell suspension must be made from a solid tumor while sustaining viability. While there may be a large volume of tumor or ascites, the actual CSCs are a rare population of that tumor; unlike blood tumors, there is no specific marker for an ovarian CSC. The first model for this process was described by Bapat et al[10] in 2005. They collected ascites from a patient sample and were able to develop 19 immortalized tumor sphere-forming clones. Two of these were passaged into nude mice and grew into tumors that closely resembled the parental tumor. A single transformed clone was able to be isolated that demonstrated increased aggressiveness from the parent tumor. This experiment was some of the first evidence to show heterogeneous growth properties of tumor cell subpopulations in ovarian cancer. Also, these tumor cells demonstrated the ability to self-renew by continuing to form tumors even after serial transplantation.
There is no specific ovarian CSC marker and researchers have relied on markers of “stemness” identified from other malignancies. Some of these proteins used as CSC markers include CD44, CD133, CD117, ALDH1A1, and EpCAM (Table 1). There are many other proteins that have been used as markers of “stemness” but are not as well defined in ovarian cancer. Discovered as a marker for breast development and breast carcinoma, CD44 is a hyaluronate receptor[11] that is involved in cell-cell and cell-matrix interactions and ultimately affects cellular growth, differentiation, and motility[12,13]. Zhang et al[14]found that CD44+/CD117+ cells had increased chemoresistance to taxane and platinum-based chemotherapy as well as the ability to self-propagate. Similarly, Alvero and colleagues showed that CD44+ cells were enriched in ovarian cancer patient ascites and once isolated and xenografted gave rise to tumor with both CD44+ and CD44- cells suggesting they can differentiate and self-renew[15]. Orian-Rosseau described various strategies to target the CD44 receptor, which included binding to hyaluronic acid and osteopontin, a protein involved in interleukin production and overexpressed in ovarian cancer, as well as contributing to receptor tyrosine kinase activation[16].
| Cancer stem cell marker | Expression | Significance |
| CD44 | Hyaluronate receptor | Cell growth, differentiation, motility, increased chemoresistance, self-propagation |
| CD133 | Transmembrane glycoprotein | Increased tumor formation, increased chemoresistance, regeneration of original tumor cells |
| CD117 | Tyrosine kinase receptor | Cell signaling, apoptosis, cell differentiation, proliferation, cell adhesion |
| ALDH1A1 | Cell protector from aldehydes | Regeneration of tumor cells, chemoresistance |
| EpCAM (CD326) | Transmembrane glycoprotein | Cell adhesion, cell proliferation, tumor formation, epithelial to mesenchymal transition |
| CD24 | Transmembrane glycoprotein | Cell adhesion, aggressive phenotype, metastasis |
CD133 is a transmembrane glycoprotein that is expressed in normal hematopoietic and epithelial stem cells, and has also been described as a CSC marker in solid tumors. Ferrandina et al[17] showed that the amount of CD133 positive cells was higher in ovarian carcinoma than in normal ovarian tissue. In 2009, Baba and colleagues reported the ability of CD133+ cancer cells to generate both CD1333+ and CD133- cells, similar to what Alvero had seen with CD44+ cell spore[18]. CD133 has also been shown to be involved in increased tumor formation, increased chemoresistance, and the ability to recapitulate the original heterogenous tumor[19].
CD117, also known as c-kit or stem cell growth factor receptor, is a proto-oncogene encoded by the KIT gene. It is a type of tyrosine kinase receptor involved in cell signal transduction. It has been shown to be involved in apoptosis, cell differentiation, proliferation, and cell adhesion[20]. CD117 was shown by Kusumbe et al[21] to have high expression in ovarian cancer cells. Interestingly, cells expressing CD117 appear to be highly tumorigenic as it only takes approximately 103 cells to be able to self-renew, differentiate, and regenerate tumor in mouse models[22] The Wnt/β-catenin pathway which has been implicated in the development of chemoresistance is activated by CD117[23].
ALDH1A1 is a member of the ALDH group of proteins, which contains 19 enzymes that function as cell protectors from carcinogenic aldehydes[24]. Landen et al[25] declared it a putative CSC marker and showed its association with chemoresistance in ovarian carcinoma. Cells that are double positive for CD133 and ALDH1A1 have a greater ability to develop tumors in mouse models as compared to CD133+/ALDH1A1 - or ALDH1A1 +/CD133 - cells[26]. Recently, Shank et al[27], showed that metformin decreased the population of ALDH+ cells in ovarian cancer cell lines as well as decreased the formation of tumor spheres in patient tumors. In vivo, they also presented that metformin would restrict the growth of whole tumor cell line xenografts[27].
