Published online Apr 26, 2014. doi: 10.4252/wjsc.v6.i2.195
Revised: January 21, 2014
Accepted: February 20, 2014
Published online: April 26, 2014
Processing time: 179 Days and 13.6 Hours
Human umbilical cord (UC) is a promising source of mesenchymal stem cells (MSCs). Apart from their prominent advantages, such as a painless collection procedure and faster self-renewal, UC-MSCs have shown the ability to differentiate into three germ layers, to accumulate in damaged tissue or inflamed regions, to promote tissue repair, and to modulate immune response. There are diverse protocols and culture methods for the isolation of MSCs from the various compartments of UC, such as Wharton’s jelly, vein, arteries, UC lining and subamnion and perivascular regions. In this review, we give a brief introduction to various compartments of UC as a source of MSCs and emphasize the potential clinical utility of UC-MSCs for regenerative medicine and immunotherapy.
Core tip: Human umbilical cord (UC) is a promising source of mesenchymal stem cells (MSCs). UC-MSCs have shown the ability of faster self-renewal and to differentiate into three germ layers, to accumulate in damaged tissue or inflamed regions, to promote tissue repair, and to modulate immune response. There are diverse protocols and culture methods for the isolation of MSCs from the various compartments of UC, such as Wharton’s jelly, vein, arteries, UC lining membrane and subamnion and perivascular regions. In this review, we introduce various compartments of UC and discuss the potential clinical utility of UC-MSCs for regenerative medicine and immunotherapy.
- Citation: Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells 2014; 6(2): 195-202
- URL: https://www.wjgnet.com/1948-0210/full/v6/i2/195.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i2.195
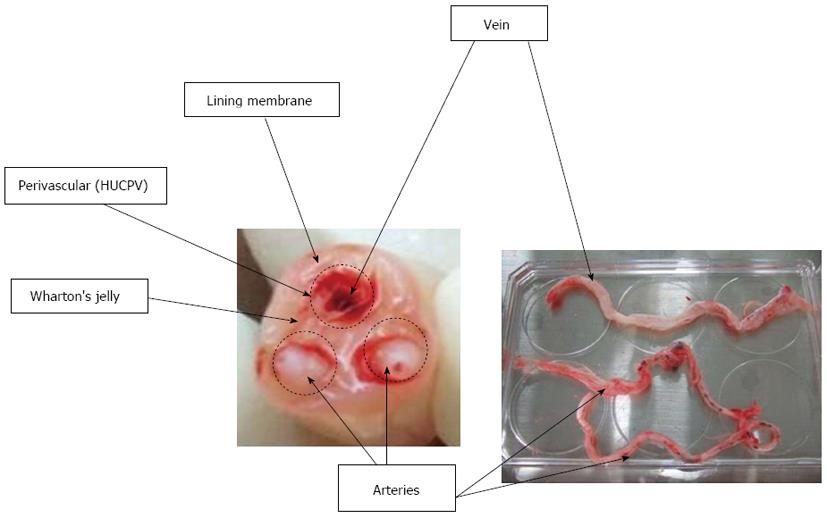
Mesenchymal stem cells (MSCs) originate in the human embryo and are considered multipotent stem cells. MSCs are a heterogeneous subset of stromal stem cells, which can be isolated from the bone marrow[1], mobilized peripheral blood[2], cord blood[3], umbilical cord (UC)[4,5], placenta[6], adipose tissue[7], dental pulp[8], and even the fetal liver[9] and lungs[10]. UC contains two umbilical arteries (UCAs) and one umbilical vein (UCV), both embedded within a specific mucous connective tissue, known as Wharton’s jelly (WJ), which is covered by amniotic epithelium (Figure 1). UC is considered medical waste and the collection of UC-MSCs is noninvasive; furthermore, the access to UC-MSCs has not been encumbered with ethical problems. UC-MSCs, similarly to MSCs derived from other sources, have distinct capacity for self-renewal while maintaining their multipotency, i.e., the ability to differentiate into adipocytes, osteocytes, chondrocytes, neurons and hepatocytes, although some differentiation abilities are known to be partial[11-13]. Moreover, UC-MSCs have also attracted great interest because of their immunomodulatory properties. Nowadays, UC-MSCs are proposed as a possible versatile tool for regenerative medicine and immunotherapy.
