Published online Sep 26, 2014. doi: 10.4252/wjsc.v6.i4.391
Revised: August 20, 2014
Accepted: August 30, 2014
Published online: September 26, 2014
Processing time: 62 Days and 1.8 Hours
A strong cohort of evidence exists that supports the localisation of corneal stem cells at the limbus. The distinguishing characteristics of limbal cells as stem cells include slow cycling properties, high proliferative potential when required, clonogenicity, absence of differentiation marker expression coupled with positive expression of progenitor markers, multipotency, centripetal migration, requirement for a distinct niche environment and the ability of transplanted limbal cells to regenerate the entire corneal epithelium. The existence of limbal stem cells supports the prevailing theory of corneal homeostasis, known as the XYZ hypothesis where X represents proliferation and stratification of limbal basal cells, Y centripetal migration of basal cells and Z desquamation of superficial cells. To maintain the mass of cornea, the sum of X and Y must equal Z and very elegant cell tracking experiments provide strong evidence in support of this theory. However, several recent studies have suggested the existence of oligopotent stem cells capable of corneal maintenance outside of the limbus. This review presents a summary of data which led to the current concepts of corneal epithelial homeostasis and discusses areas of controversy surrounding the existence of a secondary stem cell reservoir on the corneal surface
Core tip: It is a long held belief that stem cells reside only at the limbus. However, there are recent reports that present evidence of corneal repair and maintenance independent of limbal involvement. These findings call to light the possibility of previously undiscovered reservoirs of corneal stem/progenitor cells located at the central and peripheral cornea. A new secondary reservoir of stem cells has a significant clinical implication as new therapeutics for corneal degenerative disorders. This review outlines the historic evidence for limbal stem cells and discusses the role of these putative central and peripheral corneal stems cells in corneal homeostasis.
- Citation: Yoon JJ, Ismail S, Sherwin T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J Stem Cells 2014; 6(4): 391-403
- URL: https://www.wjgnet.com/1948-0210/full/v6/i4/391.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i4.391
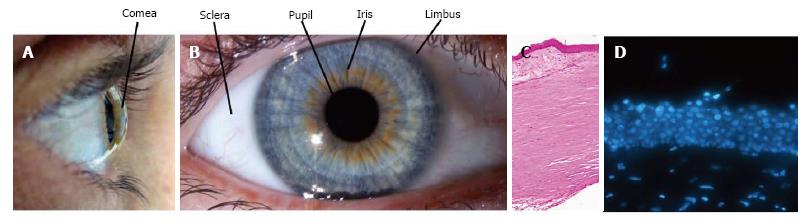
The transparent front surface of the eye, the cornea (Figure 1A) overlies the iris, pupil and anterior chamber. The structures that compose the anterior chamber are surrounded by the white opaque sclera with the tissues meeting at the limbus. Maintenance of corneal integrity is imperative to light entry and refraction onto the correct position on the retina.
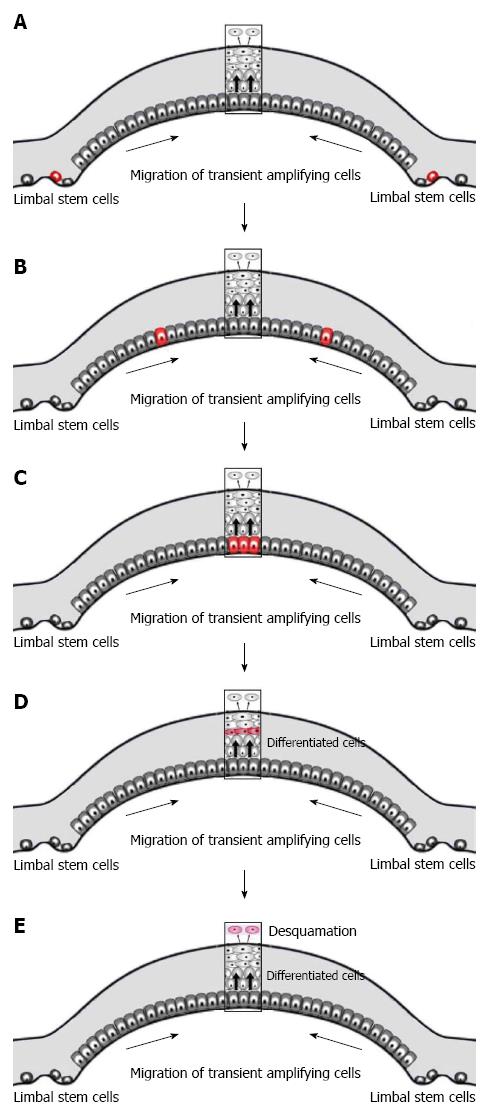
The anterior-most ocular surface is composed of corneal and conjunctival epithelia with the limbus at the transition zone between the two (Figure 1B and C). The corneal epithelium undergoes continuous renewal throughout life (Figure 1D). The central dogma of corneal homeostasis states that the mass of the epithelium remains constant so that the rate of cellular addition must equal that of cellular loss[1]. The predominant theory for corneal homeostasis is the XYZ hypothesis proposed by Thoft et al[2] in 1983. This theory proposes that the limbus serves as a reservoir of ocular stem cells. Asymmetric division of these stem cells produces a stem-like daughter cell which remains within the limbus and a transient-amplifying cell (TAC) (Figure 2A) which migrates centripetally and anteriorly (Figure 2B). TACs undergo multiple rounds of replication and progressively lose “stemness” (Figure 2C) as they migrate anteriorly and progress to post-mitotic suprabasal wing cells, and then terminally differentiated superficial squamous cells (Figure 2D). The superficial cells are lost from the surface by normal exfoliation (squamification) or traumatic injury (Figure 1E). Therefore anterior migration from cells of the basal epithelium “X” and centripetal migration from the limbus “Y” equals desquamation from the surface “Z”. The entire human corneal epithelium is renewed in 9 to 12 mo[3].
Whilst the research underpinning the limbus as the main reservoir for corneal epithelial stem cells has been consolidated with sophisticated cell tracking assays, an additional emerging view of the existence of stem cells outside of the limbus is supported by findings from several independent groups. This review analyses the data in support of limbal stem cells (LSCs) and looks at the possibility of a secondary reservoir of stem cells for the corneal epithelium.
Studies reporting differences between central corneal and limbal cells were published as early as the 1940s. These early studies showed increased frequency of mitoses in the basal layer of peripheral cornea using mitotic figure counts and radiated thymidine[4,5]. Centripetal migration of cells expressing melanin pigment was observed in rabbit as well as human corneas, suggesting the limbus as a source of new cells[6,7]. Since then, various studies have established the limbus as the location of corneal epithelial stem cells based on a set of unique properties observed within this cell population:
DNA label-retention studies have shown the limbus contains cells in a growth-arrested or slow cycling state. Retention of radiated thymidine or 5-bromo-2’-deoxyuridine (BrdU) has been reported in limbal cells of mice cornea in situ[8-10], human limbal explant cultures[11] and whole cornea organ cultures[12]. The retention of DNA label was observed for up to nine weeks in these studies. The labelling index, or the percentage of BrdU-retaining cells, was 1%-4% in mice corneas[9,10,13], and approximately 4% in human limbal explant cultures[11]. The nuclear label was lost progressively as the labelled cells moved towards the central cornea, indicating increased cell division during centripetal migration[8].