EpCAM (CD326) is a transmembrane glycoprotein involved in cell adhesion. EpCAM has been shown to have oncogenic signaling properties which result in cell proliferation and tumor formation[28]. Higher expression of EpCAM has also been seen in metastatic ovarian tumors[29] and it is involved in epithelial to mesenchymal transition leading to metastasis[30].
Another glycoprotein identified as an ovarian CSC is CD24 which is a cell membrane glycoprotein involved in cell adhesion. In 2005, the movement of CD24 from the cell membrane to the cytoplasm in borderline ovarian tumors was associated with microinvasion and omental implants as well as shorter survival time in adenocarcinoma of the ovary[31]. Moulla et al[32] also demonstrated that the transition from membrane to cytoplasmic CD24 expression was associated with a more aggressive phenotype in borderline tumors.
While it is interesting to utilize proteins to identify CSCs in various tissues, the clinical significance of these markers is still being determined. In 2012, Meng and colleagues reported on CD44+/CD24- cells in ovarian cancer cell line studies and patient ascites samples. Ovarian cancer cell line studies confirmed that increased numbers of CD44+ cells increased chemoresistance. Patient ascites samples with > 25% CD44+ cells had significantly decreased median progression-free survival (6 mo vs 18 mo, P = 0.01) as well as propensity to recur (83% vs 14%, P = 0.003)[33]. Zhang and colleagues studied 400 ovarian cancer tissue samples for CD133 positivity. They found associations between CD133+ and higher grade ovarian tumors, advanced stage disease, and decreased response to chemotherapy. They also found that CD133+ tumors are associated with decreased overall survival (P = 0.007) and shorter disease free interval (P < 0.001)[34]. In a study by Chau et al[23], they evaluated 3 patient samples in a xenograft mouse model and it was found that there was increased chemoresistance in patients with CD117+ tumor cells. In 65 ovarian cancer patients with advanced stage disease, greater than 20% of ALDH1A1+ cells correlated with decreased progression-free survival (6 mo vs 14 mo, P = 0.035)[25]. Recently, Zhu et al[35] reported on overexpression of CD24 in epithelial ovarian cancer and found that it was an independent variable associated with a low survival rate, increased metastasis, and decreased survival time.
Recent studies have indicated an enriched population of CSCs in ovarian cancer patients with recurrent carcinoma as compared to patients with primary cancer. Rizzo et al[36] noted an increased percentage of side population cells (generally accepted to be CSCs) in the ascites of patients with first recurrence after platinum-based chemotherapy as compared to ascites of chemo-naive patients. Steg et al[37] compared 45 matched primary and recurrent ovarian cancer patient samples for expression of stem cell markers including ALDH1A1, CD44, and CD133. Primary samples showed low densities of the markers, but samples collected after primary therapy showed higher densities of ALDH1A1, CD44, and CD133 due to the death of the non-stem cells. Stem cell markers were also examined in this study and 14% of recurrent tumors showed overexpression of these markers compared to primary tumors.
Stem cell markers have been implicated in chemoresistance and recurrence of ovarian cancer; therefore, it is reasonable to evaluate agents that could target these cells. CD44 has been studied with phase I trials in head and neck cancer via an antibody drug conjugate, BIWI 1[38]. There have also been several monoclonal antibodies designed to target CD44 in squamous cell cancers which could be extrapolated to adenocarcinomas[39]. CD44+ cells have been targeted in an intraperitoneal (IP) mouse model with cisplatin via a conjugate of hyaluronic acid and cisplatin which was then internalized more efficiently than CD44+ cells in ovarian cancer cell lines (A2780 and OV2008). Li and Howell[40] also demonstrated decreased growth in IP inoculated A2780 ovarian cancer cells treated with a hyaluronic acid-cisplatin conjugate when compared to free cisplatin. A hyaluronic acid-paclitaxel (HA-TXL) conjugate to target CD44+ cancer cells has also been studied in an IP mouse model with ovarian cancer cell lines (SKOV3ip1 or HeyA8) and showed significantly reduced tumor weights and nodules[41]. Similarly, CD133 has been targeted by IP administration of an anti-CD133 targeted toxin (dCD133KDEL), in an ovarian cancer cell line (NIH:OVCAR5-luc) in a mouse model, which resulted in significant decrease in progression of CD133 expressing tumors[42].
