Published online Apr 26, 2014. doi: 10.4252/wjsc.v6.i2.230
Revised: January 25, 2014
Accepted: April 11, 2014
Published online: April 26, 2014
Processing time: 117 Days and 7 Hours
Glioblastoma Multiforme (GBM) is a grade IV astrocytoma, with a median survival of 14.6 mo. Within GBM, stem-like cells, namely glioblastoma stem cells (GSCs), have the ability to self-renew, differentiate into distinct lineages within the tumor and initiate tumor xenografts in immunocompromised animal models. More importantly, GSCs utilize cell-autonomous and tumor microenvironment-mediated mechanisms to overcome current therapeutic approaches. They are, therefore, very important therapeutic targets. Although the functional criteria defining GSCs are well defined, their molecular characteristics, the mechanisms whereby they establish the cellular hierarchy within tumors, and their contribution to tumor heterogeneity are not well understood. This review is aimed at summarizing current findings about GSCs and their therapeutic importance from a molecular and cellular point of view. A better characterization of GSCs is crucial for designing effective GSC-targeted therapies.
Core tip: Stem-like cells in glioblastoma, a malignant brain tumor, have increased tumorigenic capacity, generate tumor lineages and exhibit marked resistance to current therapies. A better understanding of these stem-like cells is necessary for designing new effective treatments. This review discusses the molecular characteristics of these cells and their therapeutic importance.
- Citation: Bayin NS, Modrek AS, Placantonakis DG. Glioblastoma stem cells: Molecular characteristics and therapeutic implications. World J Stem Cells 2014; 6(2): 230-238
- URL: https://www.wjgnet.com/1948-0210/full/v6/i2/230.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i2.230
Glioblastoma Multiforme (GBM), classified by World Health Organization (WHO) as grade IV astrocytoma, is a deadly primary brain malignancy with more than 10000 new cases in the United States annually (http://www.cbtrus.org). Despite the aggressive treatment options involving surgery and concomitant chemoradiotherapy, median survival is 14.6 mo[1]. The fact that survival has improved by only a few months over the past 50 years highlights the need for a better understanding of the disease and the design of informed therapies[2].
GBM is a highly heterogeneous tumor with distinctive histologic hallmarks including high cell density, intratumoral necrosis, vascular hyperplasia and invasion through brain parenchyma[3]. This heterogeneity is also displayed at the microscopic level, where a cellular hierarchy is dominated by the presence of stem-like cells, namely glioblastoma stem cells or GSCs[4]. In this review we will discuss the molecular and phenotypic characteristics of GSCs and their therapeutic implications.
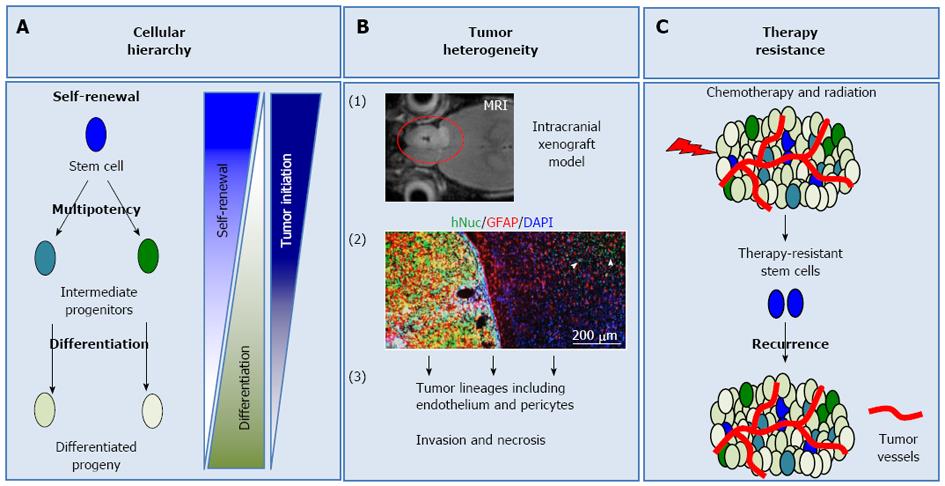
Within multi-cellular systems, cells specialize to undertake different responsibilities, in order to maintain homeostasis. As a consequence of this specialization, every cell is not equal in its self-renewal and differentiation ability. Some cells are more stem-like, meaning that they can self-renew and give rise to different progeny through more restricted intermediate progenitors (Figure 1A)[5]. The extent of self-renewal is dictated by the developmental stage that cells are in and varies from tissue to tissue. For example, in tissues such as the gastrointestinal tract or hematopoietic system, where cellular turnover is high, adult stem cells self-renew more often, compared to more quiescent tissues such as the brain[6,7]. On the other hand, as cells differentiate, their self-renewal ability decreases and they adopt properties related to their tissue (Figure 1A)[8]. The differences in differentiation potential define a cellular hierarchy within these systems, where stem cells represent the top of this hierarchy. Lineage restriction and differentiation during physiological processes are mostly believed to be irreversible. However, pathologic conditions or experimental manipulations can cause de-differentiation[4,9]. Therefore, it is important to understand how cellular hierarchy is established and maintained in tumors in order to understand tumor biology.