Slow turnover rate in the limbus has also been demonstrated by resistance to 5-fluorouracil (an anti-metabolite which specifically targets proliferative cells)[14], cytoplasmic staining for cyclins D, E and A (indicator of a growth-arrested state)[15] and susceptibility to malignant transformation[16-18]. The susceptibility to tumour formation is thought to be a property of stem cells as oncogenic mutations are more likely to accumulate in cells with long life span[19].
Life-long maintenance of any stratified epithelium necessitates a self-renewing pool of stem cells, asymmetric division of precursor cells and a rapid proliferative response upon injury[20]. Studies have suggested that these attributes are unique to the limbal cell population.
Self-renewal capacity or clonogenicity of limbal cell populations has been shown by their ability to form sphere colonies on a 3T3 fibroblast feeder layer[21]. These authors showed that the holoclone, meroclone and paraclone colony formation system previously identified in human skin could be translated into spheres derived from human corneal biopsies. The single cell-derived sphere colonies from the limbus (equivalent to holoclones) were capable of undergoing 80 to 100 cell division cycles and could be propagated up to 14 passages before senescence. Single cell isolates from central cornea only formed paraclones (mostly consisting of terminally differentiated cells and capable of 15 cell divisions at maximum) and meroclones (intermediate form between holoclones and paraclones).
Asymmetric cell division has been suggested by uneven distribution of cell fate determinants across the corneal epithelium. Molecules implicated in asymmetric cell division and early cell fate decision, such as Musashi-1[22], Notch-1[23], p75[24], C/EBPδ[25] and ∆Np63α[26] have been almost exclusively localised in the mouse and human limbus.
Proliferative potential of limbal cells has been demonstrated by both in vitro and in vivo studies. Primary human limbal epithelial cell cultures showed high proliferative potential with a mean of 23 population doublings in vitro, while central corneal cells could not be propagated[27]. Explant cultures of human limbal epithelium showed larger outgrowth and higher mitotic rate compared to explants from central epithelia[28,29]. When transplanted into the flanks of athymic mice, single cell suspensions from limbal cell culture produced cysts which had more organised structure and longer life span than those derived from central corneal cell suspensions[30]. Furthermore, in vivo animal studies have shown that the slow cycling limbal basal cells can rapidly divert to proliferative status upon damage to cornea[8,13].
Morphological differences between limbal and corneal cells have been highlighted using a variety of imaging technologies including synchrotron infrared microspectroscopy[31], morphometric analysis of DAPI-stained nuclei[9], transmission electron microscopy[32,33], in vivo confocal microscopy and flow cytometry[34]. These studies commonly identified cuboidal cells 10 µm in diameter with a high nucleus-to-cytoplasm ratio in the limbal basal layer. The sparse cytoplasm in these cells appears smooth due to the paucity of organelles and intracellular junctions, another indicator of low metabolic activity and protein turnover. In contrast, basal cells of the central epithelium are more columnar and have a lower nucleus-to-cytoplasm ratio[31].
The identification of exclusive biochemical markers of corneal stem cells has been for many years a highly desirable endeavour. A number of putative stem cell markers have been suggested based on the biochemical transition that takes place in the basal cell layer of the corneo-limbal junction[35-37]. Limbal basal cell layers preferentially express certain structural proteins (vimentin, cytokeratin 14, 15 and 19), cell adhesion molecules (integrin α6, β1, β4, P-cadherin and N-cadherin), enzymes (α-enolase, aldehyde dehydrogenase, cytochrome oxidase, Na+/K+-ATPase and carbonic anhydrase), metallothionein, growth factor receptors (KGF-R and NGF-R), cell fate/cycle regulators (notch-1, Musashi-1, ∆Np63α, p75, Bmi-1 and C/EBPδ) and ABCG2, an ATP-binding cassette transporter protein. ABCG2 has been shown to be responsible for the efflux of the nuclear dye Hoechst 33342, enabling isolation of ABCG2-positive cells using flow cytometry[38]. This dye efflux property is an established marker of a stem cell in many cell lineages including haematopoietic[39], neuronal[40], muscle[41], and epithelium[42]. The ABCG2 proteins are thought to protect LSCs from oxidative stress by transporting small regulatory molecules required for their proliferation, differentiation and apoptosis[43]. ABCG2-positive cells are termed side population (SP) cells, and only a small proportion of limbal basal cells are SP cells. The SP cells have been shown to possess a number of stem cell properties including up-regulation in response to central corneal wounding[44], small cells with high nucleus-to-cytoplasm ratio, slow cycling, expression of ∆Np63α and ABCG2, absence of cytokeratin 3, 12 and involucrin, and increased colony-forming efficiency and growth capacity[45,46].
As limbal basal cells migrate out of the limbus, their protein expression profile gradually changes. Central corneal epithelium is characterised by the loss of α-enolase and melanin pigmentation and the expression of cytokeratin 3 and 12, connexin 43 and 50, involucrin and CLED, a Ca2+-linked protein associated with early epithelial differentiation. The expression of a large amount of metabolic enzymes and proteins in the central corneal cells is thought to contribute to the increase in cell size[47]. Furthermore, increase in cell size has been correlated with loss of colony-forming efficiency[48].
Centripetal migration of corneal epithelial cells is a well-documented phenomenon[49,50]. Imaging studies have directly visualised centripetal migration of limbal cells towards the centre of the cornea. One of earliest studies used India ink to mark limbal cells which then migrated centripetally over the wounds of the mice cornea[51,52]. Centripetal migration was observed in rabbit lamellar keratoplasty model where the host corneal epithelial cells invaded the grafted donor tissue[53]. Similar results were obtained in the explants of human donor corneal buttons, where all donor corneal epithelial cells were replaced by recipient cells as early as three months post-penetrating keratoplasties[54]. Both Collinson et al[55], and Nagasaki et al[56] used transgenic mice with reporter genes to visualise centripetal migration in normal mice cornea. Interestingly, Matsuda et al[57] and Srinivasan et al[58] found that wounds close to the limbus or repeated insult to the central epithelium accelerated the healing rate, the latter implying that rapidly dividing TACs of the periphery have moved to more central areas after the first trauma and respond more quickly to the second.
