Published online Feb 26, 2012. doi: 10.4252/wjsc.v4.i2.9
Revised: December 21, 2011
Accepted: December 28, 2011
Published online: February 26, 2012
AIM: To investigate the interaction between mesenchymal stem cells (MSCs) and bone grafts using two different cultivation methods: static and dynamic.
METHODS: MSCs were isolated from rat bone marrow. MSC culture was analyzed according to the morphology, cell differentiation potential, and surface molecular markers. Before cell culture, freeze-dried bone (FDB) was maintained in culture for 3 d in order to verify culture medium pH. MSCs were co-cultured with FDB using two different cultivation methods: static co-culture (two-dimensional) and dynamic co-culture (three-dimensional). After 24 h of cultivation by dynamic or static methods, histological analysis of Cell adhesion on FDB was performed. Cell viability was assessed by the Trypan Blue exclusion method on days 0, 3 and 6 after dynamic or static culture. Adherent cells were detached from FDB surface, stained with Trypan Blue, and quantified to determine whether the cells remained on the graft surface in prolonged non-dynamic culture. Statistical analyses were performed with SPSS and a P < 0.05 was considered significant.
RESULTS: The results showed a clear potential for adipogenic and osteogenic differentiation of MSC cultures. Rat MSCs were positive for CD44, CD90 and CD29 and negative for CD34, CD45 and CD11bc. FDBs were maintained in culture for 3 d and the results showed there was no significant variation in the culture medium pH with FDB compared to pure medium pH (P > 0.05). In histological analysis, there was a significant difference in the amount of adhered cells on FDB between the two cultivation methods (P < 0.05). The MSCs in the dynamic co-culture method demonstrated greater adhesion on the bone surface than in static co-culture method. On day 0, the cell viability in the dynamic system was significantly higher than in the static system (P < 0.05). There was a statistical difference in cell viability between days 0, 3 and 6 after dynamic culture (P < 0.05). In static culture, cell viability on day 6 was significantly lower than on day 3 and 0 (P < 0.05).
CONCLUSION: An alternative cultivation method was developed to improve the MSCs adhesion on FDB, demonstrating that dynamic co-culture provides a superior environment over static conditions.
- Citation: Gonçalves FDC, Paz AHDR, Lora PS, Passos EP, Cirne-Lima EO. Dynamic culture improves MSC adhesion on freeze-dried bone as a scaffold for bone engineering. World J Stem Cells 2012; 4(2): 9-16
- URL: https://www.wjgnet.com/1948-0210/full/v4/i2/9.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v4.i2.9
Various methods have been studied for the restoration of bone defects resulting from traumatic injury, tumor resection, degenerative diseases, and congenital deformities[1]. It is known that successful treatment of bone loss requires a combination of an osteoinductive signal, an osteoconductive matrix, and cell response to the osteogenic potential[2]. Even so, major clinical need has stimulated the development of strategies to replace diseased bone tissue with a graft capable of integration with surrounding healthy tissue[3].
Autogenous bone grafts have the capacity to form new bone and serve as a scaffold for new bone formation. However, the availability of bone donor sites can become restricted in some patients, because the autogenous graft available may not be sufficient to treat large bone defects. In addition, the donor site is frequently a source of morbidity, including possible infection, hematoma, pain, irritability and hypersensitivity tissue limbs[2,4-6].
Freeze drying is employed for the preparation of nonautologous grafts. Allogenous and xenogenous bone grafts have been shown to be a viable option for bone tissue replacement, after adequate treatment to reduce immunogenicity and risk of disease transmission[7]. Furthermore, there are many advantages in the use of this source of bone including the convenience in storage, sterility, and low biochemical change[8,9]. However, sterilization techniques decrease the osteoinductive properties of these grafts[7,10,11]. Therefore, an association between stem cells and freeze-dried bone (FDB) could promote better bone regeneration, since these cells have osteogenic and osteoinductive capacity[12].
The maintenance of stable bone is the result of a controlled balance between the activities of bone forming (osteoblast) and bone resorbing (osteoclast) cells. Molecular interactions between osteoblasts and osteoclasts are influenced by the actions of the precursor cells of the osteoblast lineage, mesenchymal stem cells (MSCs)[13]. MSCs have the potential to differentiate in to mesenchymal tissue lineages, including bone, cartilage, fat, tendon/ligament, muscle, and marrow stroma, and have been receiving widespread attention because of their potential utility in tissue-engineering applications[5,14]. These multipotent cells are present in the bone marrow and in many other tissues and exhibit great plasticity[15]. Nevertheless, MSCs from bone marrow and periosteum have a higher potential for bone tissue regeneration than MSCs from adipose tissue[16,17]. MSCs are capable of autocrine and paracrine stimulation to produce their own scaffold and recruit additional cells required for bone formation and vasculogenesis[4]. In addition, MSCs have immunosuppressive properties and low immunogenicity, helping to reduce graft-versus-host reactions in allogeneic transplantation[18-21].
Some studies have suggested that pre-culture of MSCs on a scaffold leads to a higher osteogenic ability than injecting MSCs cultured into a scaffold during bone reconstruction surgery[22,23]. Although there are experimental results demonstrating the effect of MSCs in combination with bone grafts, there are few publications about co-cultivation methods (cell culture associated with FDB)[11,24]. Also, there is no consensus in the literature on the method, the amount or period of cells exposure to the grafts to promote adequate cell adhesion. In addition there are no in vitro histological studies examining the presence of MSC on the FDB surface.