Noguera et al[43] evaluated imatinib mesylate, a CD117 specific inhibitor, in low grade recurrent platinum resistant tumors of the ovary in a single site phase II trial. Thirteen patients were enrolled and 48% of those had c-kit positive tumors. Eleven patients were eligible for evaluation of response, and though well-tolerated, no antitumor activity was seen in these low-grade tumors[43]. An anti-EpCAM monoclonal antibody, catumaxomab, was evaluated in a phase II/III trial in 258 patients with malignant ascites from epithelial cancer, half of which were ovarian carcinomas. When compared to paracentesis alone for treatment of ascites, addition of catumaxomab increased the median time to next paracentesis (11 d vs 77 d, P < 0.0001). Patients who received catumaxomab also had decreased signs and symptoms of ascites. The safety profile was acceptable[44]. Catumaxomab was evaluated in conjunction with steroid premedication (Catumaxomab Safety Phase IIIb Study with Intraperitoneal Infusion in Patients with Malignant Ascites Due to Epithelial Cancer) as well as in retreatment with IP therapy (SECIMAS), but results from these studies have not yet been posted (http://www.clinicaltrials.gov). It is also being evaluated in combination with cytotoxic chemotherapy in a phase II trial [ENGOT-ov8][45].
Another method of targeting CSCs is to target their signaling pathways, which include Notch, Wnt/β-catenin, TGF-β, and Hedgehog pathways. McAuliffe and colleagues demonstrated this concept with the Notch pathway and platinum resistant ovarian cancer[46]. In particular they looked at Notch3, and showed that it was overexpressed in ovarian CSCs and was correlated with increased platinum resistance. A pan Notch inhibitor, gamma-secretase inhibitor (GSI), when used in combination with cisplatin, had a synergistic cytotoxic effect, and led to decreased numbers of CSCs (12.8% side population cells in the control, 2.31% with Notch inhibitor alone, and 0.81% with GSI and cisplatin). A Notch ligand, Jagged 1, was targeted in taxane-resistant ovarian cancer cell lines by Steg et al[47]. They showed that targeting Jagged1 induced chemosensitivity to docetaxel in vivo and reduced tumor weights. They implicated the Hedgehog pathway in these experiments with Jagged1 by showing that rather than the chemoresistance being mediated by MDR1 as expected, it was GLI2, a Hedgehog downstream marker, that was downregulated. Another study with Jagged1 found that inhibition of the Wnt/β-catenin signaling pathway reduced its expression[48]. Wnt/β-catenin pathways have previously been demonstrated to produce self-renewal in ovarian cancer and appear to be a driving force behind ovarian cancer progression[49]. The Hedgehog signaling pathway has been implicated in the growth regulation of spheroid-forming cells in ovarian cancer. This was demonstrated by Ray et al[50], in four ovarian cancer cell lines (ES2, TOV112D, OV90, and SKOV3) where spheroid volume was increased up to 46-fold with Hedgehog agonists. Cyclopamine, a Hedgehog inhibitor, was used to prevent further growth of spheroid-forming cells in these cells lines and showed up to a 10-fold reduction in growth in ES2 cells[50]. Multiple groups are actively working to target these signaling pathways in hopes of altering ovarian cancer chemoresistance and recurrence.
Although there is growing evidence that ovarian CSCs are relevant, there are still many who debate the existence of these cells. At the forefront of this debate, remains the fact that a specific ovarian CSC marker has not been identified. None of the markers discovered are exclusively found in ovarian cancer cells. CD133 is recognized as the putative CSC marker for many human solid tumors, however, signaling pathways that regulate its behavior remain unknown[51]. Some studies presented in this review may be showing that CSCs are more “tumorigenic” based on ability of preferential or improved grafting. It will give much more credence to the argument if some of the pathways or markers being targeted show significant clinical results.