During pregnancy, the fetus and mother are connected by UC. UC prevents umbilical vessels from compression, torsion and bending, while providing good blood circulation. McElreavey et al[4] for the first time reported isolation of fibroblast-like cells from WJ of human UC in 1991. The UC-derived cells have the similar surface phenotype, plastic adherence and multipotency as those of MSCs derived from other sources. It was 3 years earlier that the first cord blood (CB) transplantation was performed in France in 1988[14]. After that, together with the development of CB banking, CB transplantation has become the alternative source of hematopoietic stem cells. Although CB-derived MSCs cannot be consistently isolated[15-18], MSCs were considered to circulate in the blood of preterm fetuses and able to be successfully isolated and expanded[3]. Where these cells home at the end of gestation is not clear[13]. Thus, UC has inevitably become a focus of attention as a source of MSCs because it contains CB[18]. One key study appeared concerning CB-derived MSCs appeared around 2003[19]. Mitchell et al[20] successfully isolated matrix cells from UC WJ using explant culture and Romanov et al[19] isolated MSCs-like cells from the subendothelial layer of UCV.
Stem cell populations can be isolated from embryonic, fetal and adult tissues. Embryonic stem cells (ESCs) are a leading candidate for tissue engineering because of their high self-renewal capacity and pluripotency (ability to differentiate into all germ layers) in vitro and in vivo. Nonetheless, in addition to ethical restrictions, the clinical applications of ESCs are severely limited by technical difficulties with the depletion of immature cells that may result in the formation of a teratoma.
In contrast, adult stem cells, such as those in the skin, bone marrow (BM) and adipose tissue, may have wider clinical applications. BM-MSCs have been used for autologous and allogeneic purposes. Recently, successful clinical application of autologous BM-MSCs was reported for conditions such as cardiac infarction[21], graft-versus-host disease (GVHD)[22,23], Crohn’s disease[24] and bone tissue engineering[25]. On the other hand, the autologous use was sometimes limited by cell numbers and age-related changes such as decreased growth and differentiation capacity[26,27].
Compared with BM-MSCs and ES cells, UC-MSCs show a gene expression profile more similar to that of ESCs and faster self-renewal rather than BM-MSCs[11,12].
It is easy to obtain a substantial number of UC-MSCs after several passages and extensive ex vivo expansion[28]. The most appreciable advantage is that the collection procedure is noninvasive and ethically acceptable.
Similar to BM-MSCs, UC-MSCs can be considered for autologous and allogeneic use. Autologous UC-MSCs might be used as gene therapy for genetic diseases and as regenerative or anti-inflammatory therapy for neonatal injury, such as cerebral palsy or hypoxic brain damage. On the other hand, allogeneic UC-MSCs can be expanded and cryopreserved in a cell bank for patients in need. The only disadvantage is that physicians need to confirm the baby’s health as a donor because it cannot be ascertained in advance whether the donor will grow normally without health problems; thus, genomic or chromosomal tests need to be performed. In contrast, in the case of a BM donor, the physician can directly see and examine the donor and then decide to collect BM. In the case of CB banking, many CB banks monitor the baby’s health after birth. Thus, it is important to know the advantages and disadvantages of UC-MSCs for each clinical application.
There are two methods to obtain MSCs from various UC compartments or from the whole UC: the explant method and the enzymatic digestion method.
UC, or its compartments, is manually minced into small fragments 1-2 mm3. These fragments are aligned and seeded regularly on the tissue culture-treated dishes. After the tissue fragments are attached to the bottom of the dish, the culture medium is poured slowly and gently, so as not to detach the fragments, and the culture is started[29-31]. The culture medium is replaced every 3-7 d for 2-4 wk until fibroblast-like adherent cells reach 80%-90% confluence. The adherent cells and tissue fragments are rinsed once with PBS and detached using a trypsin solution, followed by washing with the medium. The cells and tissue fragments are filtered to remove the tissue fragments.
The disadvantage of this method is that the fragments often float in the medium, resulting in poor cell recovery. No MSCs can be obtained from the floating fragments. To collect a consistent number of MSCs each time, it is important to prevent the exfoliation of the tissue fragments from the bottom of plastic dishes.
