Published online Apr 26, 2014. doi: 10.4252/wjsc.v6.i2.163
Revised: December 20, 2013
Accepted: March 3, 2014
Published online: April 26, 2014
Processing time: 180 Days and 17.9 Hours
Islet cell transplantation has therapeutic potential to treat type 1 diabetes, which is characterized by autoimmune destruction of insulin-producing pancreatic islet β cells. It represents a minimal invasive approach for β cell replacement, but long-term blood control is still largely unachievable. This phenomenon can be attributed to the lack of islet vasculature and hypoxic environment in the immediate post-transplantation period that contributes to the acute loss of islets by ischemia. Moreover, graft failures continue to occur because of immunological rejection, despite the use of potent immunosuppressive agents. Mesenchymal stem cells (MSCs) have the potential to enhance islet transplantation by suppressing inflammatory damage and immune mediated rejection. In this review we discuss the impact of MSCs on islet transplantation and focus on the potential role of MSCs in protecting islet grafts from early graft failure and from autoimmune attack.
Core tip: Type 1 diabetes is caused by a cell-mediated autoimmune destruction of pancreatic β cells. The transplantation of pancreatic islets provides a cure for this disorder. In this review, we first summarize the current knowledge on the pathogenesis of type 1 diabetes and on the therapeutic options for this disorder. Next we discuss the impact of mesenchymal stem cells on vascularization and immunomodulation of islet transplantation.
- Citation: Figliuzzi M, Bonandrini B, Silvani S, Remuzzi A. Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J Stem Cells 2014; 6(2): 163-172
- URL: https://www.wjgnet.com/1948-0210/full/v6/i2/163.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i2.163
Type 1 diabetes results from the autoimmune destruction of insulin-producing pancreatic islet β cells and is usually diagnosed in children and young adults. β cell replacement therapies using either pancreas or islet transplantation represent a therapeutic alternative to the administration of exogenous insulin.
Islet transplantation is advantageous compared with whole pancreas transplantation because it is relatively non-invasive. However, a decline in islet cell survival, after transplantation, remains a significant obstacle in successful islet transplantation. It has been suggested that the complete lack of islet vasculature and hypoxic environment in the immediate post-transplantation period contribute to the acute loss of islet by ischemia[1]. Moreover, graft failure continues to occur because of immunological rejection, despite the use of potent immunosuppressive agents.
Mesenchymal stem cells (MSCs) are non-hematopoietic multipotent stromal cells that can differentiate in a variety of tissues[2]. The ability of MSCs to secrete trophic and angiogenic factors may help to prevent early islet damage and assist islet engraftment. MSCs may also attenuate autoimmunity through their immunomodulatory properties while secreting cytokines to control autoreactive T cells. All these properties could be used for in vivo co-transplantation to improve islet engraftment[3]. Here we discuss the impact of MSCs on islet transplantation from both early graft failure and from autoimmune attack, to prevent immune rejection and promote long-term islet allograft survival.
Type 1 diabetes is a fast-growing global problem with enormous social, health, and economic consequences. This metabolic disorder is characterized by the irreversible destruction of insulin-secreting β cells. Death of the pancreatic β cells is associated with hyperglycaemia, which is the main determinant of long-term complications in diabetic patients. Exogenous insulin administration is required to control glucose levels in the blood. The pancreatic islets are the targets of an autoimmune assault, where islets are invaded by mononuclear cells that cause an inflammatory reaction called “insulitis”, leading to loss of most of β cells. β cell death in the course of insulitis is probably caused by direct contact with activated macrophages and T-cells, and/or exposure to soluble mediators secreted by these cells, as cytokines, nitric oxide (NO), and oxygen free radicals[4].
Type 1 diabetes is a multifactorial disease where a genetic predisposition combines with environmental factors to induce the activation of the specific autoimmune destruction of β cells. Different known genetic risk factors can predict type 1 diabetes but autoantibodies are the most important preclinical markers. Autoantibodies include: islet cell autoantibodies, autoantibodies to insulin, autoantibodies to GAD (GAD 65), and autoantibodes to the tyrosine phosphatases IA-2 and IA-2β. In 85%-90% of patients affected by juvenile diabetes, these autoantibodies are detectable[5]. Several genetic loci have been associated with type 1 diabetes but the HLA (human leukocyte antigen) region, located on chromosome 6p, with its multiple genes is the strongest link to immune-mediated diabetes susceptibility. More than 200 identified genes are located in the HLA region, over half of which are predicted to be expressed[6]. Non-genetic factors also contribute to the risk of type 1 diabetes. This is supported by the fact that the overall concordance rate for type 1 diabetes among monozygotic twins is only about 10%-40%[7]. Environmental factors play a substantial role in the development of type 1 diabetes. They include specific infectious agents, dietary factors, perinatal factors, socioeconomic factors, and psychosocial factors[8].
The treatment of type 1 diabetes mellitus includes different therapeutic approaches. The aim of clinical intervention is to arrest or prevent the β cell destruction due to autoimmunity, reverse this process and restore normal blood glucose level and immune homeostasis. Insulin therapy was the first therapy and represented the primary breakthrough treatment for type 1 diabetes, however, frequent hyper- and hypo-glycaemia episodes seriously affect the quality of life of these patients. Recent technological innovations such as insulin analogue formulation, devices for insulin delivery and glucose monitoring systems have allowed diabetic patients to improve their glycaemic control[9]. Intensive insulin therapies via insulin pens, subcutaneous or intraperitoneal insulin infusions using pumps reduce the onset and progression of diabetic complications, risks of hypo- or hyper-glycaemia, and increase the patient’s quality of life.
β cell replacement is the only way to restore euglycaemia and ameliorate the progression of diabetic complications. Pancreas or pancreatic islet transplantation represents therapeutic alternatives to the administration of exogenous insulin to treat patients with type 1 diabetes. At the current time pancreas transplantation can produce long-term exogenous insulin independence, however, it remains a major surgical undertaking, associated with sizeable early morbidity and mortality, and with mandatory life-long immunosuppression[10]. Islet transplantation also offers glycaemic control and prevention of hyperglycaemia without the need for exogenous insulin administration[11]. As islets make up only 1%-2% of the pancreas, islet transplantation provides a much smaller transplant mass than whole pancreas transplant and is therefore a much less invasive procedure, and presents a smaller load of immunogenic tissue.
New therapeutic strategies for type 1 diabetes focus on three important points: residual β cell prevention, β cell restoration and β cell immune protection[12]. An achievable goal could be to develop a new cellular source for β cell. Different studies focus on immortalization and expansion of β cells from deceased donor pancreas[13,14], reprogramming or transdifferentiation of other pancreatic cells to β cells[15], differentiation from pancreatic progenitor cells in the adult pancreas[16] and differentiation and maturation from embryonic stem cells and induced pluripotential stem cells[17]. All these cellular sources appear promising in developing potential new candidates for beta-cell substitution and therapy for patients.
Immunoprotection strategies include immunomodulatory therapies and immunoisolation techniques. Immunotherapies aim to down-regulate the autoimmune response to pancreatic self-antigens and arrest beta-cell destruction. Ideally, this type of technique would induce prolonged remission from type 1 diabetes and achieve a cure[18]. As regards the separation of implanted cells from the host immune system, this has been recognized as an experimental strategy to prevent immunorecognition, rejection and avoid lifelong immune suppression. A bioartificial pancreas tries to create a barrier to immune cells while allowing sufficient oxygen and nutrients transfer. Immunoisolation strategies facilitate islet transplantation without the need of immunosuppression[19].
Transplantation of pancreatic islets is a less invasive procedure than pancreas transplantation, with a shorter hospital stay and lower morbidity. This therapeutic option is reserved for patients with severe glycaemic variability, progressive diabetic complications and life threatening hypoglycemia[19]. Successful islet transplantation was established in the early 70s in diabetic rats[20] and rhesus monkeys[21]. Najaran et al[22] reported the first significant case of human islet transplantation in patients with chronic pancreatitis. These patients underwent total or near total pancreatectomy, followed by autologous islet transplantation which prevented the development of diabetes. Thereafter, allograft was attempted in selected patients with type 1 diabetes. Unfortunately, out of the 237 allografts transplanted from 1990 to 1999, only 16% have resulted in insulin-independence for more than 1 week, and only 11% for more than 1 year[23]. Important progress was made thanks to improvements in techniques for isolating human islets[24,25] and to the availability of new and more effective immunosuppressive agents.
A positive turn in islet transplantation occurred in 2000, when James Shapiro and his colleagues treated 7 diabetic patients with severe hypoglycemia with allogeneic islets and a novel immunosuppressive regimen, obtaining insulin-independence in all the transplanted patients at a median follow-up period of 11.9 mo[11]. This success was due to several changes in the transplantation procedure, such as the large number of infused islets (from 2-4 donors for each recipient), an immunosuppressive regimen with inclusion of sirolimus and without glucocorticoids and the limited cold ischemia time of the recovered pancreases. A follow-up report monitored 65 transplant recipients for a period of 5 years. This study showed that 80% of the transplanted patients remained insulin-independent at 1 year, but only 10% retained an insulin-free status at 5 years. However partial graft function allowed improvement of glycaemic control with a decreased occurrence of hypoglycemic episodes. Recent results for islet transplantation demonstrate major improvement in outcomes. Analysis of transplantations performed by Collaborative Islet Transplant Registry (CITR) from 1999 to 2010 showed that the insulin independence rate at 3 years after transplantation increased from 27% in 1999-2003 to 44% in 2007-2010 and at 4 years approximately 90% of the grafts showed some degree of function[26].
All these studies indicated that islet transplantation is a promising strategy for treatment of type 1 diabetes. However, there are several challenges limiting widespread application. The disadvantages of the current approach is the limited supply and suboptimal yields of procurement and isolation of islets, graft failure and relatively high requirements to achieve prolonged insulin independence and glucose stability[27]. Poor vascularization and hypoxia of the transplanted islets[28], destruction by autoimmunity and allorejection[29] and exposure to the toxic effects of immunosuppressive agents[30] are thought to contribute to early graft failure. Better protection of the transplanted islets and improved immunosuppression are current strategies under investigation that could substantially advance islet transplantation as an acceptable alternative treatment. Mesenchymal stromal cells have been proposed to be one possible means to enhance islet transplantation protocols[31].
MSCs are multipotent, self-renewing cells that reside mainly in the bone marrow, representing only 0.001%-0.01% of nucleated marrow cells. They can be also isolated from other tissues, including skeletal muscle[32], adipose tissue[33], amniotic fluid[34] and umbilical cord blood[35] and expanded for several passages without losing their self-renewing capacity[36,37]. The International Society for Cellular Therapy has defined criteria to define the MSC population, including adherence to plastic in culture, expression of cell surface markers, such as CD105, CD73 and CD90, and lack of expression of CD45, CD34, CD14 or CD11b, CD79a or CD19 and HLA-DR surface molecules[38]. MSCs have been well characterized for their ability to differentiate into several cell types of mesenchymal origin, such as osteoblasts, adipocytes and chondrocytes[38], but it has been also demonstrated that they have the capacity to differentiate into cell types of endodermal and ectodermal lineages, including lung epithelial cells[39], retinal pigment[40], skin[41], sebaceous duct cells[42], renal tubular cells[43], neural cells[44], hepatocytes[45] and insulin producing cells[46]. However, an intense debate about the contribution of MSCs to form functional tissue through transdifferentiation processes is still open[47]. Aside to their ability to differentiate into many types of cells, MSCs can also have a reparative effect through the migration to the site of injury[48] and the release of paracrine factors that affect cell migration, proliferation and survival of the surrounding cells[49]. In addition, MSCs have been shown to contribute to repair processes through the secretion of pro-angiogenic molecules, thus promoting the formation of new blood vessels in vivo[50]. Moreover, MSCs have emerged as a useful cell population for immunomodulation therapy thanks to their ability to secrete a large amount of bioactive molecules that affect immune and inflammatory responses[51]. The combination of tissue regenerative potential and immunomodulatory or immunosuppressive activity has prompted therapeutic interest.
Normally pancreatic islets have a rich vascular supply within the pancreas to support their metabolic activity and to facilitate rapid dispersal of secreted hormones. Large islets are supplied by 1-3 arterioles that penetrate the B cell-rich islet core and distribute into a dense network of sinusoidal capillaries connected to venules in the islet periphery[52]. Islets receive considerably more blood flow than surrounding pancreatic exocrine tissue[53] and islet capillaries are much more permeable than exocrine capillaries due to the presence of 10 times as many small pores within their endothelial cells[54]. Relatively strong expression of VEGF by islets is probably responsible for the high degree of vascularization and fenestration. Depletion of VEGF in β cells in mice reduces vascular density and permeability to the level of exocrine tissue and partly impairs insulin secretion[55]. The islet vasculature degenerates during the process of isolation and transplantation and the islets must initially rely on diffusion of oxygen and nutrients from the culture medium and from vessels in the transplant environment for their survival[56,57]. This condition leads to prolonged hypoxia that, at the early post-transplant stages, is considered a major reason for early islet graft loss. The vessel density and oxygen tension in transplanted islets are less than half compared with islets in the native pancreas[58]. Further compromising islet graft survival in this context is their vulnerability to oxidative stress, a consequence of relatively low expression of antioxidant enzymes[59]. Thus, transgenic islet expression of antioxidant enzymes, such as glutathione peroxidase, could be a possible solution. However, a potential drawback of this approach is that glutathione peroxidase removes H2O2, an inducer of VEGF synthesis[60], and thus may further impair islet graft revascularization. The net result of oxidative and other challenges is that more than 70% of islets transplanted intraportally fail to become stably engrafted[61].
VEGF is a multi-functional angiogenic regulator that stimulates blood vessel formation, endothelial cell survival and epithelial cell proliferation[62]. The receptors of VEGF are predominantly expressed on vascular endothelial cells[62] and are also expressed in pancreatic islets[63]. Several line of evidence indicates that insufficient expression of VEGF limits the rate and extent of islet graft revascularization. Transplanted islets show a significant reduction of VEGF expression at day 3-4 after transplantation[64] while an over expression of VEGF markedly improves the degree of revascularization and function of islet grafts. Mouse islets transfected with an adenovirus carrying the cDNA for the human VEGF165 isoform were transplanted under the kidney capsule of diabetic nude mice. Vascular endothelial growth factor (VEGF) expression resulted in an increase in both islet graft mass and revascularization and, unlike vector-transfected grafts, rapidly returned recipient to stable normoglycaemia[65].
Several bone marrow subpopulations, such as endothelial progenitor cells and MSCs may be able to differentiate into one or more of the cellular compartments of the vascular bed[66,67]. MSCs are known to secrete VEGF and other growth factors and to enhance proliferation of endothelial cells and smooth muscle cells[68]. MSCs release a wide array of cytokines that support hematopoietic stem and progenitor cell development, as well as the secretion of other cytokines that are relevant to increasing blood flow to ischemic tissue[69]. Moreover, MSCs secrete several important arteriogenic cytokines, including VEGF and monocyte chemoattractant protein-1 (MCP-1). In mice undergoing distal femoral artery ligation, a model of hind limb ischemia, local injection of MSCs increased adductor muscle levels of VEGF and fibroblast growth factor (FGF) proteins compared with controls, and co-localization of VEGF and transplanted MSCs within adductor tissue was demonstrated[68].
Recently it has been reported that in animal models, MSCs are able to enhance survival and function of islet graft by increasing islet revascularization[70]. Consistent with these studies, our group showed that cultured MSCs express high level of VEGF and that transplantation of those MSCs elicited a robust host angiogenic response leading to neovascularization of syngeneic islet grafts in diabetic rats. This effect may serve to increase local perfusion of the islets and ameliorate their metabolic activity[71]. Similar results were obtained in a preclinical model by Berman et al[72] that demonstrated enhanced islet engraftment and function at 1 mo post-transplant in a cynomologus monkey model of allogeneic islet-MSCs transplantation. The authors hypothesized that MSCs enhance islet engraftment by staying in proximity to the islets at the time of cotransplantation, providing revascularization and regenerative signals. MSCs provided an important approach for enhancement of islet engraftment, thereby decreasing the numbers of islets needed to achieve insulin independence[72].
In summary, MSCs cotransplanted with islets in type 1 diabetic recipients can facilitate islet revascularization, engraftment and improved islet function: Consequently, the presence of MSCs permit to reduce the islet number required for reversal of diabetes. Therefore, cotransplantation of MSCs with islets could facilitate islet engraftment and improve islet graft function in clinical islet transplantation.
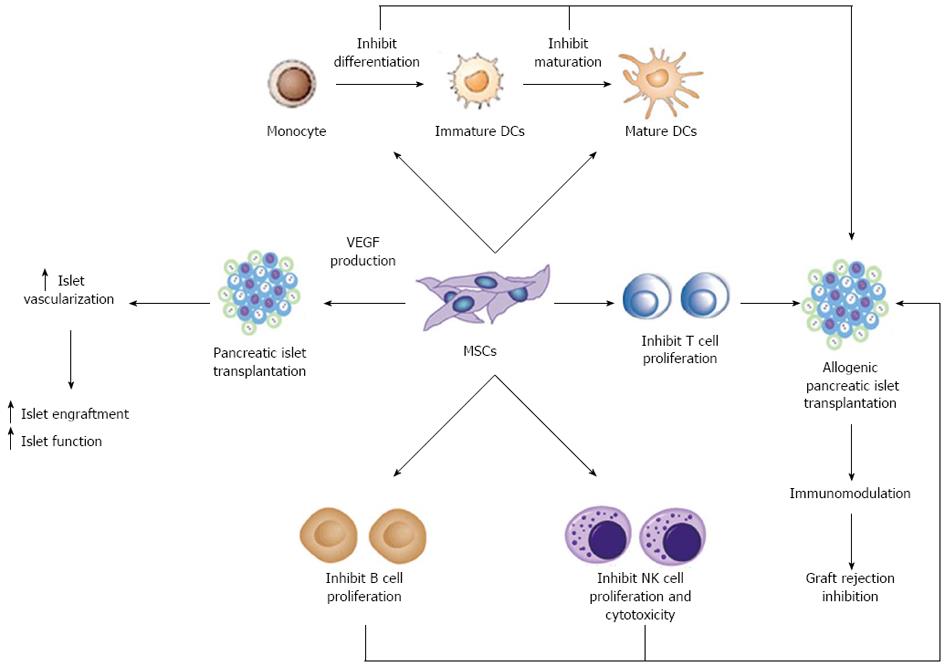
One of the most promising aspects of MSCs regards their dynamic role in modulating the immune system. MSCs are not only immunoprivileged cells, due to the low expression of class II Major Histocompatibilty Complex (MHC-II) and co-stimulatory molecules in their cell surface, but they also interfere with different pathways of the immune response by means of direct cell-to-cell interactions and soluble factor secretion. As schematically represented in Figure 1, it is well established that MSCs can exert immunosuppressive activity on T cells[73] and interfere with dendritic cell (DC) maturation[74]. Furthermore, MSCs may modulate natural killer (NK) cell cytotoxic activity, B cell proliferation and immunoglobulin production.
MSCs have been shown to suppress autoreactive T-cell responses in models of autoimmunity such as experimental autoimmune encephalomyelitis[75], collagen-induced arthritis[76] and autoimmune enteropathy[77]. Type 1 diabetes is one of the most prevalent autoimmune diseases in childhood. The effector mechanisms of immune-mediated destruction of islet β cells are complex, but an essential early event is the activation of islet cell antigen reactive T cells. Recently, the therapeutic benefit of MSCs has been evidenced in the treatment of type 1 diabetes. Lee et al[78] used immunodeficient recipient mice chemically induced by streptozotocin to study the effect of human MSCs in the development of diabetes. Infusion of hMSCs reduced glycaemic levels and increased peripheral insulin levels. In the pancreas of these mice the islets appeared larger compared with islets from untreated diabetic mice[78]. In experimental mouse models, intravenously infused MSCs are capable of migrating to pancreatic islets[48]. However, the role of MSCs in β cell replacement is controversial. Some evidence suggests the possibility that MSCs differentiate into islet β cells[48]. In addition, similar results were reported by Ezquer et al[79] in a model of streptozotocin-induced diabetes. Reversion of hyperglycemia and glycosuria was observed after injection of MSCs, with increased morphologically normal β pancreatic islets. Other reports have contradicted these findings suggesting that MSCs could be feeder cells for islet differentiation, proliferation and vascularization, but do not differentiate into β cells[80].
MSCs may also offer therapeutic opportunities in transplantation by directly targeting alloreactive T cells. MSCs are immunosuppressive in vitro and, in mixed-lymphocyte reactions, suppress T-cell proliferation[73] through soluble factors, including 2,3-dioxygenase (IDO), prostaglandin-E2 (PGE2), nitric oxide, transforming growth factor β (TGF β) and hepatocyte growth factor (HGF)[81,82]. Neutralizing antibodies against TGF β and HGF can restore the MSC-induced suppression of T cell proliferation[73]. In a model of allogenic pancreatic islet transplantation, the administration of MSCs resulted in the prolonged survival of islets and led to long-term stable normoglycemia[83]. In this study MSCs were colocalized at the graft site where they locally produced immunosuppressive matrix metalloproteinase-2 and-9 that block the activation and expansion of alloreactive T cells[83]. In a most recent paper, using a rat model of streptozotocin induced diabetes, the authors found that MSCs significantly improved glycemic control and reduced graft infiltration by immune cells in either allogenic or syngeneic pancreatic islet transplantation[84]. They found that MSCs were effective when administrated either locally or systematically. The modulation of acute rejection that the authors observed after islet transplantation may indicate that soluble factors are released by MSCs to several organs after their systemic administration.
Additional studies revealed that MSCs might produce this anti-proliferative effect via induction of anergy in the T cell population[85], T cell tolerance[75], or by inducing proliferation of regulatory T cell populations[86]. Berman et al[72] first reported increased numbers of Treg in a MSC allogeneic islet transplant preclinical model. MSCs treatment significantly enhanced islet engraftment and function at 1 mo post-transplant, as compared with animals that received islets without MSCs. Additional infusions of donor or third-party MSCs resulted in reversal of rejection episodes and prolongation of islet function. Stable islet allograft function was associated with increased numbers of regulatory T-cells in peripheral blood[72].
The immune response is related not only to T cells, but to the interaction between DC cells and T cells[87]. DCs are antigen-presenting cells (APCs) capable of stimulating both naïve and memory T cells. MSCs affect the differentiation, maturation and function of DCs at different levels[74,88]. MSCs have also been shown to alter the cytokine secretion profile of DCs toward up-regulation of regulatory cytokines, such as IL-10, and down regulation of inflammatory cytokines such as IFNγ, IL-12 and TNFα, inducing a more anti-inflammatory or tolerant dendritic cell phenotype[74,89]. Studies in animal models suggest that DC based immunotherapeutic strategies might also be utilized to facilitate islet transplant tolerance[90,91]. Li et al[92] demonstrated that in mice with combined transplantation of pancreatic islets and MSCs, the expression of CD11c (DCs phenotype derived from monocytes) and CD83 (mature DCs phenotype) was down regulated markedly. This finding showed that MSCs inhibit the maturation of DCs and the stimulation of T cell was weakened, resulting in survival of transplanted pancreatic islets.
Autoimmunity also involves B cells by antibody production. The interaction between MSCs and B cells is not yet completely understood. However, co-culture experiments with these two cells using both mouse and human cells showed that MSCs inhibit B cell proliferation[93]. They also observed that MSCs affect chemotactic properties of B cells while B-cell co-stimulatory molecule expression and cytokine production were unaffected by MSCs.
Finally, NK cells are key effector cells of innate immunity. MSCs alter the function of NK cells by suppressing their proliferation, and cytotoxicity. Spaggiari et al[88] demonstrated that cytokine induced proliferation of freshly isolated NK cells was inhibited in the presence of MSCs.
Thanks to their interactions with many different types of immune cells, MSCs administrated in conjunction with islet cell transplantations could prevent immune rejection and promote long term islet allograft survival.
In summary, current data suggest that MSCs have the potential to aid in the treatment of type 1 diabetes and overcome some of the current limitations to islet transplantation. These cells may exert beneficial pro-angiogenic and immunomodulatory effects when co-transplanted with pancreatic islets. The pro-angiogenic effects result from the release of angiogenic factors from MSCs that have been shown to improve islet vascularization and graft function in islet transplantation. The immunomodulatory properties of MSCs may help in reducing inflammatory damage to the islets in the early peritransplant period. MSCs may also reduce autoimmunity through their capacity to inhibit T cell proliferation and suppress differentiation and maturation of dendritic cells.
These data encourage further preclinical co-transplantation of MSCs and pancreatic islets to improve the outcome of allogeneic islet transplantation in the treatment of type 1 diabetes. However, some key issues need to be addressed before MSC based therapies become a safe option for clinical studies. Most importantly, it is unclear if co-transplanted MSCs engraft and differentiate at the implantation site. Thus, the long-term stability of MSC activity and function after transplantation should be assessed in vivo. In addition, the selection of a suitable donor MSC source may differ if the treatment aims at modulating the autoimmune disease or enhancing pancreatic islet engraftment and vascularization. Therefore, whether autologous or allogeneic MSCs are suitable as a donor source should be selected according to the specific aim of the study.
| 1. | Miao G, Ostrowski RP, Mace J, Hough J, Hopper A, Peverini R, Chinnock R, Zhang J, Hathout E. Dynamic production of hypoxia-inducible factor-1alpha in early transplanted islets. Am J Transplant. 2006;6:2636-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15362] [Article Influence: 569.0] [Reference Citation Analysis (2)] |
| 3. | Hematti P, Kim J, Stein AP, Kaufman D. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev (Orlando). 2013;27:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 Suppl 2:S97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1172] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 5. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67-S74. [PubMed] |
| 6. | Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Nokoff N, Rewers M. Pathogenesis of type 1 diabetes: lessons from natural history studies of high-risk individuals. Ann N Y Acad Sci. 2013;1281:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Peng H, Hagopian W. Environmental factors in the development of Type 1 diabetes. Rev Endocr Metab Disord. 2006;7:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Golden SH, Sapir T. Methods for insulin delivery and glucose monitoring in diabetes: summary of a comparative effectiveness review. J Manag Care Pharm. 2012;18:S1-17. [PubMed] |
| 10. | Boggi U, Rosati CM, Marchetti P. Follow-up of secondary diabetic complications after pancreas transplantation. Curr Opin Organ Transplant. 2013;18:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3880] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 12. | Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med. 2013;2:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Narushima M, Kobayashi N, Okitsu T, Tanaka Y, Li SA, Chen Y, Miki A, Tanaka K, Nakaji S, Takei K. A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol. 2005;23:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Ravassard P, Hazhouz Y, Pechberty S, Bricout-Neveu E, Armanet M, Czernichow P, Scharfmann R. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121:3589-3597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 15. | Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific α- to-β-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011;25:1680-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1317] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 18. | Waldron-Lynch F, Herold KC. Immunomodulatory therapy to preserve pancreatic β-cell function in type 1 diabetes. Nat Rev Drug Discov. 2011;10:439-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | O’Sullivan ES, Vegas A, Anderson DG, Weir GC. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev. 2011;32:827-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175-186. [PubMed] |
| 21. | Scharp DW, Murphy JJ, Newton WT, Ballinger WF, Lacy PE. Transplantation of islets of Langerhans in diabetic rhesus monkeys. Surgery. 1975;77:100-105. [PubMed] |
| 22. | Najarian JS, Sutherland DE, Baumgartner D, Burke B, Rynasiewicz JJ, Matas AJ, Goetz FC. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg. 1980;192:526-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Corrêa-Giannella ML, Raposo do Amaral AS. Pancreatic islet transplantation. Diabetol Metab Syndr. 2009;1:9. [PubMed] |
| 24. | Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes. 1997;46:1120-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 181] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM, Rajotte RV. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999;8:285-292. [PubMed] |
| 26. | Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35:1436-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 545] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 27. | Hatziavramidis DT, Karatzas TM, Chrousos GP. Pancreatic islet cell transplantation: an update. Ann Biomed Eng. 2013;41:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Ritz-Laser B, Oberholzer J, Toso C, Brulhart MC, Zakrzewska K, Ris F, Bucher P, Morel P, Philippe J. Molecular detection of circulating beta-cells after islet transplantation. Diabetes. 2002;51:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Blondet JJ, Carlson AM, Kobayashi T, Jie T, Bellin M, Hering BJ, Freeman ML, Beilman GJ, Sutherland DE. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am. 2007;87:1477-501, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Alejandro R, Barton FB, Hering BJ, Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Vija L, Farge D, Gautier JF, Vexiau P, Dumitrache C, Bourgarit A, Verrecchia F, Larghero J. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999;65:22-26. [PubMed] |
| 33. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5835] [Article Influence: 233.4] [Reference Citation Analysis (1)] |
| 34. | In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR, Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 585] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 36. | Polak JM, Bishop AE. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci. 2006;1068:352-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Raghunath J, Salacinski HJ, Sales KM, Butler PE, Seifalian AM. Advancing cartilage tissue engineering: the application of stem cell technology. Curr Opin Biotechnol. 2005;16:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13044] [Article Influence: 686.5] [Reference Citation Analysis (12)] |
| 39. | Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407-8411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 536] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 44. | Muñoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585-4595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 255] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 46. | Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 47. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1423] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 48. | Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 444] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 49. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2126] [Cited by in RCA: 2250] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 50. | Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1192] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 51. | Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 52. | Konstantinova I, Lammert E. Microvascular development: learning from pancreatic islets. Bioessays. 2004;26:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Lifson N, Lassa CV, Dixit PK. Relation between blood flow and morphology in islet organ of rat pancreas. Am J Physiol. 1985;249:E43-E48. [PubMed] |
| 54. | Bearer EL, Orci L. Endothelial fenestral diaphragms: a quick-freeze, deep-etch study. J Cell Biol. 1985;100:418-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 300] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 56. | Emamaullee JA, Rajotte RV, Liston P, Korneluk RG, Lakey JR, Shapiro AM, Elliott JF. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54:2541-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 723] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 60. | Grzenkowicz-Wydra J, Cisowski J, Nakonieczna J, Zarebski A, Udilova N, Nohl H, Józkowicz A, Podhajska A, Dulak J. Gene transfer of CuZn superoxide dismutase enhances the synthesis of vascular endothelial growth factor. Mol Cell Biochem. 2004;264:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Boker A, Rothenberg L, Hernandez C, Kenyon NS, Ricordi C, Alejandro R. Human islet transplantation: update. World J Surg. 2001;25:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2741] [Cited by in RCA: 2698] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 63. | Kuroda M, Oka T, Oka Y, Yamochi T, Ohtsubo K, Mori S, Watanabe T, Machinami R, Ohnishi S. Colocalization of vascular endothelial growth factor (vascular permeability factor) and insulin in pancreatic islet cells. J Clin Endocrinol Metab. 1995;80:3196-3200. [PubMed] |
| 64. | Vasir B, Jonas JC, Steil GM, Hollister-Lock J, Hasenkamp W, Sharma A, Bonner-Weir S, Weir GC. Gene expression of VEGF and its receptors Flk-1/KDR and Flt-1 in cultured and transplanted rat islets. Transplantation. 2001;71:924-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, Zhang H, Song K, Meseck M, Bromberg J. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 66. | Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6624] [Cited by in RCA: 6380] [Article Influence: 220.0] [Reference Citation Analysis (1)] |
| 67. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585-592. [PubMed] |
| 70. | Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 71. | Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, Remuzzi A, Benigni A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 72. | Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O’Connor DH, Bartholomew AM. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59:2558-2568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 73. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2378] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 74. | Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080-2087. [PubMed] |
| 75. | Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1061] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 76. | Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 441] [Article Influence: 23.2] [Reference Citation Analysis (1)] |
| 77. | Parekkadan B, Tilles AW, Yarmush ML. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008;26:1913-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438-17443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 551] [Article Influence: 27.6] [Reference Citation Analysis (1)] |
| 79. | Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631-640. [PubMed] |
| 80. | Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes. 2004;53:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 81. | Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 82. | Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 83. | Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 84. | Longoni B, Szilagyi E, Quaranta P, Paoli GT, Tripodi S, Urbani S, Mazzanti B, Rossi B, Fanci R, Demontis GC. Mesenchymal stem cells prevent acute rejection and prolong graft function in pancreatic islet transplantation. Diabetes Technol Ther. 2010;12:435-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 841] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 86. | Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516-525. [PubMed] |
| 87. | Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;39:2325-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 782] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 89. | Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 486] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 90. | Oluwole OO, Depaz HA, Gopinathan R, Ali A, Garrovillo M, Jin MX, Hardy MA, Oluwole SF. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific permanent acceptance of rat islets by adoptive transfer of allopeptide-pulsed host myeloid and thymic dendritic cells. Diabetes. 2001;50:1546-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60:1366-1370. [PubMed] |
| 92. | Li FR, Wang XG, Deng CY, Qi H, Ren LL, Zhou HX. Immune modulation of co-transplantation mesenchymal stem cells with islet on T and dendritic cells. Clin Exp Immunol. 2010;161:357-363. [PubMed] |
| 93. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1277] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
P- Reviewers: Chang FC, Sanlioglu AD, Sumi S S- Editor: Song XX L- Editor: O’Neill M E- Editor: Zhang DN