If progression and development of chemoresistance is due to the ovarian CSCs, then specific therapy for CSCs must be developed. In ovarian cancer, the use of monoclonal antibodies to many surface markers for CSCs has proven of some potential value. The most utilized monoclonal antibody, bevacizumab, an anti-vascular endothelial growth factor agent, has been shown to improve progression-free survival in advanced ovarian cancer[52]. Recently, CSCs have been implicated in the hypoxic environment that bevacizumab creates, but this relationship has not yet been well defined[53]. In addition to those mentioned previously, the anti-CD44 antibody, A3D8 was shown to produce significant apoptosis and arrest of cell cycle in the S phase for the SKOV3 ovarian cancer cell line by Du et al[54] and may represent a therapeutic option. Patients taking metformin for diabetes have previously been reported to have improved survival and some groups postulate that this relationship is due to the downregulation of CSC growth. A phase II trial is currently underway to evaluate this relationship (NCT01579812) (http://www.clinicaltrials.gov). There are over 3000 results when searching for clinical trials related to CSCs on Clinicaltrials.gov. While the majority of these are not specific for ovarian cancer, many are for breast cancer or other solid tumors, which have traditionally led to findings applicable to ovarian cancer.
It appears that ovarian CSCs are involved in chemoresistance and likely contribute to an overall poor prognosis in ovarian cancer patients. Researchers continue to study the role of ovarian CSCs and develop targeting agents for specific identification and therapeutic treatment. Clinical trials are ongoing for agents targeting ovarian CSCs and data from these trials will be important to determine future research directions aimed at improving survival in women with ovarian cancer.
| 1. | National cancer institute. Oxford: Oxford University Press 2014; . |
| 2. | Hoskins W PC YR, Baraket R, Markman M, Randall M. Principles and practice of gynecologic oncology. 4th ed. Philadelphia: Lippincott Williams & Wilkins 2005; . |
| 3. | Leitao MM, Chi DS. Surgical management of recurrent ovarian cancer. Semin Oncol. 2009;36:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Jordan CT. Cancer stem cell biology: from leukemia to solid tumors. Curr Opin Cell Biol. 2004;16:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Hope KJ, Jin L, Dick JE. Human acute myeloid leukemia stem cells. Arch Med Res. 2003;34:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Bapat SA. Human ovarian cancer stem cells. Reproduction. 2010;140:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007;1:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Kitamura H, Okudela K, Yazawa T, Sato H, Shimoyamada H. Cancer stem cell: implications in cancer biology and therapy with special reference to lung cancer. Lung Cancer. 2009;66:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Valent P, Bonnet D, De Maria R, Lapidot T, Copland M, Melo JV, Chomienne C, Ishikawa F, Schuringa JJ, Stassi G. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 539] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 10. | Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 570] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 11. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7800] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 12. | Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241-319. [PubMed] |
| 13. | Marhaba R, Klingbeil P, Nuebel T, Nazarenko I, Buechler MW, Zoeller M. CD44 and EpCAM: cancer-initiating cell markers. Curr Mol Med. 2008;8:784-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311-4320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1023] [Cited by in RCA: 1016] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 15. | Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158-166. [PubMed] |
| 16. | Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 17. | Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 322] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden DT, Rueda BR. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 20. | Miettinen M, Lasota J. Kit (cd117): A review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Applied immunohistochemistry & molecular morphology: AIMM/official publication of the Society for Applied Immunohistochemistry. Appl Immunohistochem Mol Morphol. 2005;13:205-220. |
| 21. | Kusumbe AP, Mali AM, Bapat SA. CD133-expressing stem cells associated with ovarian metastases establish an endothelial hierarchy and contribute to tumor vasculature. Stem Cells. 2009;27:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Luo L, Zeng J, Liang B, Zhao Z, Sun L, Cao D, Yang J, Shen K. Ovarian cancer cells with the CD117 phenotype are highly tumorigenic and are related to chemotherapy outcome. Exp Mol Pathol. 2011;91:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Chau WK, Ip CK, Mak AS, Lai HC, Wong AS. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene. 2013;32:2767-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 618] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 25. | Landen CN, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186-3199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 26. | Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991-4001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 415] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 27. | Shank JJ, Yang K, Ghannam J, Cabrera L, Johnston CJ, Reynolds RK, Buckanovich RJ. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol. 2012;127:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 28. | van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010;31:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 29. | Bellone S, Siegel ER, Cocco E, Cargnelutti M, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: implications for epithelial cell adhesion molecule-specific immunotherapy. Int J Gynecol Cancer. 2009;19:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7902] [Article Influence: 464.8] [Reference Citation Analysis (1)] |