The chemotactic signal for centripetal migration may be provided in the form of cytokines and/or the difference between the composition of extracellular matrix between the limbus and the cornea[59]. KGF, a paracrine hormone secreted by stromal cells, has been shown to enhance outgrowth in rabbit limbal explant culture on human amniotic membrane[60]. While the inflammatory cytokine interleukin-6[61], fibronectin[62], and hyaluronan[63], all of which are highly up-regulated upon injury, have been shown to play a role in drawing rabbit limbal cells towards the wound.
Recently, a very elegant study by Di Girolamo et al[64] has shown the centripetal movement of cells generated in the limbus using inducible multicolour tagging technology in vivo. Furthermore, this study linked the inducible multicolour tagging system with K14, one of the cytokeratin molecules that has been shown to mark an association with limbal stem cells. This study clearly showed that coloured K14 positive cells originated from the basal limbal epithelium and formed narrow corridors of epithelial cells that radiated centripetally onto the corneal surface. These authors do acknowledge that K14 is not an absolute limbal stem cell marker and that they could not exclude the existence of stem cells outside the limbal niche as K14 was targeted because of its limbal location.
Limbal basal cells characteristically lack differentiation markers indicating they are in an undifferentiated state. Several studies however, have implied a high multipotent differentiation potential when appropriate combinations of cellular signalling molecules are encountered: Rabbit limbal epithelial cell sheets transformed into fibroblasts when transplanted onto limbal stroma[65]; during the culture of human limbal explants, the limbal epithelial cells which invaded into the stroma underwent epithelial-mesenchymal transition[66]; mouse limbal epithelial cells expressed opsin when transplanted onto mice retina, indicating their potential to differentiate into rod photoreceptors[67]; and the potential to transdifferentiate to neuronal cells was demonstrated by Zhao et al[68]. In their study, rat limbal cell isolates maintained in growth factor-driven culture system expressed neuronal progenitors, β-tubulin, nestin and neurofilament. When subject to serum-containing differentiation medium, the limbal cell isolates expressed glial markers such as GFAP and O4. The limbus-derived neuron-like cells not only expressed neuronal markers and neurotransmitter receptors, but also exhibited electrical responses to GABA and kainic acid[69].
A stem cell niche is an anatomically defined area that is thought to provide a variety of intrinsic and extrinsic factors such as the physical protection, survival factors and cytokines and deemed essential to the maintenance of a stem cell population while preventing entry into differentiation[70,71]. Over the past decade, much progress has been made in characterising the putative niche in the limbus. The limbal areas are rich in melanin pigments, highly innervated, well-vascularised and have a different array of extracellular matrix components than the central epithelium. Melanocytes or melanin granules within the cytoplasm of progenitor cells are thought to play a role in protection against ultraviolet radiation[8,72]. Blood-derived growth factors and nutrients provide for the active cell division[8,73].
The epithelial-stromal interface in the limbus differs from that in the central cornea. Bowman’s layer, a densely interwoven collagen sheet lying between the basement membrane of the central corneal epithelium and the stroma, is absent in the limbus[74]. In the limbus, stroma directly underlies the epithelial basement membrane. The limbal epithelial basement membrane also differs from that of central cornea in its composition[75-80]. The limbal basement membrane labelled positive for type IV collagen α1 chain, laminin α2, β1, β2, γ1, γ3 chains, nidogen, agrin, BM40/SPARC, tenascin-C and thrombospondin-4, whereas central cornea showed positive immunoreactivity to type IV collagen α3 chain, type V collagen, thrombospondin-1 and endostatin. Limbal-specific basement membrane components were co-localised with putative stem cell markers such as ABCG2, p63 and cytokeratin 19, but not with differentiation markers including cytokeratin 3, connexin 43, desmoglein and integrin α2. In addition, the cornea-limbal transitional zone showed strong immunostaining to type XVI collagen, fibrillin-2, tenascin-C/R, vitronectin, bamacan, chondroitin sulfate and versican, and were co-localised with vimentin-positive cell clusters.
To date, four anatomic structures have been proposed as the corneal stem cell niche; Palisades of Vogt, limbal epithelial crypts, limbal crypts and focal stromal projections. The Palisades of Vogt are ridges of epithelium in the limbus that extend centripetally from the bulbar conjunctiva, and are easily visible by slit lamp microscopy, especially in young donors or those with dark skin[7,81,82]. More recently, Shanmuganathanet al[83] and Dua et al[84] identified limbal epithelial crypts located at the inter-palisade epithelial rete ridges of the Palisades of Vogt. The limbal epithelial crypts radiate either peripherally into conjunctival stroma or circumferentially into limbal stroma. Shortt et al[85] proposed two additional niches using in vivo confocal microscopy; limbal crypts which are projections of limbal epithelium from the peripheral cornea into the limbal stroma, and focal stromal projections which are finger-like projections of limbal stroma with central blood vessels extending upward into the epithelium. These papillary structures offer physical protection for the deeply seated cells from injuries and shearing forces, and a large surface area that can accommodate increased cell numbers, blood vessels, and other supportive cells such as melanocytes, macrophages and stromal cells. Limbal crypts and focal stromal projections predominantly occur within regions of the cornea normally covered by the eyelids, which is a potential protective mechanism of these proposed niches[85]. Some of the putative stem cell features such as expression of ABCG2, p63 and p75, and high nucleus-to-cytoplasm ratio have been identified in the limbal basal cells lining these papillary structures[24,77]. In patients with limbal stem cell deficiency (LSCD), these four proposed niche structures are absent[84,85].
Recent studies have identified stromal stem cells which are directly subjacent to limbal basal cells[86,87]. An arising view of the limbal niche environment is that the limbal basal cells, stromal stem cells and the extracellular matrix molecules function as one unit to maintain the reservoir of ocular stem cells[88-90]. Human limbal epithelial cells co-cultured with stromal stem cells produced colonies with average diameter five times as large as those obtained with murine 3T3 feeder layer, indicating enhanced proliferation of limbal cells in the presence of stromal stem cells[91]. Recently, it was shown that limbal epithelial cells actively merge with stromal cells via chemokine receptor-mediated signalling in sphere-forming conditions, and this interaction seemed crucial for the maintenance of stem cell phenotype[92].
The ability of limbal cells to regenerate corneal epithelium is robust evidence for the existence of stem cells in the limbus. Limbal stem cell deficiency (LSCD) is a complex corneal disorder resulting from functional and/or anatomical loss of limbus due to chemical or thermal burn, radiation, genetic/autoimmune disorders, multiple surgeries, contact lens use, infection or drug use[93,94]. Signs and symptoms of LSCD include conjunctivalisation, corneal vascularisation, pain, tear, redness, oedema, poor vision and blindness, which are thought to be associated with failure of epithelial regeneration[95,96]. Similar symptoms and a delayed wound healing response could be reproduced in rabbits by surgically removing the limbus[95,97]. The degree of loss of limbal tissue has been shown to correlate with the severity of pathology[98]. Clinical studies have shown that LSCD can be successfully treated with application of limbal cells[99-102]. Currently the sources of limbal cells are limbal autograft for unilateral LSCD, allogenic limbal graft from living related or cadaveric donors and ex vivo expanded limbal cells on transplantable substrate[93]. The overall success rate of limbal cell transplant is estimated at 76%, ranging from 50% to 100%[103]. The success rate varies between studies because outcome parameters, ex vivo expansion protocol, length of follow-up and aetiology of LSCD are different in each study[103]. Standard corneal transplants do not appear to provide a cure for patients with LSCD[104].