WJ is either directly exposed to enzymatic solutions to release the cells or it is cut into small pieces followed by enzymatic digestion. The enzymes used for digestion vary from simple collagenase[31,32] to a combination of either collagenase and hyaluronidase with or without trypsin[33,34] or collagenase, dispase II and hyaluronidase[33]. The digestion time and concentrations varied by researchers.
There are four compartments of UC as a source of MSCs: (1) Whole UC-MSCs: the whole UC is cut into smaller pieces followed by either an explant procedure or enzyme digestion[30,35,36]; (2) UCWJ-, UCA- and UCV-MSCs: UCWJ-MSCs are obtained after removing umbilical vessels. Umbilical vessels [two arteries (UCAs) and one vein (UCV)] can also be minced into 1-2 mm3 fragments. The fragments are aligned regularly on the plate and cultured until MSCs start growing; (3) UC lining and subamnion-derived MSCs: the subamnion region of UC lining membrane is removed with a razor blade and then cut into small pieces. These fragments are plated in plastic culture dishes until MSCs start growing (explant method). With this method, however, it might be difficult to remove the adjacent region underneath the amniotic epithelium completely[37,38]; and (4) Human UC perivascular stem cells (HUCPVC): the vessels are extracted from UC and tied at both ends into loops. The loops are then placed into an enzymatic solution for a defined period of time to allow the cells to separate from the perivascular region. The detached cells are cultured and collected as HUCPVCs[26,28,39].
It is still controversial whether the isolation of the cells from the whole or some compartment is superior to others with respect to their proliferation ability, differentiation ability and immunosuppressive capacity.
The frequency of colony-forming unit fibroblasts (CFU-F) is significantly higher in whole UC-derived MSCs than in BM-MSCs with limiting dilution[26,30,40]. The authors first compared UCWJ-MSCs, UCA-MSCs and UCV-MSCs. UCV-MSCs exhibited a significantly higher frequency of CFU-F than UCWJ-MSCs and UCA-MSCs, but the doubling time was not different among these cell types[5]. The Mennan group also reported that there are no significant differences among the various compartments of UC, although the cells derived from any UC compartment proliferate significantly faster than BM-MSCs, with mean doubling times of 2-3 d at P0 through P3[41]. Depending on the purpose, researchers need to select either a compartment or the whole UC.
The immunoprofile of UC-MSCs is analyzed using flow cytometry, according to the standard definitions for MSCs described by the position paper of the International Society for Cellular Therapy[42]. There are no single specific markers that can be used to identify multipotent MSCs. MSCs are positive for adhesion markers such as CD29 and CD44; mesenchymal markers such as CD90, CD73 and CD105; and human leukocyte antigen class I (HLA-ABC). MSCs are negative for endothelial cell marker CD31; hematopoietic cell markers such as CD34, CD45 and CD117; and human leukocyte differentiation antigen class II (HLA-DR)[43]. Among the different UC compartments, UCWJ-, UCV- and UCA-derived MSCs show a similar fibroblast-like spindle shape and the MSCs from these three types of tissues demonstrate no significant differences in the immunoprofile. These cells are positive for CD13, CD29 (integrin β1), CD73 (SH3), CD90 (Thy-1), CD105 (SH2; endogrin) and HLA-ABC at the cellular frequency greater than 90% and are negative for CD34, CD45, CD133 and HLA-DR, with the cellular frequency less than 1%[5]. Mennan et al[41] also confirmed that MSC immunophenotypes showed no significant differences among different sources: BM, umbilical cord arteries, vein, WJ and UC lining membrane. Even although the authors could not find any major differences in their immunophenotypes, the cell populations derived from the different compartments may consist of different proportions of multipotent stem cells. Karahuseyinoglu et al[44] demonstrated that CD73 is expressed throughout the vessels and endothelium and is absent in the perivascular region, but the strongest expression is observed in the epithelial and subepithelial regions of WJ. CD90 is positive in most compartments but negative in the endothelial lumen lining. A high expression of vimentin, CKs (1, 4, 5, 6, 8, 10, 13, 18 and 19), desmin and SMA has been detected in the subamniotic layer and the perivascular region. Schugar et al[45] reported that CD146 (endothelial progenitor marker) is expressed in the vessel walls (100%) and the perivascular region of UC (62%) but is no longer expressed in UCWJ-MSCs[26,46]. These markers might aid in determining the multipotency of the isolated cell population. Phenotypic characterization of UC-MSCs might be influenced by the culture passage number, culture medium and culture method.
Furthermore, ESC markers such as Oct4, Nanog, Sox-2 and KLF4 are expressed only at low levels in UC-MSCs[47]. This suggests that MSCs are primitive stem cells, somewhere between ESCs and mature adult stem cells. Nonetheless, a precise isolation of pluripotent MSCs using specific markers remains a challenge.
The role of SSEA3 and SSEA4 in MSCs remains controversial. Gang et al[48] reported that SSEA4+ cells proliferate predominantly when the culture is initiated from primary BM cells in the medium supplemented with special cocktails of cytokines. In contrast, the authors failed to reproduce the same phenomena in UCWJ-MSCs in the medium consisting of α-MEM and 10% FBS. Furthermore, SSEA4 expression in UCWJ-MSCs significantly correlates with the FBS concentration in the culture medium, whereas SSEA3 expression was inversely correlated. We concluded that SSEA4 in UCWJ-MSCs is not a marker of either proliferation capacity or multipotency[31]. Schrobback et al[49] assessed SSEA4 expression in human articular chondrocytes, osteoblasts and BM-derived MSCs and characterized their differentiation potential. Their results showed that SSEA4 levels in these cells are not related to the capacity for chondrogenic and osteogenic differentiation and the proliferation potential in vitro[49].
UC-MSCs originating from the extraembryonic mesoderm and their capacity for differentiation into adipogenic, chondrogenic and osteogenic lineages have been extensively studied[50]. Regarding the osteogenic differentiation ability, Hsieh et al[11] demonstrated that the gene profiles of UC-MSCs are close to ESCs; UC-MSCs show delayed and insufficient differentiation into osteocytes. On the other hand, BM-MSCs have already expressed an osteogenic gene profile and can easily differentiate into osteocytes. Among the three compartments, UCWJ, UCV and UCAs, UCWJ-MSCs demonstrate an obviously defective ability to differentiate into osteocytes, even although the expression of osteocyte-related genes is detected by reverse-transcriptase PCR, at levels similar to those in the other two tissues/compartments[5]. Mennan et al[41] compared the osteogenic differentiation among cord regions in six samples and found that the best differentiation is seen with UCWJ-MSCs and whole UC-derived MSCs, rather than with UCA-, UCV- and UC lining MSCs.
As for adipocytic differentiation, Mennan et al[41] reported that UC-MSCs produce small lipid vacuoles, whereas BM-MSCs produce more mature adipocytes (unilocular lipid vacuoles). UC-MSCs might maintain their multipotency for longer periods than BM-MSCs can[51], although there were no obvious differences among MSCs derived from UC compartments in our research[5].
With respect to chondrogenic differentiation, UC-MSCs show no apparent differences among the different cord regions (sources)[41]. Moreover, the comparison of the chondrogenic potential between BM-MSCs and UC-MSCs revealed that UC-MSCs produce thrice as much collagen as BM-MSCs; this finding indicates that the former may be a better option for fibrocartilage tissue engineering[52].
In relation to other differentiation abilities, UCWJ-MSCs are the most studied cell type among various UC compartments and many papers have been published[53,54]. In addition to differentiating into osteocytes, chondrocytes and adipocytes, UCWJ-MSCs can differentiate into cardiomyocytes (with the gene expression of N-cadherin and cardiac troponin I[55]), neurons and glia[20], oligodendrocytes[56] and hepatocytes[57]. Recently, clinical trials have been conducted using UC-MSCs for neurogenic disorders (spinocerebellar ataxia and multiple system atrophy of the cerebellar type)[58] and liver disorders[59,60].