| 31. | Choi YL, Kim SH, Shin YK, Hong YC, Lee SJ, Kang SY, Ahn G. Cytoplasmic CD24 expression in advanced ovarian serous borderline tumors. Gynecol Oncol. 2005;97:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Moulla A, Miliaras D, Sioga A, Kaidoglou A, Economou L. The immunohistochemical expression of CD24 and CD171 adhesion molecules in borderline ovarian tumors. Pol J Pathol. 2013;64:180-184. [PubMed] |
| 33. | Meng E, Long B, Sullivan P, McClellan S, Finan MA, Reed E, Shevde L, Rocconi RP. CD44+/CD24- ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis. 2012;29:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol. 2012;25:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 35. | Zhu J, Zhang G, Lu H. CD24, COX-2, and p53 in epithelial ovarian cancer and its clinical significance. Front Biosci (Elite Ed). 2012;4:2745-2751. [PubMed] |
| 36. | Rizzo S, Hersey JM, Mellor P, Dai W, Santos-Silva A, Liber D, Luk L, Titley I, Carden CP, Box G. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther. 2011;10:325-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 37. | Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, Zhang K, Conner M, Landen CN. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012;18:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 297] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 38. | Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Heider KH, Kuthan H, Stehle G, Munzert G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother. 2004;53:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Li SD, Howell SB. CD44-targeted microparticles for delivery of cisplatin to peritoneal metastases. Mol Pharm. 2010;7:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Lee SJ, Ghosh SC, Han HD, Stone RL, Bottsford-Miller J, Shen de Y, Auzenne EJ, Lopez-Araujo A, Lu C, Nishimura M. Metronomic activity of CD44-targeted hyaluronic acid-paclitaxel in ovarian carcinoma. Clin Cancer Res. 2012;18:4114-4121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Skubitz AP, Taras EP, Boylan KL, Waldron NN, Oh S, Panoskaltsis-Mortari A, Vallera DA. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol Oncol. 2013;130:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Noguera IR, Sun CC, Broaddus RR, Branham D, Levenback CF, Ramirez PT, Sood AK, Coleman RL, Gershenson DM. Phase II trial of imatinib mesylate in patients with recurrent platinum- and taxane-resistant low-grade serous carcinoma of the ovary, peritoneum, or fallopian tube. Gynecol Oncol. 2012;125:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 45. | Eskander RN, Tewari KS. Epithelial cell-adhesion molecule-directed trifunctional antibody immunotherapy for symptom management of advanced ovarian cancer. Clin Pharmacol. 2013;5:55-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | McAuliffe SM, Morgan SL, Wyant GA, Tran LT, Muto KW, Chen YS, Chin KT, Partridge JC, Poole BB, Cheng KH. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA. 2012;109:E2939-E2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 47. | Steg AD, Katre AA, Goodman B, Han HD, Nick AM, Stone RL, Coleman RL, Alvarez RD, Lopez-Berestein G, Sood AK. Targeting the notch ligand JAGGED1 in both tumor cells and stroma in ovarian cancer. Clin Cancer Res. 2011;17:5674-5685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Chen X, Stoeck A, Lee SJ, Shih IeM, Wang MM, Wang TL. Jagged1 expression regulated by Notch3 and Wnt/β-catenin signaling pathways in ovarian cancer. Oncotarget. 2010;1:210-218. [PubMed] |
| 49. | Gatcliffe TA, Monk BJ, Planutis K, Holcombe RF. Wnt signaling in ovarian tumorigenesis. Int J Gynecol Cancer. 2008;18:954-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Ray A, Meng E, Reed E, Shevde LA, Rocconi RP. Hedgehog signaling pathway regulates the growth of ovarian cancer spheroid forming cells. Int J Oncol. 2011;39:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Puglisi MA, Tesori V, Lattanzi W, Gasbarrini GB, Gasbarrini A. Colon cancer stem cells: controversies and perspectives. World J Gastroenterol. 2013;19:2997-3006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1658] [Cited by in RCA: 1852] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 53. | Sun H, Jia J, Wang X, Ma B, Di L, Song G, Ren J. CD44+/CD24- breast cancer cells isolated from MCF-7 cultures exhibit enhanced angiogenic properties. Clin Transl Oncol. 2013;15:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Du YR, Chen Y, Gao Y, Niu XL, Li YJ, Deng WM. Effects and mechanisms of anti-CD44 monoclonal antibody A3D8 on proliferation and apoptosis of sphere-forming cells with stemness from human ovarian cancer. Int J Gynecol Cancer. 2013;23:1367-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
P- Reviewer: Iavazzo CR, Gardner Mutch D S- Editor: Song XX L- Editor: A E- Editor: Lu YJ