The body of evidence for the presence of stem cells at the limbus is impressive and convincing if largely circumstantial. The final piece of the jigsaw that remains to be revealed is the identification of an absolute stem cell marker that is definitive of stem cell functionality. Likewise the body of evidence of the origin of epithelial cells at the limbus and their contribution to corneal epithelial homeostasis through the centripetal movement over the corneal surface has been elegantly shown by several research groups in several mammalian systems both in vitro and in vivo. However, despite this body of evidence, the proof that stem cells of the corneal epithelium reside only at the limbus and nowhere else is lacking and several pieces of knowledge remain unexplained by our current understanding of corneal maintenance by limbal stem cells:
The traditional defining features of stem cells of the corneal epithelium include slow turnover rate, clonogenicity, proliferative potential, characteristic morphology, expression of certain proteins, centripetal migration in vivo, multipotency, specialised niche structures and ability to regenerate corneal epithelium. Despite the obvious biochemical changes at the cornea-limbal junction, selection of a consensus LSC marker has not been straightforward because each of these candidate markers has limitations resulting in inevitable ambiguities in separating stem cells from early progenitors[33,105]. In fact, there is mounting evidence showing that some of the putative markers of LSCs are not unique to the limbal basal cells.
Slow turnover rate has been demonstrated by label retaining studies in animal models. However, there are several pitfalls related to the use of label retention as a marker of stem cells[106]. The duration of the DNA labelling period was typically less than one week in most label retaining studies[8-11]. Cells quiescent during the labelling period will not take up DNA label and never be identified by this method. On the other hand, cells that have undergone a few rounds of cell division may still show DNA label albeit at a weaker level. Furthermore, label retention is not an essential property of stem cells as stem cells such as those underlying mammalian intestinal mucosa have short cycle time[107]. Not all label retaining cells are stem cells and vice versa.
The slow cycling property of the limbal cells has also been inferred from their resistance to 5-fluorouracil and predisposition to cancer. However, cells resistant to 5-fluorouracil are also found in the central epithelium although smaller in number than in the limbus[14]. Predisposition to cancer is also common in cells at the transitional zone where two types of epithelia unite in non-ocular tissue systems. The endo-ectocervical and oesophagus-stomach junctions are such examples.
Clonogenicity and asymmetric division are not unique properties of the limbal cells. Central corneal cells isolated from various mammalian species including humans have been shown to form clonogenic spheres in vitro although the number of spheres formed was smaller than when limbal cells isolates were used[108,109].
Asymmetric division as a means of self-renewal of stem cells is a widely accepted concept, but is difficult to show in experimental settings, and therefore it is as yet largely hypothetical due to a lack of compelling evidence. Recent evidence suggests mitotic spindle orientation and direction of asymmetric division are under the influence of specific environmental cues from the limbus rather than intrinsic polarity[110,111]. Possible environmental cues include growth factors, adhesion molecules and components of basement membrane that are specifically found in the limbus[112].
In terms of morphological criteria for LSCs, different groups have reported contradictory results. The amount of melanin granules[8,32,33], prominence of nucleoli and basal membrane invaginations[9,32,33,73] appear to vary from study to study. The reason for this contradiction is unknown but the lack of clear morphological distinction between stem cells and TACs could be responsible. As yet, TACs cannot be distinguished from true stem cells based on cellular morphology alone.
The expression of the protein markers of the LSCs either occurs in other cell types of the ocular surface, or is subject to change depending on environmental input. Cytokeratin 19, a well-established marker of limbal basal cells is also expressed in conjunctival epithelial cells[113]. ∆Np63α was identified in the corneal panni excised from patients with LSCD using western blot[114]. The free-floating spheres generated from human central corneal cells expressed ∆Np63α and ABCG2[109]. ABCG2 was found to be weakly expressed in the central cornea with what appeared to be an increasing gradient of expression towards peripheral cornea and finally the limbus[109,115].
Furthermore, the link between limbal location and stem cell indicators is further compounded as several studies have indicated that the components of the niche influence the expression of LSC markers. Espana et al[116] transplanted rabbit central corneal or limbal epithelial sheets onto either limbal or corneal stroma, and investigated the expression profile of two differentiation markers, cytokeratin 3 and connexin 43. Regardless of the type of epithelium transplanted, corneal stroma promoted expression of cytokeratin 3 while limbal stroma suppressed it. Expression of connexin 43 and apoptosis only occurred when corneal epithelium was cultured on corneal stroma. Li et al[87] showed that when human limbal epithelial cells were co-cultured with stromal stem cells, p63α was up-regulated and cytokeratin 12 down-regulated. The opposite expression pattern was observed when corneal fibroblasts were used instead of stromal stem cells. Kurpakus et al[117] showed that bovine conjunctival cells on corneal substrate expressed the differentiation marker cytokeratin 12 only when the basement membrane was left attached to the substrate, suggesting corneal basement membrane may encourage differentiation.
Since there is not one consensus marker for LSCs, a combination of functional, morphological and immunohistochemical markers is perhaps the most useful identifier for LSCs at present. To date, the “SP” property is the only marker that has been aligned with functionality. ABCG2-positive cells in the limbus exhibited proliferative capacity, label retention and clonogenicity. However, heterogeneity exists even within the limbal SP cells as suggested by the lack of intracellular complexities in 60% to 80% of limbal SP cells[47].
At the time of writing this article, a newly published study in Nature has defined a new gene, ABCB5, as a novel limbal stem cell marker[118]. The authors have shown ABCB5 positive cells were predominantly BrdU label retaining cells from the limbus and co-localised with ∆Np63α in both mice and humans. Furthermore, the authors showed that ABCB5 positive cell numbers were reduced in LSC deficient patients and that ABCB5 positive cells isolated from mouse and human corneas had the ability to rescue the cornea in LSC deficient mice in both syngeneic and xenogeneic transplant models. Finally, the paper demonstrated that ABCB5 knockout mice showed disorganised corneal epithelial organisation and reduced wound healing capabilities, although bizarrely the knockout mouse was indistinguishable from wild type littermates by physical examination and contained all anterior and posterior segment components.
This appears to be the first description of a molecular limbal marker with stem cell functionality, and may be the missing jigsaw piece required to define limbal stem cells beyond doubt.
A number of independent studies have challenged the long held belief that the limbus is the sole repository of stem cells in the corneal epithelium. These studies show that wound healing and normal corneal homeostasis can take place in the absence of limbus.
In 1994, Sandvig et al[119] showed that small lesions made in the rat central corneas did not evoke proliferative responses in the limbus, while medium-sized and large lesions did. This suggests wound healing of small lesions does not require limbal input. Our laboratory developed a “donut” excimer laser ablation model to demonstrate that human corneal epithelial regrowth occurs bi-directionally from both central and peripheral cornea[115]. In our model, the cell proliferation and migration response to wounding appeared to be as rapid from the central cornea as from the limbus, with central corneal epithelial cells fully capable of corneal epithelial regeneration. When the limbus was also ablated to remove any LSC’s, re-growth occurred from the remaining central corneal epithelium and extended right out to the limbus.
Corneal maintenance without limbal input has also been observed by several other researchers. Huang et al[97] created a rabbit LSCD model by performing 360°cornea-limbal peritomy. After six months, two thirds of the corneas were completely normal while one third showed mild vascularisation. Kawakita et al[120] blocked communication and migration between the limbus and the cornea by transplanting a stainless steel ring on rabbit peripheral corneas. In their study, the isolated central corneas remained free of epithelial defects for at least six months. In a mouse LSCD model where the limbus was cauterised, the corneas remained transparent for four months[108]. In this study, portions of athymic mice limbus were excised and replaced with limbal grafts from β-gal-ROSA26 mice whose cells were β-galactosidase labelled. After four months they observed that β-galactosidase-labelled limbal cells never migrated out of the grafts and hence made no contribution to corneal homeostasis. However, when the eyes with limbal transplants were chemically or physically wounded, the labelled cells rapidly migrated out of the graft, along with unlabelled recipient limbal cells, to create a mosaic in the resulting healed corneal epithelium.
One criticism that these studies commonly face is that their observations may be due to the result of a TAC response as the periods of observation were rather short. If stem cells do exist in the central cornea, one would expect to see long-term corneal maintenance in the animal LSCD models.
Indeed, long-term corneal maintenance in the absence of limbal input has been described in a few case reports. Some patients who had 360° LSCD were found to have normal corneas for up to 12 years[121]. Also in LSCD patients who received ex vivo expanded limbal cell transplants, donor limbal cells that only lasted for 28 wk[122] or 9 mo[123] still resulted in the long-term restoration of the central corneal epithelium. What is maintaining the central cornea in these cases? Assuming desquamation of superficial cell layer occurs constantly, there are a few possible scenarios; (1) the amount of limbal stem cells remaining is undetectable but just enough to maintain homeostasis; (2) TACs in the basal cell layer of the central epithelium have an unexpected life span and a greater than previously thought proliferative potential; or (3) a self-renewing pool of precursor cells exist in the central cornea. Two independent groups have proposed the existence of a conceptual type of cell in the central corneal epithelium which is a TAC with more stem cell-like characteristics[121,124]. Further research efforts are required to explore and clarify these possibilities although a TAC cell with more stem cell-like characteristics sounds uncommonly similar to a stem cell. Thus the question arises - is there a different type of stem cell that exists on the corneal surface that may be activated by different mechanisms, may serve different purposes and may be defined by different markers than the limbal stem cells?
A further strong argument against the existence of stem cells in the central cornea is the absence of anatomic niche structure in the central cornea to maintain stemness. However, there is evidence for survival and self-maintenance of LSCs outside of the described limbal niches.
The most frequently used substrate for limbal stem cell expansion is human amniotic membrane, the innermost wall of the placenta consisting of an epithelial monolayer, basement membrane and avascular stroma[125]. Isolated limbal cells, when cultivated on amniotic membrane, formed stratified epithelium much resembling cornea in situ and exhibited limbal stem cell phenotype such as increased expression of ∆Np63, p75, p63, ABCG2, integrin β1, Pax6, cytokeratin 3 and 19, decreased expression of connexin 43, increased resistance to phorbol ester-induced differentiation[126], label retention and clonogenicity[127]. Paulkin et al[128] analysed corneal buttons from LSCD patients who had previously received limbal cell transplants on amniotic membrane. The regenerated epithelial specimens had normal stratified structures and expressed central corneal markers cytokeratin 3 and 12 but not 19. These techniques provide evidence that limbal stem cells can survive, proliferate and expand outside of their niche which has been previously thought to be necessary for LSC maintenance.
It is not fully understood how an avascular structure like amniotic membrane can maintain the phenotype and metabolic needs of the LSCs[36,129]. The amniotic basement membrane is thought to promote adhesion, migration and differentiation of limbal epithelial cells, while amniotic stroma provide growth factors and anti-angiogenic and anti-inflammatory cytokines such as KGF, HGF, NGF, TGF-β and bFGF that prevent apoptosis and help maintain the stem cell phenotype.
Cytokine signalling is becoming increasingly recognised as a key component of a niche, regulating stem cell morphology and behaviour[130]. The Wnt/β-catenin signalling system has been shown to be responsible for preventing apoptosis of limbal cells in vitro[131]. The authors suggested that as long as survival factors are present, limbal stem cells are likely to survive outside their niche. Indeed, in a mouse model, LSCD was successfully treated with human limbal fibroblast-conditioned culture medium but not with skin fibroblast-conditioned medium, again emphasising the importance of chemical signals produced in the limbus[132].
There are studies which question the longevity of ex vivo expanded limbal epithelial cells. Li et al[66] showed progressive loss of clonogenicity and proliferative potential of limbal explant cultures on intact amniotic membrane in subsequent passages. The reason for this contradictory result is unknown but slight differences in expansion protocol and donor tissue variability might be responsible.
Furthermore, one study has proposed the existence of compound niches of cells that exist in the limbus of the mouse in unwounded corneas[133]. However, after wounding these compound niches were able to migrate onto the surface of the cornea and express corneal epithelial cytokeratins while also retaining both features of the compound niche and features of goblet cells. This study serves to illustrate that a niche may not be an immovable structure to which cells attach but may be inherent to the cellular components and therefore able to migrate with those components.
Epithelia of skin, gut wall and cornea are outer most coverings of our body and share the same developmental origin. In all types of epithelia, with the exception of cornea, desquamated cells are replaced with newly generated cells from stem cells located in the basal layer[8]. Only corneal epithelium is thought to be renewed from a distant repository of stem cells. This is somewhat peculiar in evolutionary sense especially when the directly adjacent conjunctiva is maintained in the same way as any other epithelia[134].
In fetal eyes, adult LSC markers are found in the basal layer across the cornea[135,136] and it is unknown how the markers become segregated in the limbus during development. Investigation of limbal organogenesis has raised a possibility that the limbal papillary structures are mere developmental remnants. The limbus does not develop until eyelids open and the ocular surface is exposed to amniotic fluid[135,136]. The papillary structures of the limbus do not form until post-natal life[137]. The question remains as to why a microenvironment essential for the support of stem cell maintenance only appears after birth and why stem cells can be maintained on the central cornea prior to birth.
A strong body of evidence has accumulated over the past few decades, showing that markers of stemness are exclusively localised at the limbus. Furthermore the centripetal migration of corneal epithelial cells after generation at the limbus has been definitively shown. Therefore, the limbus has been designated as the single repository of stem cells of the corneal epithelium. However, there is mounting evidence showing that the expression of the stem cell markers are largely determined by extrinsic signals provided by the regional microenvironment[130,138], and the markers themselves do not indicate intrinsic stemness. As shown by the clinical success of LSC transplant on amniotic membrane in LSCD, a niche structure is not an absolute requirement for the survival of ocular stem cells, as long as the right survival signals are provided. The existence of the limbus as the sole repository of corneal epithelial stem cells also does not explain a number of clinical observations which have demonstrated corneal wound healing without limbal input and also does not explain the developmental origin of the limbus.
A vast majority of studies consider central cornea as a lineage-committed, post-mitotic tissue, but some groups have independently suggested a possibility that stem cells exist outside the limbus. Until more definitive data becomes available, the possibility of the existence of progenitor cells outside the limbus should not be excluded as central cornea may provide a new source of stem cells that can serve as a sustainable repository of high quality, evaluated, optimised tissue for the treatment of corneal degenerative disorders.
We would like to thank current and previous members of the laboratory for their input into this manuscript.
| 1. | Sharma A, Coles WH. Kinetics of corneal epithelial maintenance and graft loss. A population balance model. Invest Ophthalmol Vis Sci. 1989;30:1962-1971. [PubMed] |
| 2. | Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442-1443. [PubMed] |
| 3. | Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41:275-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 359] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Buschke W, Friedenwald JS, Fleischmann W. Studies on the mitotic activity of the corneal epithelium; methods; the effects of colchicine, ether, cocaine and ephedrin. Bull Johns Hopkins Hosp. 1943;73:143-167. |
| 5. | Hanna C, O’Brien JE. Cell production and migration in the epithelial layer of the cornea. Arch Ophthalmol. 1960;64:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Mann I. A study of epithelial regeneration in the living eye. Br J Ophthalmol. 1944;28:26-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 405] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 990] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 9. | Zhao J, Mo V, Nagasaki T. Distribution of label-retaining cells in the limbal epithelium of a mouse eye. J Histochem Cytochem. 2009;57:177-185. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Pajoohesh-Ganji A, Pal-Ghosh S, Simmens SJ, Stepp MA. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells. 2006;24:1075-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Arpitha P, Prajna NV, Srinivasan M, Muthukkaruppan V. A subset of human limbal epithelial cells with greater nucleus-to-cytoplasm ratio expressing high levels of p63 possesses slow-cycling property. Cornea. 2008;27:1164-1170. [PubMed] |
| 12. | Figueira EC, Di Girolamo N, Coroneo MT, Wakefield D. The phenotype of limbal epithelial stem cells. Invest Ophthalmol Vis Sci. 2007;48:144-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111:2867-2875. [PubMed] |
| 14. | Tseng SC, Zhang SH. Limbal epithelium is more resistant to 5-fluorouracil toxicity than corneal epithelium. Cornea. 1995;14:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Joyce NC, Meklir B, Joyce SJ, Zieske JD. Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci. 1996;37:645-655. [PubMed] |
| 16. | Waring GO, Roth AM, Ekins MB. Clinical and pathologic description of 17 cases of corneal intraepithelial neoplasia. Am J Ophthalmol. 1984;97:547-559. [PubMed] |
| 17. | Kruse FE, Tseng SC. A tumor promoter-resistant subpopulation of progenitor cells is larger in limbal epithelium than in corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34:2501-2511. [PubMed] |
| 18. | Lavker RM, Wei ZG, Sun TT. Phorbol ester preferentially stimulates mouse fornical conjunctival and limbal epithelial cells to proliferate in vivo. Invest Ophthalmol Vis Sci. 1998;39:301-307. [PubMed] |
| 19. | Miller SJ, Lavker RM, Sun TT. Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. Biochim Biophys Acta. 2005;1756:25-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 754] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 21. | Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769-782. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 514] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Raji B, Dansault A, Leemput J, de la Houssaye G, Vieira V, Kobetz A, Arbogast L, Masson C, Menasche M, Abitbol M. The RNA-binding protein Musashi-1 is produced in the developing and adult mouse eye. Mol Vis. 2007;13:1412-1427. [PubMed] |
| 23. | Thomas PB, Liu Y-H, Zhuang FF, Selvam S, Song SW, Smith RE, Trousdale MD, Yiu SC. Identification of notch-1 expression in the limbal basal epithelium. Mol Vis. 2007;13:337-344. |
| 24. | Di Girolamo N, Sarris M, Chui J, Cheema H, Coroneo MT, Wakefield D. Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J Cell Mol Med. 2008;12:2799-2811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, De Luca M. C/ebpδ regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol. 2007;177:1037-1049. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of deltanp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523-9528. [RCA] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Lindberg K, Brown ME, Chaves HV, Kenyon KR, Rheinwald JG. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993;34:2672-2679. [PubMed] |
| 28. | Ebato B, Friend J, Thoft RA. Comparison of central and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1987;28:1450-1456. [PubMed] |
| 29. | Ebato B, Friend J, Thoft RA. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1988;29:1533-1537. [PubMed] |
| 30. | Wei ZG, Sun TT, Lavker RM. Rabbit conjunctival and corneal epithelial cells belong to two separate lineages. Invest Ophthalmol Vis Sci. 1996;37:523-533. [PubMed] |
| 31. | German MJ, Pollock HM, Zhao B, Tobin MJ, Hammiche A, Bentley A, Cooper LJ, Martin FL, Fullwood NJ. Characterization of putative stem cell populations in the cornea using synchrotron infrared microspectroscopy. Invest Ophthalmol Vis Sci. 2006;47:2417-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355-366. [RCA] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Schlötzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 336] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, Tseng SC. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci. 2003;44:5125-5129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Chee KY, Kicic A, Wiffen SJ. Limbal stem cells: the search for a marker. Clin Experiment Ophthalmol. 2006;34:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Takács L, Tóth E, Berta A, Vereb G. Stem cells of the adult cornea: from cytometric markers to therapeutic applications. Cytometry A. 2009;75:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Mort R, Douvaras P, Morley S, Dorà N, Hill R, Collinson J, West J. Stem cells and corneal epithelial maintenance – insights from the mouse and other animal models. Results Probl Cell Differ. 2012;55:357-394. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2213] [Cited by in RCA: 2128] [Article Influence: 70.9] [Reference Citation Analysis (8)] |
| 40. | Murayama A, Matsuzaki Y, Kawaguchi A, Shimazaki T, Okano H. Flow cytometric analysis of neural stem cells in the developing and adult mouse brain. J Neurosci Res. 2002;69:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 1720] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 42. | Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97-104. [PubMed] |
| 43. | Kubota M, Shimmura S, Miyashita H, Kawashima M, Kawakita T, Tsubota K. The anti-oxidative role of ABCG2 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:5617-5622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Park KS, Lim CH, Min BM, Lee JL, Chung HY, Joo CK, Park CW, Son Y. The side population cells in the rabbit limbus sensitively increased in response to the central cornea wounding. Invest Ophthalmol Vis Sci. 2006;47:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | de Paiva CS, Pflugfelder SC, Li DQ. Cell size correlates with phenotype and proliferative capacity in human corneal epithelial cells. Stem Cells. 2006;24:368-375. [RCA] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li D-Q. Abcg2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 47. | Wolosin JM, Budak MT, Akinci MA. Ocular surface epithelial and stem cell development. Int J Dev Biol. 2004;48:981-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Barrandon Y, Green H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci USA. 1985;82:5390-5394. [RCA] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 245] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Ho PC, Elliott JH. Kinetics of corneal epithelial regeneration. II. Epidermal growth factor and topical corticosteroids. Invest Ophthalmol. 1975;14:630-633. [PubMed] |
| 50. | Pfister RR, Burstein N. The alkali burned cornea I. Epithelial and stromal repair. Exp Eye Res. 1976;23:519-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Buck RC. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985;26:1296-1299. [PubMed] |
| 52. | Buck RC. Cell migration in repair of mouse corneal epithelium. Invest Ophthalmol Vis Sci. 1979;18:767-784. [PubMed] |
| 53. | Kinoshita S, Friend J, Thoft RA. Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1981;21:434-441. [PubMed] |
| 54. | Lagali N, Stenevi U, Claesson M, Fagerholm P, Hanson C, Weijdegård B. Survival of donor-derived cells in human corneal transplants. Invest Ophthalmol Vis Sci. 2009;50:2673-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Collinson JM, Chanas SA, Hill RE, West JD. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6(+/-) mouse. Invest Ophthalmol Vis Sci. 2004;45:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Nagasaki T, Zhao J. Centripetal movement of corneal epithelial cells in the normal adult mouse. Invest Ophthalmol Vis Sci. 2003;44:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Matsuda M, Ubels JL, Edelhauser HF. A larger corneal epithelial wound closes at a faster rate. Invest Ophthalmol Vis Sci. 1985;26:897-900. [PubMed] |
| 58. | Srinivasan BD, Eakins KE. The reepithelialization of rabbit cornea following single and multiple denudation. Exp Eye Res. 1979;29:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001;226:653-664. [PubMed] |
| 60. | Cheng CC, Wang DY, Kao MH, Chen JK. The growth-promoting effect of kgf on limbal epithelial cells is mediated by upregulation of deltanp63alpha through the p38 pathway. J Cell Sci. 2009;122:4473-4480. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Nishida T, Nakamura M, Mishima H, Otori T. Interleukin 6 promotes epithelial migration by a fibronectin-dependent mechanism. J Cell Physiol. 1992;153:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Kimura K, Hattori A, Usui Y, Kitazawa K, Naganuma M, Kawamoto K, Teranishi S, Nomizu M, Nishida T. Stimulation of corneal epithelial migration by a synthetic peptide (PHSRN) corresponding to the second cell-binding site of fibronectin. Invest Ophthalmol Vis Sci. 2007;48:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Nishida T, Nakamura M, Mishima H, Otori T. Hyaluronan stimulates corneal epithelial migration. Exp Eye Res. 1991;53:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 136] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Di Girolamo N, Bobba S, Raviraj V, Delic NC, Slapetova I, Nicovich PR, Halliday GM, Wakefield D, Whan R, Lyons GJ. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2014;Jun 25; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 65. | Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol. 2005;167:381-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Li W, Hayashida Y, He H, Kuo CL, Tseng SC. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Zhao X, Das AV, Bhattacharya S, Thoreson WB, Sierra JR, Mallya KB, Ahmad I. Derivation of neurons with functional properties from adult limbal epithelium: implications in autologous cell therapy for photoreceptor degeneration. Stem Cells. 2008;26:939-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Zhao X, Das AV, Thoreson WB, James J, Wattnem TE, Rodriguez-Sierra J, Ahmad I. Adult corneal limbal epithelium: a model for studying neural potential of non-neural stem cells/progenitors. Dev Biol. 2002;250:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Seigel GM, Sun W, Salvi R, Campbell LM, Sullivan S, Reidy JJ. Human corneal stem cells display functional neuronal properties. Mol Vis. 2003;9:159-163. [PubMed] |
| 70. | Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7-25. [PubMed] |
| 71. | Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1230] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 72. | Higa K, Shimmura S, Miyashita H, Shimazaki J, Tsubota K. Melanocytes in the corneal limbus interact with K19-positive basal epithelial cells. Exp Eye Res. 2005;81:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Gipson IK. The epithelial basement membrane zone of the limbus. Eye (Lond). 1989;3:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Wilson SE, Hong JW. Bowman’s layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea. 2000;19:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Tuori A, Uusitalo H, Burgeson RE, Terttunen J, Virtanen I. The immunohistochemical composition of the human corneal basement membrane. Cornea. 1996;15:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Cleutjens JP, Havenith MG, Kasper M, Vallinga M, Bosman FT. Absence of type IV collagen in the centre of the corneal epithelial basement membrane. Histochem J. 1990;22:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Schlötzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, Kruse FE. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Wiley L, SundarRaj N, Sun TT, Thoft RA. Regional heterogeneity in human corneal and limbal epithelia: an immunohistochemical evaluation. Invest Ophthalmol Vis Sci. 1991;32:594-602. [PubMed] |
| 79. | Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461-473. [PubMed] |
| 80. | Kolega J, Manabe M, Sun TT. Basement membrane heterogeneity and variation in corneal epithelial differentiation. Differentiation. 1989;42:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Townsend WM. The limbal palisades of vogt. Trans Am Ophthalmol Soc. 1991;89:721-756. |
| 82. | Goldberg MF, Bron AJ. Limbal palisades of vogt. Trans Am Ophthalmol Soc. 1982;80:155-171. |
| 83. | Shanmuganathan VA, Foster T, Kulkarni BB, Hopkinson A, Gray T, Powe DG, Lowe J, Dua HS. Morphological characteristics of the limbal epithelial crypt. Br J Ophthalmol. 2007;91:514-519. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 84. | Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529-532. [RCA] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 85. | Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, Daniels JT. Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 86. | Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53:5109-5116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 87. | Li G, Zhu Y, Xie H, Chen SY, Tseng SCG. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53:5686-5697. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 88. | Zieske JD. Perpetuation of stem cells in the eye. Eye (Lond). 1994;8:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Pinnamaneni N, Funderburgh JL. Concise review: Stem cells in the corneal stroma. Stem Cells. 2012;30:1059-1063. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 90. | Ordonez P, Di Girolamo N. Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells. 2012;30:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 91. | Bray LJ, Heazlewood CF, Atkinson K, Hutmacher DW, Harkin DG. Evaluation of methods for cultivating limbal mesenchymal stromal cells. Cytotherapy. 2012;14:936-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Xie HT, Chen SY, Li GG, Tseng SC. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011;29:1874-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 93. | Menzel-Severing J. Emerging techniques to treat limbal epithelial stem cell deficiency. Discov Med. 2011;11:57-64. [PubMed] |