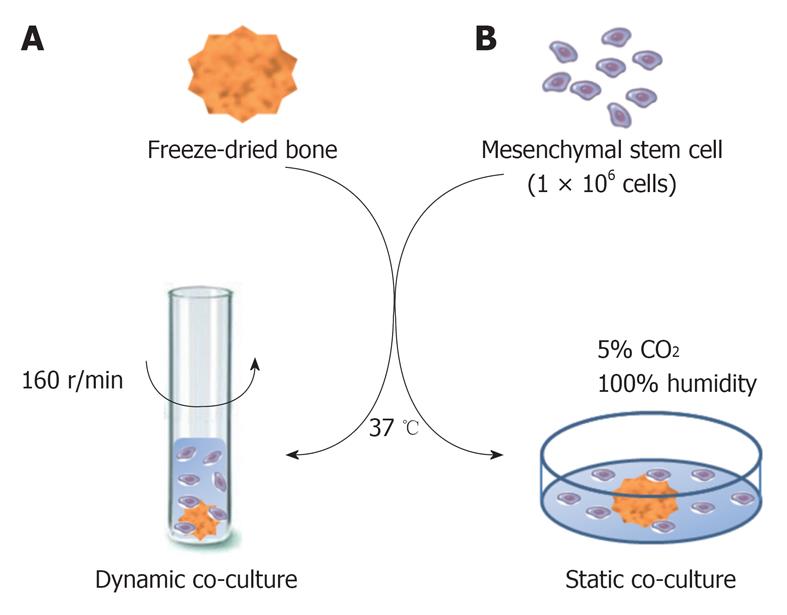
This study comprised an in vitro analysis to evaluate the integration of MSCs co-cultured with FDB fragments in two different cultivation methods: a static co-culture (two-dimensional - 2D) and a dynamic co-culture (three-dimensional - 3D). The static system is the conventional method of cell culture. A cell suspension is added to the culture medium with FDB for posterior cell sedimentation on the bone surface. In the dynamic system, the culture medium containing FDB and cells is constantly in agitation. We suggested the dynamic co-culture method of MSCs in order to establish a better interaction between cells and FDB fragments.
The protocol of isolation, characterization, and in vitro expansion of MSCs was performed according to Paz et al[25]. Eight-week-old Wistar rats were purchased from the Centro de Reprodução e Experimentação de Animais de Laboratório - CREAL/UFRGS. The procedures were performed in accordance with the guidelines for animal experimentation of UFRGS University and the Brazilian Federal Law 11.794/08 that establishes procedures for the scientific use of animals and regulates the registration of experimentation centers. This study was approved by the Research Ethics Committee of the Hospital de Clínicas de Porto Alegre and is registered under the number 09-015.
Bone marrow cells were obtained from femurs and tibias. After isolation, 1 × 107 bone marrow derived cells were cultured (37 °C, 5% CO2) in T25 culture flasks (TPP, Schaffhausen, Switzerland) with D-MEM (Invitrogen, CA, USA) medium containing 15 mmol/L Hepes (Gibco, NM, USA), 15% inactivated fetal bovine serum (FBS) (Invitrogen, CA, USA), 100 units/mL penicillin and 100 mg/mL streptomycin (Gibco, NM, USA). On the third day of culture, the medium was changed and non-adherent cells were removed. Adherent cells gaining 80% of confluence were passaged using 0.05% Trypsin-EDTA solution (Gibco, NM, USA) and then maintained in D-MEM with 10% FBS (complete medium).
In order to characterize MSCs in accordance with The International Society for Cellular Therapy Statement[26], two different experimental procedures were employed. Adipogenic differentiation was induced by culturing MSCs for up to 3 wk in D-MEM 10% FBS, 15 mmol/L Hepes, supplemented with 10-8 mol/L dexamethasone (Sigma, MO, USA), 5 μg/mL Insulin and 50 μg/mL Indomethacin (Sigma, MO, USA). Adipocytes were easily discerned from the undifferentiated cells by phase-contrast microscopy. To further confirm their identity, cells were fixed with 40 g/L paraformaldehyde and stained with Oil Red (Sigma, MO, USA) after 21 d of adipogenic differentiation. Secondly, to induce osteogenic differentiation, MSCs were cultured in D-MEM 10% FBS, 15 mmol/L Hepes, supplemented with 10-8 mol/L dexamethasone, 5 μg/mL ascorbic acid 2-phosphate (Sigma, MO, USA) and 10 mmol/L β-glycerolphosphate (Sigma MO, USA). To observe calcium deposition, cultures were fixed with 40 g/L paraformaldehyde and stained with Alizarin Red S stain (Nuclear, SP, Brazil) after 21 d of osteogenic differentiation.
In order to characterize the cell population according to surface molecular markers, immunophenotyping was performed. Approximately 1 × 106 MSCs were prepared. They were placed in sterile tubes and washed 2 times by centrifugation at 300 ×g for 5 min at 4 °C. MSCs were then resuspended in phosphate-buffered saline (PBS) and incubated for 20 min at 4 °C with phycoerytrin (PE) or fluorescein isothiocyanate (FITC) conjugated antibodies against rat CD34, CD45, CD11bc, CD44, CD90 and CD29. All assays were conducted using antibody concentrations as recommended by the manufacturers. Phycoerythrin-PE and FITC mouse anti-rat IgG1, IgG2a and IgM were used as isotype controls. Cells were collected and washed with PBS by centrifugation, and fluorescence analysis was carried out with the BD FACS-Calibur flow cytometer (Becton-Dickinson, NJ, USA) with a one-laser system that is capable to detect three fluorochromes excited by the 488nm laser in a multiparameter manner. Data samples were analyzed using Cellquest and PAINT-A-GATE software.
FDB from bovine was obtained from the Tissue Bank of the Orthopedics and Traumatology Service, Hospital de Clínicas de Porto Alegre (HCPA). This bone graft is used in surgery for bone repair in patients at HCPA. Briefly, the freeze drying protocol consists of fat removal and subsequent bone dehydration in chemical baths followed by washings and centrifugations. The final step is freeze drying (-40 °C) for 7 d. At the end of the process, the material is packaged in gas permeable packing and sterilized in an autoclave. For this experiment, the FDB was fragmented in a volume of approximately 45 mm3.
FDB was maintained in culture for 3 d in order to determine any possible change in pH. Some studies have reported that FDB could alter the medium pH, and this could potentiall impair MSC viability[11]. For the analysis, pH was measured on days 0, 1, 2 and 3 after the beginning of the experiment.
MSCs were co-cultured with FDB fragments using two different cultivation methods: a static co-culture method (two-dimensional - 2D) and a dynamic co-culture method (three-dimensional - 3D). In both methods, cell culture with FDB was maintained in D-MEM medium containing 15 mmol/L Hepes, 10% inactivated FBS, 100 units/mL penicillin and 100 mg/mL streptomycin.
The groups studied were: (1) cells and bone (CB); and (2) cells, bone and matrix (CBM). Additionally, two control groups were used in both co-cultivation methods: a group containing only bone and another group containing only cells. Three independent experiments were performed for each co-culture system and method of analysis.
Dynamic co-culture method: FDB was co-cultured with MSCs in culture tubes with a non-adherent surface and maintained in an orbital incubation system (Certomat BS-1, B. Braum Biotech International) at 37 °C with agitation (160 r/min) for 24 h, without humidity control and CO2 supply. One FDB and 1.0 × 106 cells/1.5 mL culture medium were added to each culture tube (Figure 1A).
Static co-culture method: FDB was co-cultured with MSCs in 24-well dishes with an adherent surface and incubated at 37 °C in a humidified atmosphere with 5% CO2. 1.0 × 106 cells/1.5mL culture medium were added to each well containing FDB for posterior cell sedimentation on the bone matrix (Figure 1B).
A commercial extracellular matrix (Matrigel™-BD, CA, USA) (dilution 1:1) was used to facilitate cell adhesion on the graft. The matrix used is liquid at 4 °C and gels at 22 to 35 °C. In the CBM groups, the extracellular matrix was previously placed on the bone surface and incubated at 37 °C for 15 min to form a gel. After, the FDB was transferred to the cell culture and incubated in a dynamic or static system.
After 24 h of cultivation of MSCs and FDB by dynamic or static methods, the bone matrix was fixed in 40 g/L formaldehyde for 24 h and decalcified (100 mL/L HNO3) for 48 h before paraffin embedding. Sections 4 μm in thickness were stained with hematoxylin and eosin. ImageJ software (National Institutes of Health, MA, USA) was used for semi quantitative analysis of cells adhesion on bone matrix surface. Cells present in nine microscopic fields were quantified in pixels at 200 × magnification.
After dynamic co-culture, FDBs (CB group) were placed in 24-well dishes and incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 6 d. The experiment goal was to determine whether the cells remain on the graft surface in prolonged non-dynamic culture. Accordingly, on days 0, 3 and 6 after dynamic culture, the adherent cells were detached by using 0.05% trypsin-EDTA and stained with Trypan Blue, which stains dead cells with a distinctive blue color under the microscope. The viable cells were quantified in a Neubauer chamber. At the same time, the static method cell viability analysis was carried out.
Quantitative data were presented as mean ± SE, and statistical comparisons were performed using repeated measurement variance analysis for culture medium pH and cell viability and two-way ANOVA analyses for cell adhesion on bone graft after log transform in PASW Statistics (SPSS version 18.0). A P-value of less than 0.05 was considered significantly different.
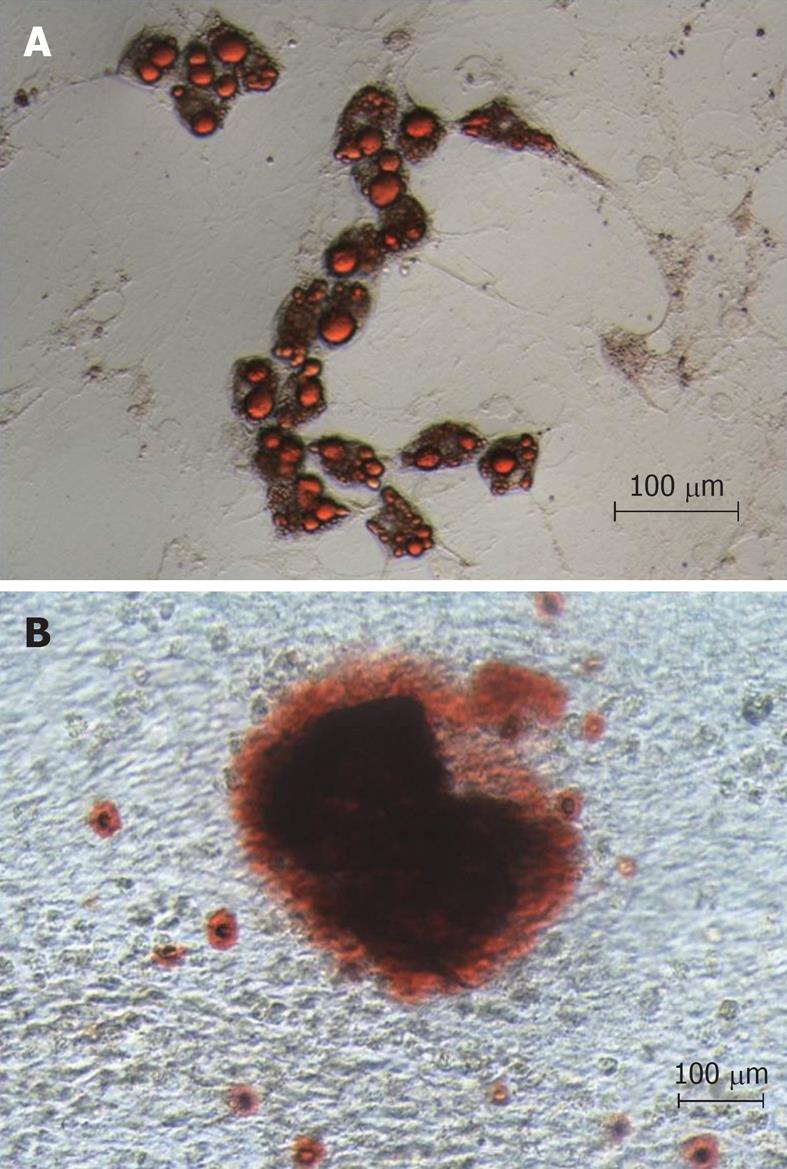
Bone marrow MSCs were obtained from rats by plating bone marrow cell suspension in tissue culture dishes and propagation of adherent cells. The isolated cells developed to visible systematic colonies of adherent fibroblast-like cells at about 7-10 d after initial plating, and became morphologically more homogeneous with further time in culture with the depletion of hematopoietic and other bone marrow stromal cells. The MSC differentiation potential was shown using protocols known to induce differentiation into bone and adipose cells. As demonstrated in Figure 2A, a clear potential for adipogenic differentiation was detected by Oil red that stains lipid vacuoles, while Figure 2B shows osteogenic differentiation as detected by Alizarin Red that stains calcium deposits.
Rat MSCs are known to be positive for CD44, CD90 and CD29 and negative for CD34, CD45 and CD11bc. Flow cytometry was performed from the moment of MSC extraction to the third trypsinization step. By the third passage the vast majority of cells stained for the markers CD44 (99.3%), CD90 (99.8%), CD29 (99.5%) and only a small proportion manifested expression of the markers CD45 (0.9%), CD11b/c (0.52%) and CD34 (0.05%). According to this pattern of cell surface marker expression, the cell population at this time point (approximately 24 d in culture) was quite uniform and can be considered bona fide MSCs.
FDB was maintained in culture for 3 d and the pH was measured on days 0, 1, 2 and 3. From our data, there was no significant variation in the culture medium pH with FDB compared to pure medium pH over time or between groups (P > 0.05). The pH measurements are presented in Table 1.
| Days | 0 d | 1 d | 2 d | 3 d |
| Culture medium | 7.377 ± 0.023 | 7.473 ± 0.067 | 7.457 ± 0.047 | 7.340 ± 0.113 |
| Culture medium with freeze-dried bone | 7.377 ± 0.023 | 7.323 ± 0.067 | 7.327 ± 0.047 | 7.493 ± 0.113 |
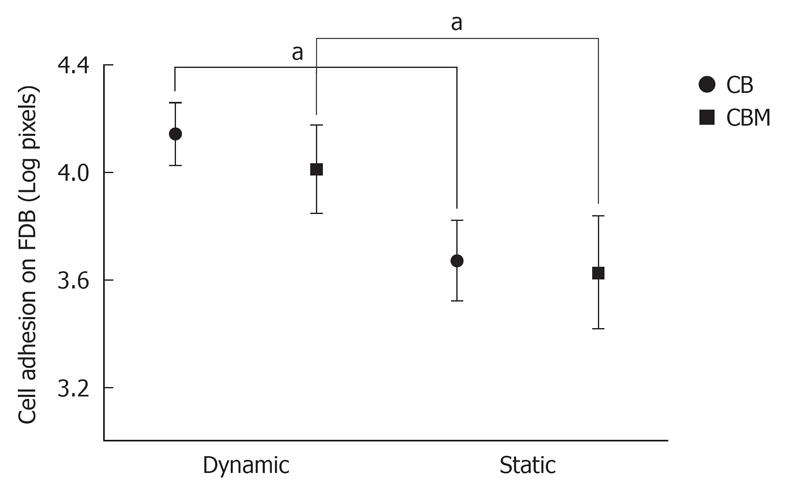
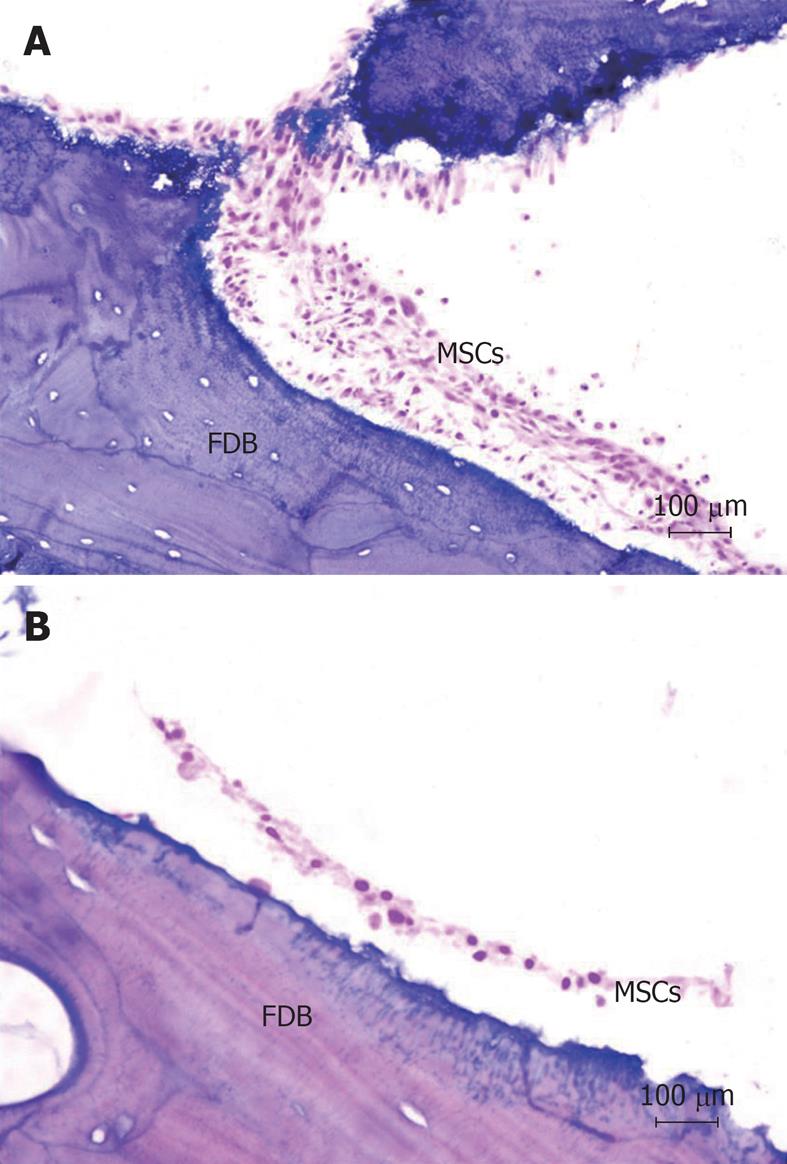
MSCs were co-cultured with FDB fragments using two different cultivation methods (a static co-culture method and a dynamic co-culture method), and in two different groups (CB and CBM). In histological analysis of cell adhesion on FDB, after 24 h of culture, there was a significant difference between the two cultivation methods (3.2 ± 0.44 CB dynamic and 3.6 ± 0.15 CB static; 4.01 ± 0.16 CBM dynamic and 4.15 ± 0.11 CBM static, P < 0.05) (Figure 3). In short, the dynamic co-culture method demonstrated greater cell adhesion on the bone surface than the static co-culture method. Meanwhile, there was no significant difference between the groups (CB and CBM) with dynamic or static methods (P > 0.05). In addition, dynamic co-culture led to the formation of multiple cell layers (Figure 4A). This cell behavior was not found in static co-culture (Figure 4B).
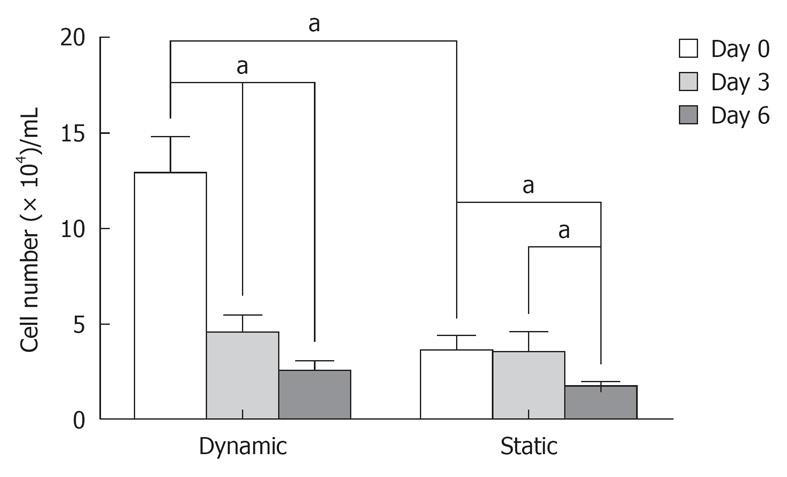
The cell viability ratio was assessed by the Trypan Blue exclusion method on days 0, 3 and 6 after dynamic or static culture. On day 0, cell viability in the dynamic system was significantly higher than in the static system (12.94 ± 1.4 × 104 dynamic method and 3.62 ± 1.4 × 104 static method, P < 0.05). Also, after dynamic culture, there was a statistical difference in cell viability between days 0, 3 and 6 (12.94 ± 1.4 × 104 on day 0, 4.55 ± 0.98 × 104 on day 3 and 2.55 ± 0.37 × 104 on day 6, P < 0.05). During this period, there was decreased cell viability on bone matrix in prolonged non-dynamic culture. In static culture, the cell viability on day 6 was significantly lower than on day 3 and 0 (1.72 ± 0.37 × 104 on day 6, 3.55 ± 0.98 × 104 on day 3 and 3.62 ± 1.4 × 104 on day 0, P < 0.05) (Figure 5).
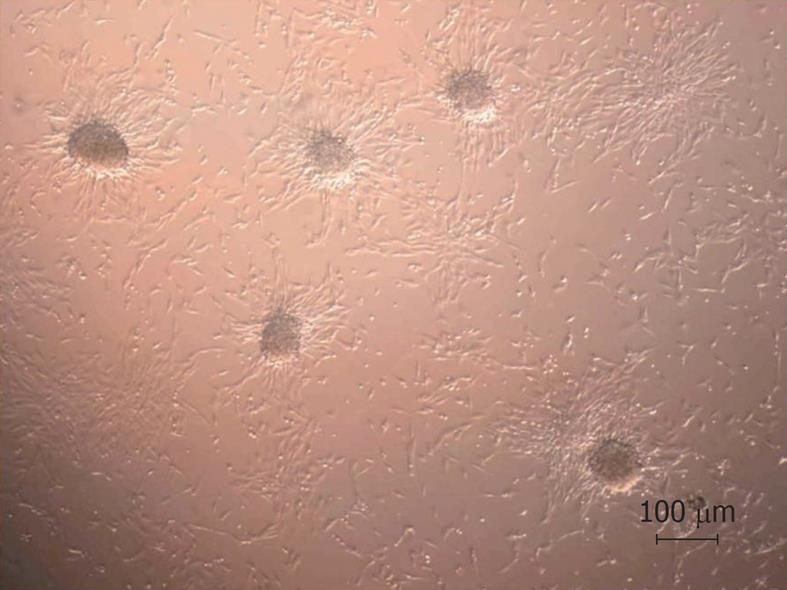
The cells that did not adhere on FDB during dynamic culture were placed in 6-well dishes for morphological analysis by optical microscopy. These cells tended to form 3-D structures (Figure 6). Conversely, there was no evidence of 3-D cellular structures in static co-culture.
FDB has been studied in vitro as a biomaterial for bone tissue engineering, because of its advantages for clinical use, material availability, biocompatibility, and long-term biofunctionality. Futhermore, it is considered that this graft has low (or no) osteoinductive property. The possible association of MSCs with FDB is important as MSCs have the ability to convey osteogenic and osteoinductive properties to biomaterials. A major concern for successful bone tissue repair treatment is adhesion of MSCs to the bone graft. Orsi et al[11] assessed the osteogenic potential of MSCs to aggregate three-dimensional structure on FDB by the conventional co-culture method, which allows the cells sedimentation on the constructs. The results showed that the addition of bone marrow cells with osteogenic potential to FDB did not improve osteogenic properties of the material in this system. This result could be due to there being insufficient cells to aggregate on the graft, since the authors did not perform any in vitro evaluation of the presence of viable cells on the graft surface. Others authors have suggested more sophisticated methods to facilitate the infiltration of culture medium into the scaffold pores in bioreactors, showing that the mechanical stimulation of MSCs on a three-dimensional osteoconductive scaffold can increase cell proliferation, differentiation, and mineralized matrix production[1,27]. Although these methods are effective for promoting an increase in the cells number infiltrating the graft and consequently a greater number of viable cells in the transplant, bioreactors are expensive and are not easily available in many laboratories. Therefore, alternative culture methods are needed that allow cell adhesion and proliferation on biomaterial.
In this study, we proposed an alternative method of three-dimensional MSC and FDB co-culture. The objective of dynamic cultivation was to make the cells adhere mechanically and proliferate on the bone surface. In tissue organization, the cells are connected to each other and with extracellular matrix. This matrix has several proteins that provide mechanical properties to tissues as well as enhanced cell communication. In two-dimensional cell culture, biological properties and mechanical and biochemical cell interaction are lost because the cells are in an environment that does not allow their natural geometry. However, three-dimensional cell cultures may mimic a microenvironment nearer to the cellular organization in tissues[28].
According to our data, it is possible to improve interaction between cells and bone biomaterial when co-culture is carried out in an orbital incubator shaker. In histological evaluation, cell clusters were observed in FDB with the formation of multiple cell layers. Although the dynamic method used has no humidity control or supply of CO2 (necessary components in the maintenance of MSCs culture), there was a greater adhesion and cell viability in the bone scaffold, when compared to the static method in the presence of CO2 and 100% humidity. It is important to note that in this work the cells were grown in the dynamic system without the presence of CO2 and humidity because the equipment we had access did not have these facilities. However, we believe that the use of equipment with CO2 and humidity could further enhance the results presented here.
The extracellular matrix used in our study did not allow higher cell adhesion on FDB, although this matrix is suitable for three-dimensional cultures and cell adhesion and differentiation[29,30]. In addition, commercially available extracellular matrices are expensive and some have components sourced from mouse sarcoma. Our results are more compatible with clinical needs in orthopedic surgery because, depending on the graft size, a significant amount of extracellular matrix may be needed to coat the bone surface for the transplant. In addition, the use in humans of a matrix from an animal source could enhance the possibility of transplant rejection.
To evaluate the period over which cells remain integrated on the graft surface, we performed the cell viability test with Trypan Blue. After dynamic co-culture, numerous cells were quantified on the bone fragment. Given that, on days 3 and 6 after the transference of dynamic cultures to the no-dynamic culture system, there was a reduction in the number of viable cells interacting with the FDB. In our view, the reduced number of viable cells on the bone matrix may be due to changes in the cultivation system from three-dimensional to two-dimensional culture, leading to an alteration in the biological and biochemical properties of cells[28]. Thus, the interaction between MSCs and FDB is satisfactory after 24 h of cultivation in dynamic culture, and does not require a longer cultivation before application of the graft. This result is in conformity with the clinical demand that the graft is made as soon as possible, avoiding prolonged cultivation which can cause genetic alterations of MSCs[31].
After dynamic co-culture, some cells which did not adhere to the graft and were placed in culture dishes for morphological assessment. The formation of spherical cell clusters was observed in culture under shaking conditions. This clustering behavior in dynamic cell culture has been described in the literature by some authors[28,32,33]. The change in morphology of MSCs is probably due to culture conditions on non-adherent surfaces, the high degree of confluence, and nutrient deprivation[28]. Nevertheless, more detailed studies on these factors is needed to better understand the formation of these spheroids aggregates. With the static method, where the quantity and viability of cells attached to FDB was low, there was no tendency to form cells spheroids in static co-culture.
In conclusion, we have developed an alternative method for culturing MSCs with a biomaterial in orbital incubator shakers. This cultivation method provides cells with a superior capacity to adhere on FDB surface, and may be applied for cell therapy associated with bone grafts in surgery for bone repair in animal models.
The authors thank Dr João Ellera Gomes for kindly providing the FDB for this study.
The freeze-dried bone (FDB) has been used as a biomaterial for bone tissue engineering and has advantages for clinical use including material availability, biocompatibility, and long-term biofunctionality. However, this graft has low (or no) osteoinductive property. The in vitro study of the association of mesenchymal stem cells (MSCs) with FDB is important, because MSCs have the ability to assign osteogenic and osteoinductive properties for biomaterial.
The interaction between MSCs and bone graft has been studied for possible use in the treatment of bone defects. Although there are experimental results demonstrating the effect of combining MSCs with bone grafts, there are few studies about cultures methods. Thus, an in vitro analysis to evaluate the integration of MSCs with bone grafts fragments in cultivation methods is potentially important.
In this study, two different cultivation methods were performed with MSCs and FDB: a static co-culture (two-dimensional - 2D) and a dynamic co-culture (three-dimensional - 3D). The static system is the conventional method of cell culture. The authors proposed that the dynamic co-culture method of MSCs could establish a better interaction between cells and FDB fragments. The dynamic co-culture method demonstrated greater cell adhesion on the bone surface and greater cell viability than the static co-culture method. In addition, we believe that this is the first histological in vitro study examining the presence of MSC cells on the FDB surface.
The authors developed an alternative method of culture MSCs with bone graft using a dynamic system, demonstrating that this cultivation provides cells with superior capacity to adhere on FDB surfaces. These results could facilitate the application of cell therapy associated with bone grafts in surgery for bone repair.
FDB: Freeze drying is employed for the preparation of nonautologous grafts. Briefly, the freeze drying protocol consists of fat removal and subsequent bone dehydration in chemical baths followed by washing and centrifugations. The last step is freeze drying (-40 °C) for 7 d. At the end of the process, the material is packaged in gas permeable packing and sterilized in an autoclave. Allogenous and xenogenous FDBs have been a viable option for bone tissue replacement.
This research describes how a new very low cost lab based ‘bioreactor’ has been developed from general lab equipment that can increase the adhesion of rat MSCs to decellurised freeze dried bone scaffolds. Results show promising increases in adhesion of cells after 24 h in the system when compared to a static culture control.
| 1. | Kasper FK, Liao J, Kretlow JD, Sikavitsas VI, Mikos AG. Flow perfusion culture of mesenchymal stem cells for bone tissue engineering. StemBook. Cambridge: Harvard Stem Cell Institute 2008; . |
| 2. | Oakes DA, Lee CC, Lieberman JR. An evaluation of human demineralized bone matrices in a rat femoral defect model. Clin Orthop Relat Res. 2003;281-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Partridge KA, Oreffo RO. Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Cuomo AV, Virk M, Petrigliano F, Morgan EF, Lieberman JR. Mesenchymal stem cell concentration and bone repair: potential pitfalls from bench to bedside. J Bone Joint Surg Am. 2009;91:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004;10:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Colterjohn NR, Bednar DA. Procurement of bone graft from the iliac crest. An operative approach with decreased morbidity. J Bone Joint Surg Am. 1997;79:756-759. [PubMed] |
| 7. | Bostrom MP, Seigerman DA. The clinical use of allografts, demineralized bone matrices, synthetic bone graft substitutes and osteoinductive growth factors: a survey study. HSS J. 2005;1:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Macedo CAS, Galia CR, Silva ALB, Cesar PC, Sanches PRS, Duarte LS. Compressive resistance of deep frozen and lyophilized bovine bone: comparative study. Rev Bras Ortop. 1999;34:529-534. |
| 9. | Oliveira ACP, Collares MVM, Galia CR, Edelweiss MI, Pinto RA, Kneibel L. Comparison between autogenous, deep-frozen homologous, and lyophilized homologous bone grafts in an experimental model of cranioplasty. Rev Soc Bras Cir Craniomaxilofac. 2007;10:140-146. |
| 10. | Baptista PPR, Polesello G, Guimaraes RP, Fernandes ML. Use of freeze-dried bone graft in bone lesions. Rev Bras Ortop. 1997;32:845-848. |
| 11. | Orsi VV, Collares MVM, Nardi NB, Pinto RA, Meirelles LS, Meurer L, Pilla C, Portinho CP, Riboldi M, Auler TB. Bovine non-demineralized lyophilized bone with mesenchymal stem cells for tissue engineering: experimental study in heterotopic site. Rev Soc Bras Cir Craniomaxilofac. 2007;10:133-139. |
| 12. | Seebach C, Schultheiss J, Wilhelm K, Frank J, Henrich D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury. 2010;41:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38 Suppl 1:S26-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 14. | Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 542] [Article Influence: 25.8] [Reference Citation Analysis (3)] |
| 15. | Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;249-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Hayashi O, Katsube Y, Hirose M, Ohgushi H, Ito H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008;82:238-247. [PubMed] |
| 17. | Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehlhorn AT, Pearce S, Kasten P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31:3572-3579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 18. | Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, Kyurkchiev DS. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Zheng ZH, Li XY, Ding J, Jia JF, Zhu P. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology (Oxford). 2008;47:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH, Cho CS. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008;153:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Jorgensen C, Djouad F, Apparailly F, Noël D. Engineering mesenchymal stem cells for immunotherapy. Gene Ther. 2003;10:928-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Portinho CP, Collares MVM, Silva FH, Nardi NB, Nance B, Pinto RA, Siqueira E, Morellato G, Sumino K. Skull reconstruction with undifferentiated mesenchymal stem cells: experimental study. Rev Soc Bras Cir Plast. 2006;21:161-165. |
| 23. | Baba S, Inoue T, Hashimoto Y, Kimura D, Ueda M, Sakai K, Matsumoto N, Hiwa C, Adachi T, Hojo M. Effectiveness of scaffolds with pre-seeded mesenchymal stem cells in bone regeneration--assessment of osteogenic ability of scaffolds implanted under the periosteum of the cranial bone of rats. Dent Mater J. 2010;29:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Castano-Izquierdo H, Alvarez-Barreto J, van den Dolder J, Jansen JA, Mikos AG, Sikavitsas VI. Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A. 2007;82:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Paz AH, Salton GD, Ayala-Lugo A, Gomes C, Terraciano P, Scalco R, Laurino CC, Passos EP, Schneider MR, Meurer L. Betacellulin overexpression in mesenchymal stem cells induces insulin secretion in vitro and ameliorates streptozotocin-induced hyperglycemia in rats. Stem Cells Dev. 2011;20:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1397] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 27. | Altmann B, Welle A, Giselbrecht S, Truckenmüller R, Gottwald E. The famous versus the inconvenient - or the dawn and the rise of 3D-culture systems. World J Stem Cells. 2009;1:43-48. [PubMed] [DOI] [Full Text] |
| 28. | Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1185] [Article Influence: 56.4] [Reference Citation Analysis (5)] |
| 30. | Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 1082] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 31. | Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 431] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 32. | de Barros AP, Takiya CM, Garzoni LR, Leal-Ferreira ML, Dutra HS, Chiarini LB, Meirelles MN, Borojevic R, Rossi MI. Osteoblasts and bone marrow mesenchymal stromal cells control hematopoietic stem cell migration and proliferation in 3D in vitro model. PLoS One. 2010;5:e9093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA. 2010;107:13724-13729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 779] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
Peer reviewers: Nicholas R Forsyth, PhD, Senior Lecturer in Stem Cell Biology, The Guy Hilton Research Laboratories, Keele University Medical School, Thornburrow Drive, Hartshill, Stoke on Trent, ST4 7QB, United Kingdom; Susan Gaynor Shawcross, PhD, Blond McIndoe Laboratories, University of Manchester, School of Biomedicine, 3.106 Stopford Building, Oxford Road, Manchester M13 9PT, United Kingdom; Juan Antonio Marchal Corrales, Professor, MD, PhD, Department of Human Anatomy and Embriology, School of Medicine, Biopathology and Regenerative Medicine Institute (IBIMER), Centre for Biomedical Research (CIBM), University of Granada, Avenida del Conocimiento s/n, Granada 18100, Spain
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM