Published online Jan 26, 2013. doi: 10.4252/wjsc.v5.i1.34
Revised: November 14, 2012
Accepted: December 20, 2012
Published online: January 26, 2013
AIM: To assess the capacity to isolate and expand mesenchymal stem cells (MSC) from bone marrow of CBA/Ca, ICR and Balb/c mice.
METHODS: Bone marrow of tibia and femur were flushed, cultured and maintained in supplemented Dulbecco’s modified Eagle’s medium. MSC immunophenotype of cultures were tracked along increasing passages for positivity to CD106, Sca-1 and CD44 and negativity to CD45, CD11b and MHC class II. Differentiation capacity of MSC towards osteogenic and adipogenic lineages were also assessed.
RESULTS: MSC were successfully cultured from bone marrow of all 3 strains, albeit differences in the temporal expression of certain surface antigens. Their differentiation into osteocytes and adipocytes were also observed. MSC from all 3 mouse strains demonstrated a shift from a haematopoietic phenotype (CD106-CD45+CD11b+Sca-1low) to typical MSC phenotype (CD106+CD45-CD11b-Sca-1high) with increasing passages.
CONCLUSION: Information garnered assists us in the decision of selecting a mouse strain to generate MSC from for downstream experimentation.
- Citation: Ooi YY, Rahmat Z, Jose S, Ramasamy R, Vidyadaran S. Immunophenotype and differentiation capacity of bone marrow-derived mesenchymal stem cells from CBA/Ca, ICR and Balb/c mice. World J Stem Cells 2013; 5(1): 34-42
- URL: https://www.wjgnet.com/1948-0210/full/v5/i1/34.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i1.34
Mesenchymal stem cells (MSC) are a great therapeutic interest for tissue regeneration and immunomodulation. To elucidate the role of MSC in these two paradigms, experimentation is often performed with MSC generated from mouse bone marrow. For this, researchers have a choice to make in selecting the mouse strain to generate their MSC from. Here, we provide a report on the comparative description of mouse MSC derived from 3 different strains under the same experimental conditions. The mouse strains assessed were CBA/Ca, ICR and Balb/c.
MSC are stromal cells with self-renewal and differentiation properties[1]. MSC have been isolated from various tissues including bone marrow, adipose, skin, bone, synovial membrane, placenta and umbilical cord[2-4] and proliferate in vitro as plastic-adherent cells with fibroblast-like morphology[5]. The long term self-renewal and multilineage differentiation properties of MSC reflect their potential for tissue regeneration and cell/gene therapy-based treatment of diseases[6] including acute graft-vs-host disease[7], osteogenesis imperfecta[8], cardiomyopathy[9] and Crohn’s disease[10]. Recent findings that MSC have immunosuppressive properties[11-14] have also increased the need for a suitable supply of MSC for experimental research. The expansion of undifferentiated MSC is a research tool for functional and genetic studies for subsequent development of preclinical protocols to treat a wide range of diseases. Murine sources of MSC are of great use for the extensive preclinical studies that are required within this research area.
Murine MSC is commonly isolated from the bone marrow aspirates of the femur and tibia. Studies demonstrate that isolation of MSC from murine bone marrow using standard methods usually results in a heterogeneous cell population with a high degree of non-mesenchymal contaminants[2,15-17]. Furthermore, there are reports of preferential growth of MSC from different mouse strains[17]. MSC isolated from four different inbred mouse strains C57Bl/6J, Balb/c, FVB/N and DBA1 showed great variation in growth rate, differentiation capacity and immunophenotype[17]. In this study, we compare phenotypic characteristics of MSC derived from bone marrow of 3 strains of mice (CBA/Ca, ICR and Balb/c) to determine a suitable source for these cells for downstream experimentation. As MSC that are cultured lack specific markers[5], cells were immunophenotyped for a range of surface markers classically associated with MSC expression. The differentiation capacity of MSC confirmed the multipotency of these stem cells. Although there were differences in temporal pattern of surface marker expression and preferential differentiation, bone marrow cultures from all 3 strains of mice tested successfully yielded MSC.
Bone marrow-derived MSC were generated from 3 different strains of mice, namely CBA/Ca, Balb/c and ICR. Mice used were male and between the ages of 6-10 wk. Animals were sacrificed by a CO2 overdose and bone marrow cells were obtained by flushing femurs and tibias with Dulbecco’s modified Eagle’s medium (DMEM). Cells were centrifuged at 289 g for 10 min, resuspended and seeded into three 25 cm² flasks in DMEM with high glucose supplemented with 10 nmol/mL GlutaMAX™-I Supplement, 100 U/mL penicillin and 100 μg/mL streptomycin, 1 mL/L gentamycin, 250 μg/mL Fungizone (all Invitrogen), 1.5 g/L sodium bicarbonate and 15% Mesenchymal Stem Cell Stimulatory Supplement (STEMCELL Technologies, Canada). Cultures were maintained at 37 °C in 95% humidified air and 5% CO2. After 48 and 72 h, cells were washed gently using 1X PBS and replaced with fresh culture media. Adherent cells were further cultured with a medium change every 3-4 d. At approximately 80% confluency (occasionally localised), cells were harvested by treating with 0.25% trypsin containing 1 mmol/L EDTA for 5 min at 37 °C. Trypsin was neutralised with culture medium and detached cells were replated in a new 25 cm2 culture flask. Cell cultures were routinely assessed using an inverted phase contrast microscope and cell viability was determined via trypan blue staining.
The following antibodies were obtained from Becton Dickinson: fluorescein isothiocyanate-conjugated anti-mouse CD106 (vascular adhesion molecule-1), CD11b, and MHC I; phycoerythrin-conjugated anti-mouse CD45, Sca-1, and MHC II and allophycocyanin-conjugated anti-mouse CD44. Cells were trypsinised, washed with 0.1% BSA/PBS and incubated with antibody (1 μL per 106 cells) for 30 min at 4 °C. Cells were then resuspended in 0.1% BSA/PBS and analysed by a FACS Calibur cytometer (BD Biosciences, San Jose, CA) using CellQuest Pro software. Ten thousand gated events were recorded. For each antibody, gating was determined based on appropriate isotype-stained controls.
Mouse bone marrow cultures of passages 10-16 were plated at a density of 6 × 104 cells/well in a 24-well plate and incubated at 37 °C in 95% humidified air, 5% CO2 till confluency was reached. For adipocytic differentiation, the Mesenchymal Stem Cell Adipogenesis Kit (Chemicon; Cat. No. SCR020) was used. Briefly, cells were stimulated with induction medium (DMEM with high glucose supplemented with 10% FBS and 1X penicillin and streptomycin, 1 μmol/L dexamethasone, 0.5 mmol/L IBMX, 10 μg/mL insulin and 50 μmol/L indomethacin) and maintained with maintenance medium (DMEM with high glucose supplemented with 10% FBS and
10 μg/mL insulin) according to kit instructions. For analysis, cells were fixed with 4% paraformaldehyde and stained with 0.36% Oil Red O solution for 50 min. For osteogenic differentiation, the Mesenchymal Stem Cell Osteogenesis Kit (Chemicon; Cat. No. SCR028) was used. Briefly, cover slips were coated with 12 μg/mL vitronectin and 12 μg/mL collagen prior to cell seeding. Cells were then stimulated with induction medium (DMEM with high glucose supplemented with 10% FBS and 1X penicillin and streptomycin, 0.1 μmol/L dexamethasone,
0.2 mmol/L ascorbic acid 2-phosphate, 10 mmol/L glycerol 2-phosphate and 1X glutamine). Cells were replaced with fresh induction medium every 2 d. For analysis, osteocytes were fixed with 70% ethanol and stained with Alizarin Red S. For both differentiation assays, controls were MSC cultures without adipocytic/osteogenic induction medium.
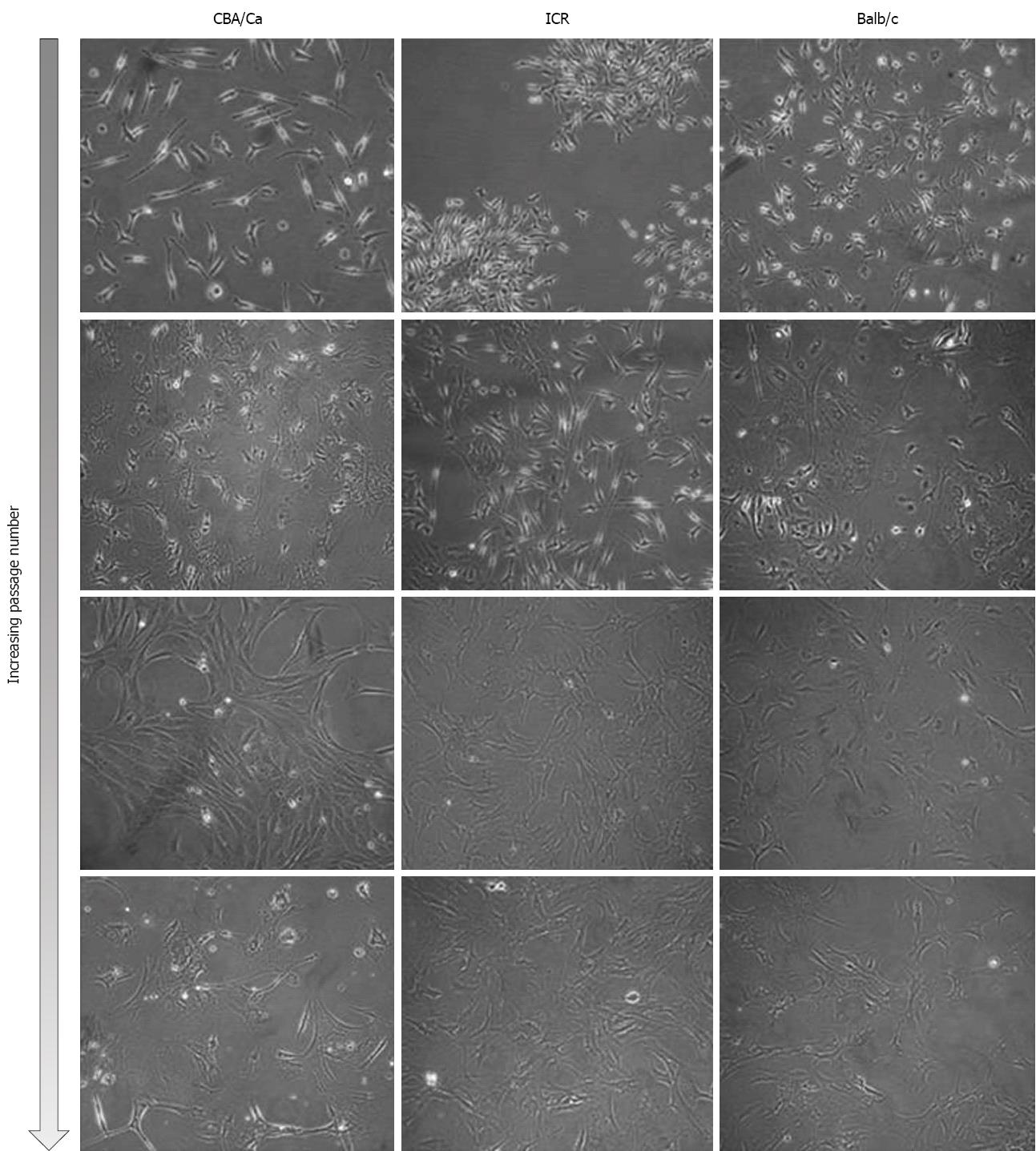
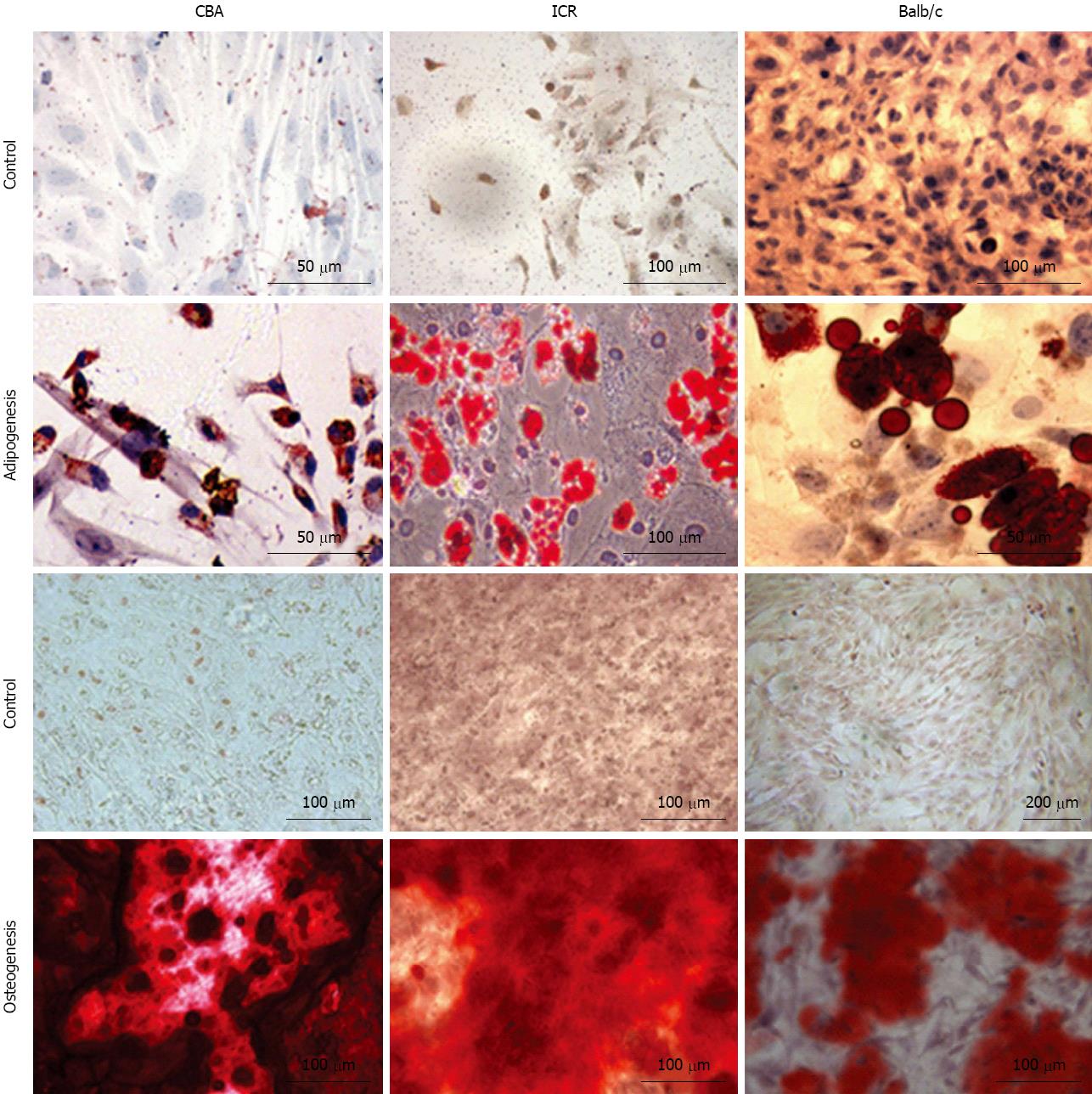
Bone marrow cells were isolated from the femurs and tibias of a total of 11 mice (4 ICR, 2 CBA/Ca and 5 Balb/c mice) and plated onto culture flasks. Non-adherent cells were removed by replacing media and cultures were observed via phase-contrast microscopy for their morphology. Initially, cells of all 3 strains of mice were small and exhibited spindle-shaped morphology (Figure 1, first and second row). Cells also tended to be locally confluent, growing in distinct colonies (as seen for ICR mice, first row; Balb/c mice, second row). A small number of round and flattened cells were observed within the colonies. As cells approached confluency within culture flasks, they were trypsinised and replated into new flasks. Bone marrow cultures became increasingly homogeneous, with cells displaying a fibroblast-shape (Figure 1, third and last row). At this point within the culture protocol, cells from all 3 mouse strains took 3-4 d to reach confluency within a 25 cm2 flask. Bone marrow cells continued to proliferate in culture beyond passage 35.
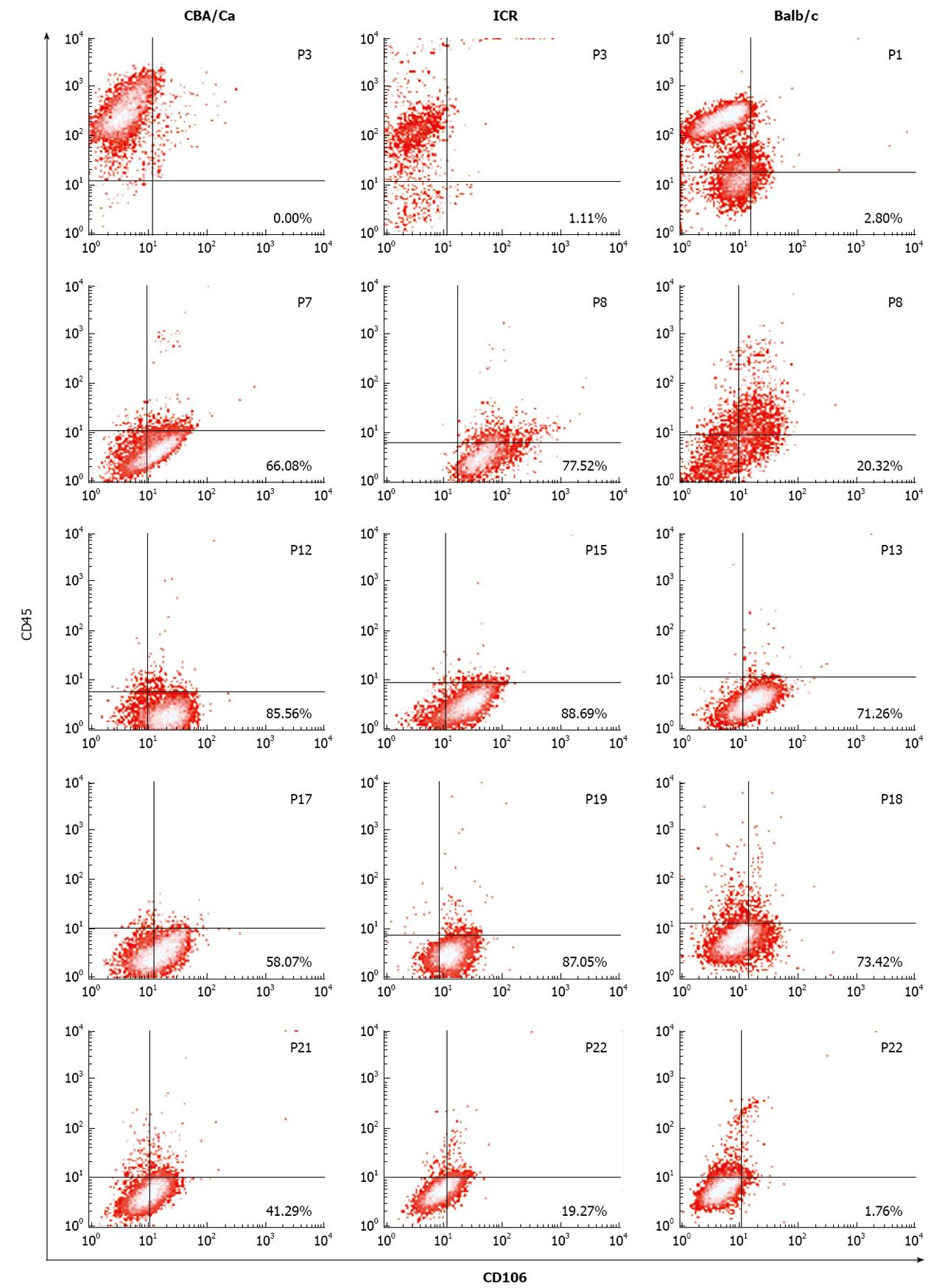
Immunophenotyping of bone marrow cultures derived from the 3 mouse strains were performed at various passages throughout the culture period. Presence of MSC were confirmed by positivity to CD106, CD44, Sca-1 and MHC I markers and negativity to CD45, CD11b, and MHC II by flow cytometry.
Flow cytometric analyses revealed that MSC demonstrated a shift from a haematopoietic phenotype to typical MSC phenotype with increasing passages. MSC of all 3 strains of mice showed this shift in phenotype from CD106-CD45+ cells at earlier passages to CD106+CD45- at later passages (Figure 2). ICR mice maintained their CD106 positivity for longer, remaining > 77% from passage 8 to 19. For CBA/Ca and Balb/c mice, CD106 expression began reverting after passage 17 and passage 18 respectively. Expression of CD45 (a common leukocyte antigen) was strong (70%-99%) at early passages for all 3 strains but decreased with increasing passages to < 9% positivity. Cultures derived from Balb/c mice took longer to lose their CD45 positivity compared to CBA/Ca and ICR strains (Figure 2).
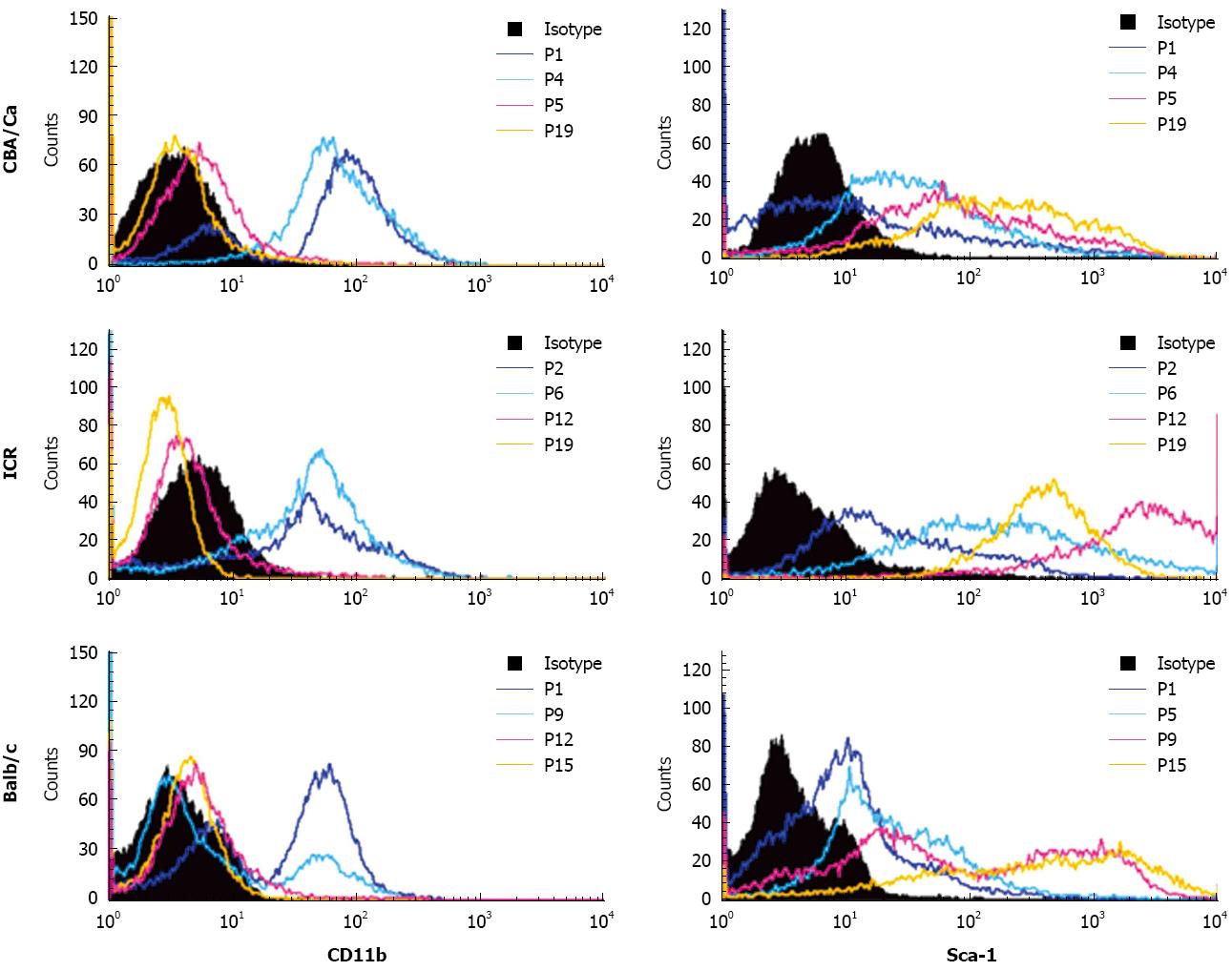
Similar to CD45, expression of haematopoietic marker CD11b also revealed a shift from CD11b+ to CD11b- with increasing passages (Figure 3). MSC cultures from all 3 strains of mice showed high positivity to CD11b (69%-99%) at early passages. Positivity for CD11b decreased with later passages. Cultures from CBA mice achieved CD11b- phenotype earlier than the other strains, beginning from passage 5. For the MSC marker Sca-1, cultures showed lower positivity to Sca-1 at early passages with only 24%-50% cells positive (Figure 3). Sca-1 expression increased to a higher percentage after passage 5 for CBA/Ca mice, passage 6 for ICR mice and passage 9 for Balb/c mice. At late passages, MSC from all 3 mouse strains showed strong positivity for Sca-1 at 84%-100%. ICR mice demonstrated a distinctly positive (96%-100%) population for Sca-1 after passage 6, while expression in CBA/Ca and Balb/c mice were 91%-97% after passage 15 and 84%-99% after passage 12 respectively.
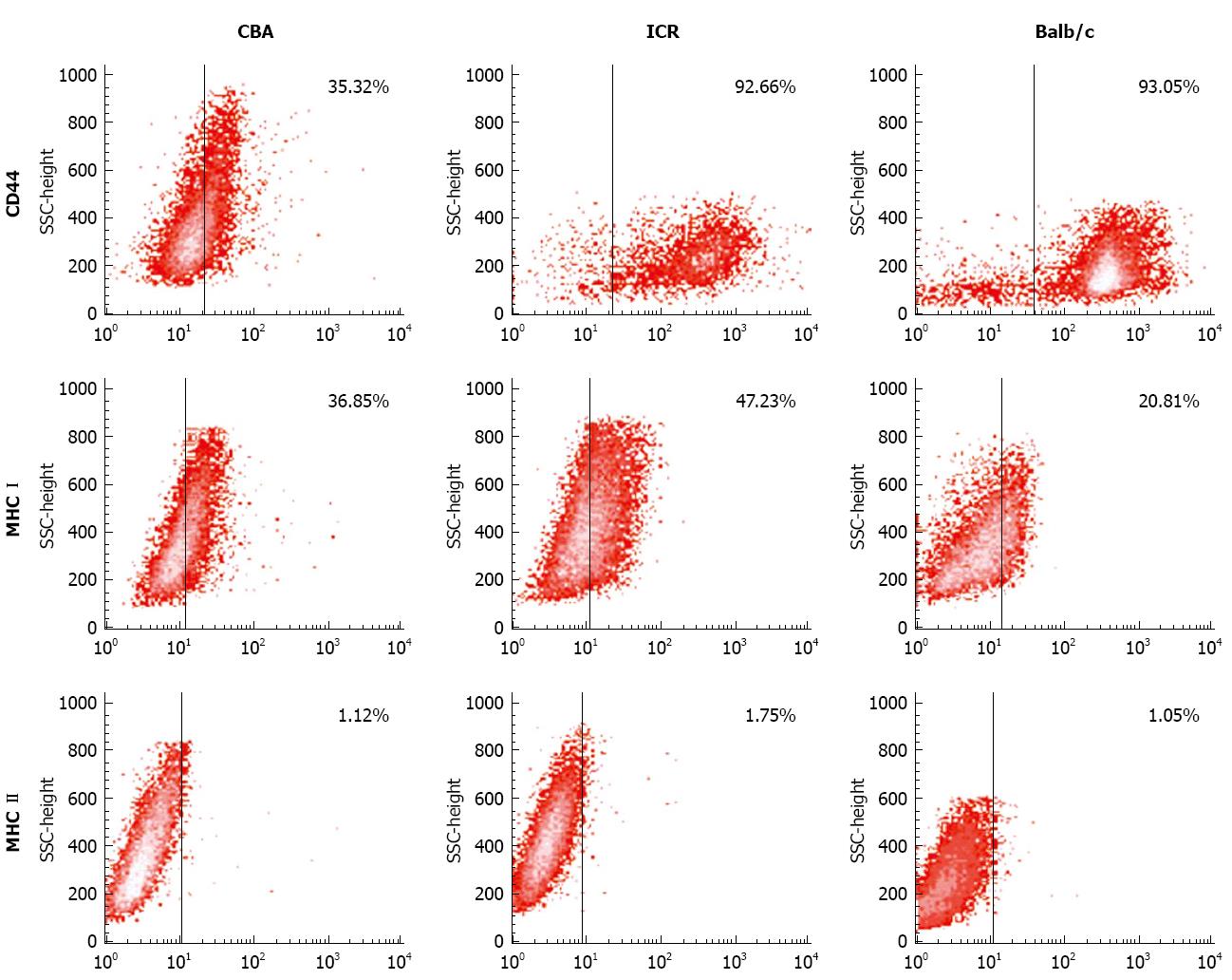
Next, CD44, MHC I and MHC II expression was examined in bone marrow cultures with CD106+CD45- phenotype. For CD44 expression, cells from ICR and Balb/c mice had the highest expression (77%-99% and 79%-99% accordingly), compared to CBA/Ca mice that had varying CD44 expression ranging from 13%-90% (Figure 4). All 3 strains of mice consistently demonstrated positivity to MHC I but not MHC II (< 2%) (Figure 4).
Results from MSC immunophenotyping shown above suggest that bone marrow cultures from all 3 strains of mice acquire an MSC phenotype (CD106+CD44+Sca-1+MHCI+CD45-CD11b-MHCII-) after multiple rounds of passaging. Also, amongst the 3 mouse strains tested, bone marrow cells for ICR and Balb/c maintained MSC phenotype for longer compared to CBA/Ca.
Bone marrow cultures from all 3 strains of mice differentiated into adipocytes and osteocytes when stimulated with adipocytic and osteogenic induction medium respectively (Figure 5). Three weeks of exposure to an adipocytic induction medium resulted in formation of lipid vacuoles in cultured MSC that could be observed with phase-contrast microscope. When cultured MSC were exposed to osteogenic induction medium, they aggregated and formed calcium deposits after 2 wk. Both of these cell differentiations were confirmed with cell-specific stains - Oil Red O for adipocytes and Alizarin Red S for osteocytes. Oil Red O-stained lipid vacuoles appeared bright red whereas Alizarin Red S stained precipitated calcium deposits dark red (Figure 5).
Isolation of mouse MSC is generally more difficult than human and rat MSC due to a high number of contaminating haematopoietic lineage cells and slow cell growth[2,15,17]. Studies have also demonstrated mouse strain-dependent variability of MSC in terms of growth kinetics, surface markers and potential for differentiation[16-18]. With the protocol described here for bone marrow-derived MSC expansion, we provide descriptive data that MSC can be successfully generated from 3 different mouse strains, namely CBA/Ca, ICR and Balb/c. Bone marrow cells were cultured and each culture was characterised for their morphology, immunophenotype and differentiation potential. MSC from all strains tested showed expression of relevant surface markers and differentiation potential into osteogenic and adipocytic cells, albeit some differences in terms of time to achieve MSC immunophenotype.
Isolation of MSC from mouse bone marrow usually results in a highly heterogenous cell population with a high degree of haematopoietic contaminants such as macrophages and fibroblasts[2,16]. Immunophenotyping analysis revealed early passages of our bone marrow cultures to be positive for haematopoietic markers CD45 and CD11b (a macrophage-specific marker). Continuous change of cell culture medium removes non-adherent haematopoietic populations from the cultures[3]. Passaging bone marrow cultures also helps eliminate haematopoietic contaminants and yield purer MSC cultures[17]. With these approaches, we accordingly observed the MSC cultures become increasingly homogenous at later passages with minimal CD45 expression profile by P7-P8, although it took a little longer for cells from Balb/c mice. Better quality MSC cultures were also obtained by discarding cells that remained attached to flasks following trypsinisation. These firmly adherent cells are probably contaminating fibroblasts and have been reported by other laboratories[17,19]. Expression of stem cell marker Sca-1 also increased at later passages. Sca-1 is a marker associated with MSC and also haematopoietic and endothelial progenitors[20]. MSC also expressed positivity to CD44 and CD106 and negativity to MHC II. To further encourage MSC growth and colony formation, we used 15% of a mouse mesenchymal supplement (MesenCult®, STEMCELL Technologies) instead of the routine 10% FBS to obtain enriched MSC cultures.
In addition to morphology and phenotype, the characterisation of MSC is complete with demonstration of their capacity for multilineage mesenchymal differentiation[15,18,19,21]. All 3 mouse strains exhibited multilineage potential in vitro, differentiating into adipocytes and osteocyte when stimulated with appropriate induction media. MSC from Balb/c mice more readily differentiate into adipocytes than the other strains, also shown by Peister and colleagues who found Balb/c more readily differentiating into adipose cells than C57Bl/6J and DBA 1 mice[17]. Conversely, MSC from Balb/c took longer to differentiate into osteocytes compared to the other 2 strains tested in our study. In view of the duration that MSC cultures remained in suitable phenotype and their ease to differentiation, our group has primarily utilised ICR mice for downstream experimentation where we show these stem cells to have immunomodulatory properties[13].
MSC from all 3 different strains of mice tested were suitable sources for bone marrow-derived MSC as they showed typical morphology, immunophenotype and differentiation capacities of MSC. However, bone marrow cultures from Balb/c mice took longer than the other strains to achieve MSC immunophenotype.
Experimental research concerning mesenchymal stem cells (MSC) relies heavily on cells isolated from animals. In this paper, we sought to determine whether MSC could be successfully generated from three different mouse strains. The ICR, CBA/Ca and Balb/c mice strains were available to us in the laboratory, and are strains commonly used in research. However, there is no literature stating the feasibility of each of these strains to yield bone marrow-derived MSC. It is important to determine the strain to use, as strain-dependent variability has already been reported by Peister and colleagues.
The therapeutic relevance for MSC is not limited to their regenerative properties, but also their capacity for modulation of immune responses. Beyond the efficacy of MSC to ameliorate disease, it is essential to test the immunogenicity and tumorigenicity of these cells. The use of murine-derived MSC for preclinical research is indisputable and their applicability in experimental settings should be determined for researchers to be able to accurately assess their use.
In this article, the authors show that bone marrow MSC can be derived from ICR, CBA/Ca and Balb/c mice strains with the protocol described. Similar information for these three mouse strains was previously unavailable. With passaging, bone marrow cultures achieved MSC immunophenotype and displayed multipotency by differentiating into osteocytes and adipocytes.
This study demonstrates that ICR, CBA/Ca and Balb/c mice strains can yield bone marrow-derived MSC for downstream experimentation. Based on the protocol described for culture of MSC, the authors describe the passage range of bone marrow cultures for each mouse strain that yields MSC (based on immunophenotyping data). This information provides data that can assist researchers faced with a choice of setting up mouse bone marrow-derived MSC cultures.
This article assessed bone marrow-derived MSC cultivated among three different mouse strains. The authors found some differences between strains with regards to the expression of certain surface antigens and differentiation capacity. These results will be useful for the selection of mouse for preclinical studies concerning bone marrow MSC.
| 1. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15369] [Article Influence: 569.2] [Reference Citation Analysis (2)] |
| 2. | Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 358] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Meirelles Lda S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009;14:4281-4298. [PubMed] |
| 4. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1715] [Cited by in RCA: 1721] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 5. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2773] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 6. | Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 746] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 7. | Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1404] [Cited by in RCA: 1329] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 9. | Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792-796. [PubMed] |
| 10. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3304] [Article Influence: 150.2] [Reference Citation Analysis (1)] |
| 12. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 841] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 13. | Ooi YY, Ramasamy R, Rahmat Z, Subramaiam H, Tan SW, Abdullah M, Israf DA, Vidyadaran S. Bone marrow-derived mesenchymal stem cells modulate BV2 microglia responses to lipopolysaccharide. Int Immunopharmacol. 2010;10:1532-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Eslaminejad MB, Nikmahzar A, Taghiyar L, Nadri S, Massumi M. Murine mesenchymal stem cells isolated by low density primary culture system. Dev Growth Differ. 2006;48:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570-585. [PubMed] |
| 17. | Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 786] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 18. | Barzilay R, Sadan O, Melamed E, Offen D. Comparative characterization of bone marrow-derived mesenchymal stromal cells from four different rat strains. Cytotherapy. 2009;11:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102-106. [PubMed] |
| 20. | Post S, Abdallah BM, Bentzon JF, Kassem M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008;43:32-39. [PubMed] |
| 21. | Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249-282. [PubMed] |
P- Reviewers Won JH, Vadalà G, de Carvalho KAT S- Editor Wen LL L- Editor A E- Editor Zheng XM