| 94. | Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48:83-92. [PubMed] |
| 95. | Chen JJ, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990;31:1301-1314. [PubMed] |
| 96. | Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 356] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 97. | Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:96-105. [PubMed] |
| 98. | Chen JJ, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2219-2233. [PubMed] |
| 99. | Shortt AJ, Secker GA, Notara MD, Limb GA, Khaw PT, Tuft SJ, Daniels JT. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52:483-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 100. | Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 848] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 101. | De Luca M, Pellegrini G, Green H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regen Med. 2006;1:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 102. | Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 966] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 103. | Baylis O, Figueiredo F, Henein C, Lako M, Ahmad S. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 104. | Gomes JA, Eagle RC, Gomes AK, Rapuano CJ, Cohen EJ, Laibson PR. Recurrent keratopathy after penetrating keratoplasty for aniridia. Cornea. 1996;15:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Di Girolamo N. Stem cells of the human cornea. Br Med Bull. 2011;100:191-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Braun KM, Watt FM. Epidermal label-retaining cells: background and recent applications. J Investig Dermatol Symp Proc. 2004;9:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 108. | Majo F, Rochat A, Nicolas M, Jaoudé GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 109. | Chang CY, McGhee JJ, Green CR, Sherwin T. Comparison of stem cell properties in cell populations isolated from human central and limbal corneal epithelium. Cornea. 2011;30:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 110. | Castro-Muñozledo F, Gómez-Flores E. Challenges to the study of asymmetric cell division in corneal and limbal epithelia. Exp Eye Res. 2011;92:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Zhang J, Li L. Stem cell niche: microenvironment and beyond. J Biol Chem. 2008;283:9499-9503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 112. | Castro-Muñozledo F. Review: Corneal epithelial stem cells, their niche and wound healing. Mol Vis. 2013;19:1600-1613. |
| 113. | Ang LP, Tan DT, Phan TT, Li J, Beuerman R, Lavker RM. The in vitro and in vivo proliferative capacity of serum-free cultivated human conjunctival epithelial cells. Curr Eye Res. 2004;28:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 114. | Espana EM, Di Pascuale MA, He H, Kawakita T, Raju VK, Liu CY, Tseng SC. Characterization of corneal pannus removed from patients with total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2004;45:2961-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Chang CY, Green CR, McGhee CN, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;49:5279-5286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 116. | Espana EM, Kawakita T, Romano A, Di Pascuale M, Smiddy R, Liu CY, Tseng SC. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130-5135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 117. | Kurpakus MA, Stock EL, Jones JC. The role of the basement membrane in differential expression of keratin proteins in epithelial cells. Dev Biol. 1992;150:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 119. | Sandvig KU, Haaskjold E, Bjerknes R, Refsum SB, Kravik K. Cell kinetics of conjunctival and corneal epithelium during regeneration of different-sized corneal epithelial defects. Acta Ophthalmol (Copenh). 1994;72:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 120. | Kawakita T, Higa K, Shimmura S, Tomita M, Tsubota K, Shimazaki J. Fate of corneal epithelial cells separated from limbus in vivo. Invest Ophthalmol Vis Sci. 2011;52:8132-8137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Dua HS, Miri A, Alomar T, Yeung AM, Said DG. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 122. | Sharpe JR, Daya SM, Dimitriadi M, Martin R, James SE. Survival of cultured allogeneic limbal epithelial cells following corneal repair. Tissue Eng. 2007;13:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Daya SM, Watson A, Sharpe JR, Giledi O, Rowe A, Martin R, James SE. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology. 2005;112:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 124. | Lauweryns B, van den Oord JJ, Missotten L. The transitional zone between limbus and peripheral cornea. An immunohistochemical study. Invest Ophthalmol Vis Sci. 1993;34:1991-1999. [PubMed] |
| 125. | Ahmad S, Kolli S, Lako M, Figueiredo F, Daniels JT. Stem cell therapies for ocular surface disease. Drug Discov Today. 2010;15:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 126. | Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Expression of Delta Np63 in response to phorbol ester in human limbal epithelial cells expanded on intact human amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:2959-2965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 127. | Mariappan I, Maddileti S, Savy S, Tiwari S, Gaddipati S, Fatima A, Sangwan VS, Balasubramanian D, Vemuganti GK. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5:1470-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 128. | Pauklin M, Steuhl KP, Meller D. Characterization of the corneal surface in limbal stem cell deficiency and after transplantation of cultivated limbal epithelium. Ophthalmology. 2009;116:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 130. | Davies EL, Fuller MT. Regulation of self-renewal and differentiation in adult stem cell lineages: lessons from the Drosophila male germ line. Cold Spring Harb Symp Quant Biol. 2008;73:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 131. | Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX. Wnt/b-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52:4734-4741. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 132. | Amirjamshidi H, Milani BY, Sagha HM, Movahedan A, Shafiq MA, Lavker RM, Yue BYT, Djalilian AR. Limbal fibroblast conditioned media: A non-invasive treatment for limbal stem cell deficiency. Mol Vis. 2011;17:658-666. |
| 133. | Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. Corneal goblet cells and their niche: Implications for corneal stem cell deficiency. Stem Cells. 2012;30:2032-2043. |
| 134. | Barrandon Y. Crossing boundaries: stem cells, holoclones, and the fundamentals of squamous epithelial renewal. Cornea. 2007;26:S10-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 135. | Davies SB, Chui J, Madigan MC, Provis JM, Wakefield D, Di Girolamo N. Stem cell activity in the developing human cornea. Stem Cells. 2009;27:2781-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 136. | Davies SB, Di Girolamo N. Corneal stem cells and their origins: significance in developmental biology. Stem Cells Dev. 2010;19:1651-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Rodrigues M, Ben-Zvi A, Krachmer J, Schermer A, Sun TT. Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation. 1987;34:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 138. | Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 677] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
P- Reviewer: Casaroli-Marano RP, Holan V, Jhanji V, Marfe G S- Editor: Song XX L- Editor: A E- Editor: Lu YJ