Immunosuppressive effects have now become the most popular property of MSCs for potential clinical use. First, MSCs themselves are weakly immunogenic owing to the lack of HLA-DR and low expression of MHC class I molecules. MSCs have been shown to have immunomodulatory properties in vitro[61]. Furthermore, MSCs lack both CD80 and CD86 proteins[36,62], which are costimulatory molecules inducing T cell activation and survival. The lack of HLA-DR, CD80 and CD86 suggests that MSCs do not elicit acute rejection and are suitable for allogeneic cell-based therapy.
Second, UC-MSCs have immunosuppressive properties in vitro and in vivo. Many studies have been published about the immunosuppressive effect of UCWJ-MSCs[63], UC lining-MSCs[37,64], HUCPV[65] and whole UC-derived MSCs[66]. The immunosuppressive effect of UC-MSCs is mediated by soluble factors and cell-to-cell contacts. PGE2, galectin-1 and HLA-G5 are released from MSCs and serve as effective factors of immunosuppression[67]. Among these factors, indoleamine 2,3-dioxygenase (IDO) is one of the most relevant because it is inducible by IFN-γ and catalyzes conversion from tryptophan to kynurenine[62,68]. This depletion of tryptophan from the environment can suppress T cell proliferation. UCWJ-MSC-mediated immunosuppression may require preliminary activation by proinflammatory cytokines, such as IFN-γ, with or without TNF-α, IL-1α or IL-1β.
It was recently suggested that the inflammatory environment produced by the upregulation of cytokines such as IFN-γ and TNF-α might alter the biological activity of MSCs from immunosuppression to immunostimulation[68]. In this case, UC-MSCs maynot prevent GVHD in vivo. It is known that upon stimulation by activated immune cells or cytokines (priming), MSCs are primed and become functional immunosuppressors. The extent of immunosuppression is greater with UCWJ-MSCs than with BM-MSCs[62]. Polchert et al[68] demonstrated that MSCs primed with IFN-γ are effective in a mouse GVHD model despite upregulated MHC class II molecules. In order to ensure the effective and safe therapeutic use of UC-MSCs, more in vivo experiments need to be conducted because of the many discrepancies with in vitro data.
Compared with the counterparts of other origins, UC-MSCs have attractive advantages as MSCs and as UC-derived cells: (1) a noninvasive collection procedure for autologous or allogeneic use; (2) a lower risk of infection; (3) a low risk of teratoma; (4) multipotency; and (5) low immunogenicity with a good immunosuppressive ability. It is still unclear which compartment in UC is the best for clinical use; nonetheless, the era of the clinical use of UC-MSCs is approaching quickly.
| 1. | Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Bieback K, Klüter H. Mesenchymal stromal cells from umbilical cord blood. Curr Stem Cell Res Ther. 2007;2:310-323. [PubMed] |
| 4. | McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem Soc Trans. 1991;19:29S. [PubMed] |
| 5. | Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, Sugimoto M, Nakauchi H, Tojo A. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton’s jelly explants of human umbilical cord. Int J Hematol. 2009;90:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [PubMed] |
| 7. | Gruber HE, Deepe R, Hoelscher GL, Ingram JA, Norton HJ, Scannell B, Loeffler BJ, Zinchenko N, Hanley EN, Tapp H. Human adipose-derived mesenchymal stem cells: direction to a phenotype sharing similarities with the disc, gene expression profiling, and coculture with human annulus cells. Tissue Eng Part A. 2010;16:2843-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ponnaiyan D, Bhat KM, Bhat GS. Comparison of immuno-phenotypes of stem cells from human dental pulp and periodontal ligament. Int J Immunopathol Pharmacol. 2012;25:127-134. [PubMed] |
| 9. | Joshi M, B Patil P, He Z, Holgersson J, Olausson M, Sumitran-Holgersson S. Fetal liver-derived mesenchymal stromal cells augment engraftment of transplanted hepatocytes. Cytotherapy. 2012;14:657-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | in ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845-852. [PubMed] |
| 11. | Hsieh JY, Fu YS, Chang SJ, Tsuang YH, Wang HW. Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton’s jelly of umbilical cord. Stem Cells Dev. 2010;19:1895-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, Chan WK, Bongso A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2011;7:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 13. | Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 564] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 14. | Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174-1178. [PubMed] |
| 15. | Zhang X, Hirai M, Cantero S, Ciubotariu R, Dobrila L, Hirsh A, Igura K, Satoh H, Yokomi I, Nishimura T. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112:1206-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Manca MF, Zwart I, Beo J, Palasingham R, Jen LS, Navarrete R, Girdlestone J, Navarrete CV. Characterization of mesenchymal stromal cells derived from full-term umbilical cord blood. Cytotherapy. 2008;10:54-68. [PubMed] |
| 17. | Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368-374. [PubMed] |
| 18. | Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146-150. [PubMed] |
| 19. | Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105-110. [PubMed] |
| 20. | Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50-60. [PubMed] |
| 21. | Minguell JJ, Lorino R, Lasala GP. Myocardial implantation of a combination stem cell product by using a transendocardial MYOSTAR injection catheter: A technical assessment. Acute Card Care. 2011;13:40-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Muroi K, Miyamura K, Ohashi K, Murata M, Eto T, Kobayashi N, Taniguchi S, Imamura M, Ando K, Kato S. Unrelated allogeneic bone marrow-derived mesenchymal stem cells for steroid-refractory acute graft-versus-host disease: a phase I/II study. Int J Hematol. 2013;98:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, Wu SJ, Luo CW, Guo R, Ling W. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45:1732-1740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 24. | Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 472] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 25. | Kagami H, Agata H, Tojo A. Bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for bone tissue engineering: basic science to clinical translation. Int J Biochem Cell Biol. 2011;43:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384-1392. [PubMed] |
| 27. | Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583-590. [PubMed] |
| 28. | Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220-229. [PubMed] |
| 29. | Marmotti A, Mattia S, Bruzzone M, Buttiglieri S, Risso A, Bonasia DE, Blonna D, Castoldi F, Rossi R, Zanini C. Minced umbilical cord fragments as a source of cells for orthopaedic tissue engineering: an in vitro study. Stem Cells Int. 2012;2012:326813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Majore I, Moretti P, Stahl F, Hass R, Kasper C. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 2011;7:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | He H, Nagamura-Inoue T, Tsunoda H, Yuzawa M, Yamamoto Y, Yorozu P, Agata H, Tojo A. Stage-Specific Embryonic Antigen 4 in Wharton’s Jelly-Derived Mesenchymal Stem Cells Is Not a Marker for Proliferation and Multipotency. Tissue Eng Part A. 2014;20:1314-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Kikuchi-Taura A, Taguchi A, Kanda T, Inoue T, Kasahara Y, Hirose H, Sato I, Matsuyama T, Nakagomi T, Yamahara K. Human umbilical cord provides a significant source of unexpanded mesenchymal stromal cells. Cytotherapy. 2012;14:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Salehinejad P, Alitheen NB, Ali AM, Omar AR, Mohit M, Janzamin E, Samani FS, Torshizi Z, Nematollahi-Mahani SN. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. In Vitro Cell Dev Biol Anim. 2012;48:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (2)] |
| 34. | Tsagias N, Koliakos I, Karagiannis V, Eleftheriadou M, Koliakos GG. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus Med. 2011;21:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Bosch J, Houben AP, Radke TF, Stapelkamp D, Bünemann E, Balan P, Buchheiser A, Liedtke S, Kögler G. Distinct differentiation potential of “MSC” derived from cord blood and umbilical cord: are cord-derived cells true mesenchymal stromal cells? Stem Cells Dev. 2012;21:1977-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 2010;19:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Zebardast N, Lickorish D, Davies JE. Human umbilical cord perivascular cells (HUCPVC): A mesenchymal cell source for dermal wound healing. Organogenesis. 2010;6:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Lü LL, Song YP, Wei XD, Fang BJ, Zhang YL, Li YF. [Comparative characterization of mesenchymal stem cells from human umbilical cord tissue and bone marrow]. Zhongguo Shi Yan Xue Ye Xue Zazhi. 2008;16:140-146. [PubMed] |
| 41. | Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013:916136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 42. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13047] [Article Influence: 686.7] [Reference Citation Analysis (12)] |
| 43. | Seo KW, Lee SR, Bhandari DR, Roh KH, Park SB, So AY, Jung JW, Seo MS, Kang SK, Lee YS. OCT4A contributes to the stemness and multi-potency of human umbilical cord blood-derived multipotent stem cells (hUCB-MSCs). Biochem Biophys Res Commun. 2009;384:120-125. [PubMed] |
| 44. | Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 356] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 45. | Schugar RC, Chirieleison SM, Wescoe KE, Schmidt BT, Askew Y, Nance JJ, Evron JM, Peault B, Deasy BM. High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J Biomed Biotechnol. 2009;2009:789526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Conconi M, Di Liddo R, Tommasini M, Calore C, Parnigotto P. Phenotype and differentiation potential of stromal populations obtained from various zones of human umbilical cord: An overview. Open Tissue Eng Regen Med J. 2011;4:6-20. |
| 47. | Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143-3154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 48. | Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 371] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 49. | Schrobback K, Wrobel J, Hutmacher DW, Woodfield TB, Klein TJ. Stage-specific embryonic antigen-4 is not a marker for chondrogenic and osteogenic potential in cultured chondrocytes and mesenchymal progenitor cells. Tissue Eng Part A. 2013;19:1316-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Huang P, Lin LM, Wu XY, Tang QL, Feng XY, Lin GY, Lin X, Wang HW, Huang TH, Ma L. Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into germ-like cells in vitro. J Cell Biochem. 2010;109:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Fong CY, Gauthaman K, Cheyyatraivendran S, Lin HD, Biswas A, Bongso A. Human umbilical cord Wharton’s jelly stem cells and its conditioned medium support hematopoietic stem cell expansion ex vivo. J Cell Biochem. 2012;113:658-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Fong CY, Subramanian A, Gauthaman K, Venugopal J, Biswas A, Ramakrishna S, Bongso A. Human umbilical cord Wharton’s jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev. 2012;8:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 53. | Kim JW, Kim SY, Park SY, Kim YM, Kim JM, Lee MH, Ryu HM. Mesenchymal progenitor cells in the human umbilical cord. Ann Hematol. 2004;83:733-738. [PubMed] |
| 54. | Anzalone R, Lo Iacono M, Corrao S, Magno F, Loria T, Cappello F, Zummo G, Farina F, La Rocca G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010;19:423-438. [PubMed] |
| 55. | Conconi MT, Burra P, Di Liddo R, Calore C, Turetta M, Bellini S, Bo P, Nussdorfer GG, Parnigotto PP. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18:1089-1096. [PubMed] |
| 56. | Zhang HT, Fan J, Cai YQ, Zhao SJ, Xue S, Lin JH, Jiang XD, Xu RX. Human Wharton’s jelly cells can be induced to differentiate into growth factor-secreting oligodendrocyte progenitor-like cells. Differentiation. 2010;79:15-20. [PubMed] |
| 57. | Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 58. | Dongmei H, Jing L, Mei X, Ling Z, Hongmin Y, Zhidong W, Li D, Zikuan G, Hengxiang W. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy. 2011;13:913-917. [PubMed] |
| 59. | Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 60. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 61. | Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33-41. [PubMed] |
| 62. | Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. [PubMed] |
| 63. | Girdlestone J, Limbani VA, Cutler AJ, Navarrete CV. Efficient expansion of mesenchymal stromal cells from umbilical cord under low serum conditions. Cytotherapy. 2009;11:738-748. [PubMed] |
| 64. | Stubbendorff M, Deuse T, Hua X, Phan TT, Bieback K, Atkinson K, Eiermann TH, Velden J, Schröder C, Reichenspurner H. Immunological properties of extraembryonic human mesenchymal stromal cells derived from gestational tissue. Stem Cells Dev. 2013;22:2619-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Ennis J, Götherström C, Le Blanc K, Davies JE. In vitro immunologic properties of human umbilical cord perivascular cells. Cytotherapy. 2008;10:174-181. [PubMed] |
| 66. | Wang D, Chen K, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Bayard F, Zhu D, Han ZC. CD14+ monocytes promote the immunosuppressive effect of human umbilical cord matrix stem cells. Exp Cell Res. 2010;316:2414-2423. [PubMed] |
| 67. | Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 437] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 68. | Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 463] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
P- Reviewers: Forte A, Kim SJ S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Zhang DN