Guided from research in liquid tumors, the idea of cancer cells with stem-like properties has revolutionized the field of cancer biology[10,11]. Although initially thought to be controversial, cancer stem cells (CSCs) are a proven concept for many liquid and solid tumors, including GBM.
In liquid tumors, cellular hierarchy is very well defined by the expression of surface markers. These hierarchically distinct populations were easily isolated by Fluorescence-Assisted Cell Sorting (FACS) via the expression of surface markers and their tumor formation ability was assessed in vivo[10]. These surface markers were then investigated in many solid tumors and some of them are still among the best-studied CSC markers.
Glioblastoma cells need to fulfill specific criteria to be classified as GSCs. In particular, they should be able to: (1) self-renew (Figure 1A); (2) differentiate into distinct lineages, a property termed multipotency (Figure 1A); and (3) initiate tumors in animal models, which recapitulate the original disease phenotype and heterogeneity (Figure 1A and B)[12,13]. Self-renewal is assessed with in vitro tumorsphere formation assay, a system borrowed form neural stem cell culture. In this assay, single cells are plated in suspension and their sphere formation ability is evaluated over serial passaging, which is an indicator of long-term self-renewal[14]. In vivo self-renewal is assayed by serial xenograft tumor formation experiments[11-13] (Figure 1B). The differentiation potential of GSCs is assessed via analysis of tumor-derived lineages in vitro and in vivo[15-17].
Evidence for GSCs first came from Dirks and colleagues, who isolated cells from human GBM samples based on expression of the cell surface glycoprotein CD133 (Prominin1/PROM1)[12,13]. They showed that these cells initiated orthotopic tumor xenografts in immunodeficient mice more efficiently than cells that did not express CD133.
Although the functional criteria defining GSCs are completely defined, the molecular characteristics of these cells are not understood. As expected by the heterogeneous histology of GBM, there is extensive cellular heterogeneity within GBM cells, and GSCs as well. The complex interplay of signaling pathways and lack of universal molecular markers identifying GSCs further complicate the study of these cells. More importantly, GSCs are resistant to chemoradiotherapeutic approaches and are, therefore, believed to cause tumor recurrence[18-20]. Thus, it is of major importance to understand the biology of these cells and their contribution to tumorigenesis, in order to overcome the problems current therapeutic approaches encounter. This review will focus on GSC markers, their molecular signatures and the signaling pathways important for their biology. Finally, we will discuss the therapeutic importance of these cells.
CD133, a pentaspan transmembrane protein of unknown function, is one of the best-studied GSC markers to date. CD133 expression has been observed during embryonic development, as well as in adult neural stem cells and ependymal cells. However, CD133 knockout mice only have a mild retinal phenotype[21-23]. When isolated and injected into immunodeficient animals, CD133+ GBM cells are more tumorigenic than CD133- cells and produce xenograft tumors that phenocopy the original patient tumor[13]. Furthermore, knockdown of CD133 with shRNA impairs GSC self-renewal[24]. However, the facts that CD133- cells can also generate tumors and that some tumors do not have a CD133+ population suggest that CD133 is not a universal GSC marker[25-31].
GSCs were also expected to share common markers with neural stem cells, their normal counterparts, based on the concept of stem cells sharing common signaling pathways. With this rationale, expression of neural stem cell markers was analyzed in GBM tumors. GSCs were shown to have increased expression of Nestin, an intermediate filament expressed in neural stem cells in neurogenic niches[18,32,33]. Besides Nestin, GSCs are enriched for Sox2, a transcription factor associated with multipotency and pluripotency[34,35].
Comparative gene expression analysis led to identification of more GSC markers, including Oct4, SSEA-1/CD15, Bmi-1, Musashi-1, Nanog, integrin-α6, L1CAM, A2B5 and ABC-type transporters, whose expression defines the side population (SP) on flow cytometric analysis, through the ability to extrude Hoechst dye[25,35-40]. Interestingly, some of these markers are expressed in embryonic stem cells, suggesting GSC overlap not only with NSCs but also with less differentiated stem cells as well. However, none of these markers are universal. Furthermore, the intracellular localization of some of these markers makes them less desirable candidates for selective therapeutic targeting.
In addition to oncogenic pathways globally important to tumor biology, signaling pathways that are important for maintenance of self-renewal and regulation of differentiation receive attention in cancer stem cell biology (Table 1). In the context of GSCs, pathways known to regulate neural development are of major interest. Various signaling pathways influence GSC biology by either maintaining self-renewal or regulating differentiation. However, certain pathways can regulate either self-renewal or differentiation in the appropriate context (Table 1).
| Signaling pathway | Function | Ref. |
| Self-renewal | ||
| Notch Signaling | Maintenance of GSCs | [50-57] |
| Tumorsphere formation | ||
| Tumorigenesis | ||
| Asymmetric division | ||
| TGF-β Signaling | Regulation of self-renewal | [34,58] |
| Maintenance of perivascular GSCs | ||
| Sonic Hedgehog Signaling | Promotion of self-renewal and migration | [56,61-66] |
| Upregulation of stem cell associated genes | ||
| Tumorigenesis | ||
| Wnt/β-catenin Signaling | Self-renewal and maintenance of GSCs | [15,66-71] |
| Tumorigenesis | ||
| Associated with bad prognosis | ||
| PI3K/Akt Signaling | Promotion of GSC self-renewal in vitro | [41-44] |
| Proliferation and survival of GSCs | ||
| Tumorigenesis | ||
| MAPK Signaling | Proliferation and survival of GSCs | [41] |
| Differentiation | ||
| BMP Signaling | Inhibition of asymmetric division | [72-74] |
| Differentiation and proliferation block | ||
| Notch Signaling | Trans-differentiation to tumor-derived endothelium | [16] |
| TGF-β Signaling | Trans-differentiation to vascular pericytes | [17] |
Studies of pathways involved in GSC self-renewal gained momentum when Fine and colleagues started culturing tumor cells in serum-free conditions[41]. By using the mitogens epidermal growth factor (EGF) and fibroblast growth factor (FGF), they limited differentiation and promoted GSC self-renewal. These mitogens act through their receptor tyrosine kinases (RTKs) and induce activation of downstream pathways such as the Phosphoinositide 3-kinase/Akt (PI3K/Akt) and Mitogen-Activated Protein Kinase (MAPK), to induce proliferation, survival and tumorigenicity[41,42]. Furthermore, blocking the PI3K/Akt pathway has been shown to impair GSC self-renewal and tumorigenicity. Finally, knockdown of CD133 in GSCs causes downregulation of Akt phosphorylation, further highlighting the role of the PI3K/Akt pathway in GSC biology[43,44].
Originally identified in genetic screens in Drosophila as a master regulator of neurogenesis, Notch signaling plays diverse roles in nervous system development, including maintenance of self-renewal and regulation of fate decisions in neural and glial lineages[45-47]. Upon binding to its ligands (Delta-like and Jagged), heterodimeric Notch receptors (Notch1-4) get cleaved by γ-secretase in the cytoplasm, releasing the Notch intracellular domain (NICD). NICD translocates into the nucleus where it acts as co-activator for transcription of the Hes and Hey families of genes[48]. These genes are transcriptional repressors of neurogenic genes, thereby causing maintenance of stemness in activated cells[49]. In GBM, Notch signaling is involved in several distinct processes in tumorigenesis, by regulating both self-renewal and differentiation of GSCs[16,50,53]. Blockage of Notch signaling with γ-secretase inhibitors inhibits self-renewal, as assayed by tumorsphere forming ability, and causes depletion of the CD133+ GSC population[54-56]. Furthermore, Numb, which prevents NICD from travelling to the nucleus and thus inhibits downstream signaling upon Notch activation, was shown to be asymmetrically distributed within GSCs and to promote asymmetric division. Asymmetric division of GSCs gives rise to two distinct daughter cells: a stem cell (GSC); and a more restricted and differentiated cell[57]. These findings support a role for Notch signaling in the maintenance of GBM’s stem cell compartment.
Inhibitors of Notch pathway components represent promising therapeutic candidates in GBM. However, the overlapping roles with normal neural and other adult stem cell maintenance raises the question of toxicity. Of note, there are ongoing phase II trials with Notch inhibitors in GBM patients (http://www.clinicaltrials.gov).
Transforming growth factor-β (TGF-β) signaling promotes GSC self-renewal through regulation of distinct mechanisms. First, it was shown to act through SRY-Related HMG-Box transcription factors Sox2 and Sox4, factors important for GSC biology, to induce self-renewal[34]. Second, blockage of TGF-β signaling decreases perivascular CD44high/Id1high GSCs, via repression of inhibitors of DNA-binding proteins Id1 and Id3[58].
Sonic Hedgehog (Shh-Gli) signaling, which is highly important for brain and spinal cord patterning during embryonic development, also plays crucial functions in GSC maintenance[59,60]. It has been shown to promote GSC self-renewal and expression of stem cell genes, whereas its blockage leads to apoptosis, delay in tumorigenesis and inhibition of GSC self-renewal and migration[56,61-66].
The Wnt/β-catenin pathway induces proliferation of progenitor cells within gliomas[15,67]. Some reports suggest that Wnt signaling is important for GSC self-renewal. Overexpression of Wnt ligands, Wnt3a and Wnt1, is observed in GSCs[67]. Other Wnt pathway components were shown to promote GSC self-renewal and tumorigenicity. Some of pathway’s downstream effectors such as β-catenin, Lgr5, Dishevelled 2 and Frizzled 4 are associated with negative prognosis[66,68-70]. FoxM1, which promotes nuclear localization of β-catenin, was also shown to be critical for GSC maintenance and tumorigenesis[71].
Bone morphogenic protein (BMP), a member of TGF-β superfamily, functions as a differentiation signal within GBM, as opposed to the previously discussed roles of other members of the TGF-β family in maintenance of self-renewal[34,72]. The difference between BMP and TGF-β’s effects on GSC biology can be ascribed to distinct signaling cascades, even though they belong to the same superfamily of ligands. Also important for astrocytic differentiation in development, BMP4 treatment inhibits asymmetric division of GSCs, thereby blocking their self-renewal and depleting the stem cell compartment of the tumor[73,74]. Treatment with BMP4 leads to differentiation and proliferation block. However, a subset of GSCs manages to escape this differentiation cue via epigenetic silencing of BMP receptor 1B (BMPR1B)[74].
Although highly important for self-renewal, reports also suggest that Notch signaling is important for trans-differentiation of GSCs into tumor-derived endothelium[16]. Similarly, TGF-β was shown to induce GSC differentiation into vascular pericytes, supporting vessel formation and leading to further tumor growth[17].
An additional level of complexity in GSC biology is exhibited by regulatory non-coding RNAs, which are fine tuners of gene expression. Among them, microRNAs (miRNAs) have the ability to modify gene expression levels by specifically binding mostly to the 3’-UTRs of genes and causing their degradation through the RNAi machinery[75]. Besides being highly important for regulation of pluripotency and reprogramming, miRNAs play important roles in GBM tumorigenesis and GSC biology. Similar to other molecular markers enriched in GSCs, miRNAs regulating neural stem cell biology are also of main interest in GSC biology. miRNAs upregulated in GBM and particularly in GSCs have anti-apoptotic, anti-differentiation, pro-proliferative and pro-invasion properties[40,76,77]. On the other hand, miRNAs promoting differentiation were shown to be downregulated in GBM, including miR-124, which is important for neural differentiation[78-81].
To better understand the interplay of different signaling pathways mentioned above and how they regulate GSC biology, we need to study the niches in which GSCs reside. Besides providing crucial signals for GSC maintenance, stem cell niches and the tumor microenvironment are critical factors in the response to therapy.
Endothelial cells provide signals required for self-renewal of neural stem cells and many other adult stem cell populations[82]. Similar to their normal counterparts, GSCs reside in a perivascular niche, where they maintain close contact with CD34+ endothelial cells[83-85]. This close contact facilitates presentation of Notch ligands on the surface of endothelial cells. These ligands activate Notch signaling in GSCs, thereby promoting self-renewal[85].
The perivascular niche is also subject to bidirectional cues coming from GSCs. CD133+ GSCs express higher levels of vascular endothelial growth factor (VEGF), leading to angiogenesis and increased vascularity of the tumor, when compared to their CD133- counterparts[86].
New evidence for trans-differentiation of GSCs into endothelial cells and pericytes further suggests that GSCs play a central role in maintaining the tumor microenvironment and their own niches, when presented with appropriate signaling cues[16,17].
As mentioned earlier, GBM is characterized not only by extensive vascular hyperplasia but also pronounced intratumoral necrosis. One of the main histologic hallmarks of GBM is a phenomenon called pseudopalisading necrosis (PPN), where densely packed tumor cells surround a necrotic area[87]. Although the etiology and biological significance of these areas are not well understood, they are believed to be regions of active tumor growth and neo-vascularization. Considering the importance of hypoxia in promoting self-renewal in embryonic stem cells and NSCs, pseudopalisades represent plausible niches for GSCs[88,89]. This hypothesis is further supported by studies showing immunoreactivity for CD133 in pseudopalisades[90]. Furthermore, hypoxia leads to activation of angiogenesis and neo-vascularization through the upregulation of VEGF in GSCs[91,92]. Some evidence also suggests that hypoxia reprograms CD133- GSCs to become CD133+ and induces Notch signaling, whose importance for GSC biology was mentioned above[88,89].
Keeping these findings in mind, the possibility of a necrotic niche for GSCs is biologically intriguing and represents a therapeutic challenge for systemic drug delivery methods, since these areas are devoid of blood vessels.
The most malignant feature of GBM is its invasion of brain parenchyma. GBM cells infiltrate normal brain tissue and can be found centimeters away from the tumor core[93]. The vast majority of recurrence after surgery and chemoradiotherapy occurs within 2 cm of the resection cavity suggesting that these invading cells also have tumorigenic capacity[94-96].
Expression of C-X-C chemokine receptor type 4 (CXCR4) and its ligand, stromal derived factor 1α (SDF-1α), which are important regulators of invasion of GBM cells, is enriched in GSCs[91]. This signaling pathway also mediates recruitment of GSCs towards endothelium, causing further invasion, differentiation and endothelial cell proliferation via VEGF expression[92].
Standard care for GBM is surgical resection, followed by concomitant temozolomide, an alkylating agent, and radiotherapy. GSCs represent important therapeutic targets because they have intrinsic machinery that overcomes current chemoradiotherapeutic approaches (Figure 1C). Some of the molecular mechanisms underlying GSC resistance to chemoradiotherapy are discussed below.
GSCs are believed to resist chemotherapy via several distinct mechanisms. One such mechanism involves the active transport of chemotherapeutic agents to the extracellular space via ABC-type transporters on the cell surface. This mechanism also defines the side population (SP) of GBM cells on flow cytometry, through the exclusion of Hoechst dye[97]. Enrichment of stem cell markers such as CD133, CD117, CD90, CD71 and CD45 is observed in cells resistant to lethal doses of chemotherapeutic drugs[98]. Furthermore, CD133 expression is increased in recurrent tumors. Transcriptional analysis of CD133+ GSCs showed that these cells have increased expression of anti-apoptotic genes, suggesting that GSCs have intrinsic mechanisms of chemoresistance[36].
In line with these observations, more compelling evidence came from Parada and colleagues, who showed that a restricted Nestin+ GSC population was able to regenerate tumors after temozolomide treatment. Selective ablation of this population led to tumor growth arrest, consistent with the notion that GSCs resist conventional chemotherapy and cause relapse[18].
Another mechanism for chemoresistance lies in the cell cycle profiles of GSCs. Most chemotherapeutic agents target actively cycling cells. However, GSCs are mostly dormant or slow-cycling cells, thereby resisting such therapies[99].
In addition to their chemoresistance, GSCs evade radiation, with radiation-resistant clones showing increased expression of GSC markers. More importantly, the Notch and TGF-β signaling pathways, which were mentioned earlier as critical for GSC self-renewal, promote radioresistance as well[51,100]. GSCs have increased DNA repair capacity. CD133+ GSCs selectively activate Chk1 and Chk2 kinases upon radiation, making them less susceptible to radiation-induced apoptosis[19].
In this review, we have summarized recent advances in understanding the biology of GSCs. We have focused on molecular markers commonly used to identify GSCs and signaling pathways that regulate important GSC characteristics, such as self-renewal, differentiation and therapy resistance. Due to their high tumorigenic potential and resistance to current therapies, GSCs represent critical drug targets. However, the lack of universal markers identifying GSCs, the complexity of signaling cascades regulating GSC biology and the large overlap between tumorigenic pathways active in both GSCs and normal stem cells complicate the development of GSC-targeted therapeutics. A better understanding of GSC biology and their contribution to cellular hierarchy and tumor heterogeneity is crucial for designing effective new therapies against gliomas and other brain malignancies.
| 1. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 16414] [Article Influence: 781.6] [Reference Citation Analysis (0)] |
| 2. | Netsky MG, August B, Fowler W. The longevity of patients with glioblastoma multiforme. J Neurosurg. 1950;7:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12:495-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Dirks PB. Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol. 2008;26:2916-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Raveh-Amit H, Berzsenyi S, Vas V, Ye D, Dinnyes A. Tissue resident stem cells: till death do us part. Biogerontology. 2013;14:573-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Sequerra EB, Costa MR, Menezes JR, Hedin-Pereira C. Adult neural stem cells: plastic or restricted neuronal fates? Development. 2013;140:3303-3309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Jackson EL, Alvarez-Buylla A. Characterization of adult neural stem cells and their relation to brain tumors. Cells Tissues Organs. 2008;188:212-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Singh S, Dirks PB. Brain tumor stem cells: identification and concepts. Neurosurg Clin N Am. 2007;18:31-8, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Chao MP, Seita J, Weissman IL. Establishment of a normal hematopoietic and leukemia stem cell hierarchy. Cold Spring Harb Symp Quant Biol. 2008;73:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1469] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 12. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. [PubMed] |
| 13. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5631] [Article Influence: 256.0] [Reference Citation Analysis (0)] |
| 14. | Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 413] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 15. | Rampazzo E, Persano L, Pistollato F, Moro E, Frasson C, Porazzi P, Della Puppa A, Bresolin S, Battilana G, Indraccolo S. Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis. 2013;4:e500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 921] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 17. | Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 709] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 18. | Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1518] [Cited by in RCA: 1796] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 19. | Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4467] [Cited by in RCA: 4855] [Article Influence: 242.8] [Reference Citation Analysis (0)] |
| 20. | Beier D, Röhrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U, Trampe-Kieslich A. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706-5715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Pfenninger CV, Roschupkina T, Hertwig F, Kottwitz D, Englund E, Bengzon J, Jacobsen SE, Nuber UA. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727-5736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Fan G. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Zacchigna S, Oh H, Wilsch-Bräuninger M, Missol-Kolka E, Jászai J, Jansen S, Tanimoto N, Tonagel F, Seeliger M, Huttner WB. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci. 2009;29:2297-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Brescia P, Ortensi B, Fornasari L, Levi D, Broggi G, Pelicci G. CD133 is essential for glioblastoma stem cell maintenance. Stem Cells. 2013;31:857-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 25. | Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 511] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 26. | Wang J, Sakariassen PØ, Tsinkalovsky O, Immervoll H, Bøe SO, Svendsen A, Prestegarden L, Røsland G, Thorsen F, Stuhr L. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 403] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 27. | Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010-4015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 839] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 28. | Lottaz C, Beier D, Meyer K, Kumar P, Hermann A, Schwarz J, Junker M, Oefner PJ, Bogdahn U, Wischhusen J. Transcriptional profiles of CD133+ and CD133- glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer Res. 2010;70:2030-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Yan X, Ma L, Yi D, Yoon JG, Diercks A, Foltz G, Price ND, Hood LE, Tian Q. A CD133-related gene expression signature identifies an aggressive glioblastoma subtype with excessive mutations. Proc Natl Acad Sci USA. 2011;108:1591-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Zarkoob H, Taube JH, Singh SK, Mani SA, Kohandel M. Investigating the link between molecular subtypes of glioblastoma, epithelial-mesenchymal transition, and CD133 cell surface protein. PLoS One. 2013;8:e64169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Campos B, Zeng L, Daotrong PH, Eckstein V, Unterberg A, Mairbäurl H, Herold-Mende C. Expression and regulation of AC133 and CD133 in glioblastoma. Glia. 2011;59:1974-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62:5551-5558. [PubMed] |
| 33. | Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. [PubMed] |
| 34. | Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 447] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 35. | Ikushima H, Todo T, Ino Y, Takahashi M, Saito N, Miyazawa K, Miyazono K. Glioma-initiating cells retain their tumorigenicity through integration of the Sox axis and Oct4 protein. J Biol Chem. 2011;286:41434-41441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1292] [Cited by in RCA: 1376] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 37. | Venugopal C, Li N, Wang X, Manoranjan B, Hawkins C, Gunnarsson T, Hollenberg R, Klurfan P, Murty N, Kwiecien J. Bmi1 marks intermediate precursors during differentiation of human brain tumor initiating cells. Stem Cell Res. 2012;8:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Lathia JD, Gallagher J, Myers JT, Li M, Vasanji A, McLendon RE, Hjelmeland AB, Huang AY, Rich JN. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PLoS One. 2011;6:e24807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Harris MA, Yang H, Low BE, Mukherjee J, Guha A, Bronson RT, Shultz LD, Israel MA, Yun K. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68:10051-10059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | González-Gómez P, Sánchez P, Mira H. MicroRNAs as regulators of neural stem cell-related pathways in glioblastoma multiforme. Mol Neurobiol. 2011;44:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1736] [Cited by in RCA: 1862] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 42. | Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 43. | Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26:3027-3036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 44. | Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL, Brem H, Riggins GJ. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Artavanis-Tsakonas S, Delidakis C, Fehon RG. The Notch locus and the cell biology of neuroblast segregation. Annu Rev Cell Biol. 1991;7:427-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 684] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 47. | Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 464] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 48. | Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 49. | Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 416] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 50. | Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der Heijden M, Moayedpardazi H, Correia AS, Soulet D, Major T, Menon J. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 51. | Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, Rich JN, Sullenger BA. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 436] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 52. | Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, Pieper RO. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Lino MM, Merlo A, Boulay JL. Notch signaling in glioblastoma: a developmental drug target? BMC Med. 2010;8:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Chen J, Kesari S, Rooney C, Strack PR, Chen J, Shen H, Wu L, Griffin JD. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010;1:822-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 472] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 56. | Ulasov IV, Nandi S, Dey M, Sonabend AM, Lesniak MS. Inhibition of Sonic hedgehog and Notch pathways enhances sensitivity of CD133(+) glioma stem cells to temozolomide therapy. Mol Med. 2013;17:103-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 57. | Jiang X, Xing H, Kim TM, Jung Y, Huang W, Yang HW, Song S, Park PJ, Carroll RS, Johnson MD. Numb regulates glioma stem cell fate and growth by altering epidermal growth factor receptor and Skp1-Cullin-F-box ubiquitin ligase activity. Stem Cells. 2012;30:1313-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 58. | Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, Rodón L, Folch G, Carmona MA, Prieto-Sánchez RM, Barba I, Martínez-Sáez E, Prudkin L. TGF-β Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 477] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 59. | Cayuso J, Ulloa F, Cox B, Briscoe J, Martí E. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development. 2006;133:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Shahi MH, Lorente A, Castresana JS. Hedgehog signalling in medulloblastoma, glioblastoma and neuroblastoma. Oncol Rep. 2008;19:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Bar EE, Chaudhry A, Farah MH, Eberhart CG. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol. 2007;170:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 62. | Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524-2533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 456] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 63. | Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 842] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 64. | Xu Q, Yuan X, Liu G, Black KL, Yu JS. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26:3018-3026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Uchida H, Arita K, Yunoue S, Yonezawa H, Shinsato Y, Kawano H, Hirano H, Hanaya R, Tokimura H. Role of sonic hedgehog signaling in migration of cell lines established from CD133-positive malignant glioma cells. J Neurooncol. 2011;104:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Rossi M, Magnoni L, Miracco C, Mori E, Tosi P, Pirtoli L, Tini P, Oliveri G, Cosci E, Bakker A. β-catenin and Gli1 are prognostic markers in glioblastoma. Cancer Biol Ther. 2011;11:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 67. | Kim Y, Kim KH, Lee J, Lee YA, Kim M, Lee SJ, Park K, Yang H, Jin J, Joo KM. Wnt activation is implicated in glioblastoma radioresistance. Lab Invest. 2012;92:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 68. | Nakata S, Campos B, Bageritz J, Bermejo JL, Becker N, Engel F, Acker T, Momma S, Herold-Mende C, Lichter P. LGR5 is a marker of poor prognosis in glioblastoma and is required for survival of brain cancer stem-like cells. Brain Pathol. 2013;23:60-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Jin X, Jeon HY, Joo KM, Kim JK, Jin J, Kim SH, Kang BG, Beck S, Lee SJ, Kim JK. Frizzled 4 regulates stemness and invasiveness of migrating glioma cells established by serial intracranial transplantation. Cancer Res. 2011;71:3066-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 70. | Pulvirenti T, Van Der Heijden M, Droms LA, Huse JT, Tabar V, Hall A. Dishevelled 2 signaling promotes self-renewal and tumorigenicity in human gliomas. Cancer Res. 2011;71:7280-7290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 72. | Voumvourakis KI, Antonelou RCh, Kitsos DK, Stamboulis E, Tsiodras S. TGF-β/BMPs: crucial crossroad in neural autoimmune disorders. Neurochem Int. 2011;59:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 903] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 74. | Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13:69-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 75. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 28208] [Article Influence: 1282.2] [Reference Citation Analysis (0)] |
| 76. | Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 421] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 77. | Kim H, Huang W, Jiang X, Pennicooke B, Park PJ, Johnson MD. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci USA. 2010;107:2183-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 78. | Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 79. | Silber J, Jacobsen A, Ozawa T, Harinath G, Pedraza A, Sander C, Holland EC, Huse JT. miR-34a repression in proneural malignant gliomas upregulates expression of its target PDGFRA and promotes tumorigenesis. PLoS One. 2012;7:e33844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 736] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 81. | Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68:9125-9130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 531] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 82. | Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 850] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 83. | Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 84. | Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1640] [Cited by in RCA: 1583] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 85. | Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061-6072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 286] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 86. | Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843-7848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 982] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 87. | Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65:529-539. [PubMed] |
| 88. | Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 825] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 89. | Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 90. | Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274-3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 598] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 91. | Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 92. | Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol. 2012;181:1126-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 346] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 93. | Teodorczyk M, Martin-Villalba A. Sensing invasion: cell surface receptors driving spreading of glioblastoma. J Cell Physiol. 2010;222:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 95. | Sampetrean O, Saga I, Nakanishi M, Sugihara E, Fukaya R, Onishi N, Osuka S, Akahata M, Kai K, Sugimoto H. Invasion precedes tumor mass formation in a malignant brain tumor model of genetically modified neural stem cells. Neoplasia. 2011;13:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 96. | Winkler F, Kienast Y, Fuhrmann M, Von Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H, Herms J. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia. 2009;57:1306-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 97. | Bleau AM, Huse JT, Holland EC. The ABCG2 resistance network of glioblastoma. Cell Cycle. 2009;8:2936-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 99. | Deleyrolle LP, Harding A, Cato K, Siebzehnrubl FA, Rahman M, Azari H, Olson S, Gabrielli B, Osborne G, Vescovi A. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain. 2011;134:1331-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 100. | Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, Barcellos-Hoff MH. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 2012;72:4119-4129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
P- Reviewers: de la Serna IL, Kan L S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN