Published online Nov 26, 2012. doi: 10.4252/wjsc.v4.i11.110
Revised: October 26, 2012
Accepted: November 2, 2012
Published online: November 26, 2012
Processing time: 163 Days and 21.3 Hours
AIM: To understand the neuroprotective mechanism of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) against amyloid-β42 (Aβ42) exposed rat primary neurons.
METHODS: To evaluate the neuroprotective effect of hUCB-MSCs, the cells were co-cultured with Aβ42-exposed rat primary neuronal cells in a Transwell apparatus. To assess the involvement of soluble factors released from hUCB-MSCs in neuroprotection, an antibody-based array using co-cultured media was conducted. The neuroprotective roles of the identified hUCB-MSC proteins was assessed by treating recombinant proteins or specific small interfering RNAs (siRNAs) for each candidate protein in a co-culture system.
RESULTS: The hUCB-MSCs secreted elevated levels of decorin and progranulin when co-cultured with rat primary neuronal cells exposed to Aβ42. Treatment with recombinant decorin and progranulin protected from Aβ42-neurotoxicity in vitro. In addition, siRNA-mediated knock-down of decorin and progranulin production in hUCB-MSCs reduced the anti-apoptotic effects of hUCB-MSC in the co-culture system.
CONCLUSION: Decorin and progranulin may be involved in anti-apoptotic activity of hUCB-MSCs exposed to Aβ42.
-
Citation: Kim JY, Kim DH, Kim JH, Yang YS, Oh W, Lee EH, Chang JW. Umbilical cord blood mesenchymal stem cells protect amyloid-β42 neurotoxicity
via paracrine. World J Stem Cells 2012; 4(11): 110-116 - URL: https://www.wjgnet.com/1948-0210/full/v4/i11/110.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v4.i11.110
Alzheimer’s disease (AD) is currently an incurable neurodegenerative disease. The proposed main causes of AD are amyloids, tau theory, mitochondria dysfunction and chronic inflammation[1,2]. These causes gradually and progressively induce neuronal loss in the brain, which progressively diminishes mental activity. Mesenchymal stem cells (MSCs) have shown promise in the treatment of AD in vitro and in vivo[3-5]. MSCs can be collected from various human sources including bone marrow, umbilical cord blood, adipose tissue and Wharton’s jelly[6,7]. Although MSCs have the capacity for differentiation of into bone, cartilage and adipose, and so are compelling candidates for regenerative therapies, the paracrine action of MSCs has been spotlighted as the major role of stem cells in disease models[8-13].
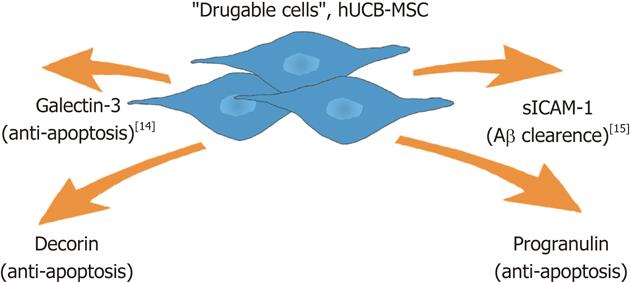
Our group has studied the paracrine effect of human umbilical cord blood-derived MSCs (hUCB-MSCs) in the treatment of AD in vitro and in vivo. We found that hUCB-MSC secretes a variety of proteins including galectin-3 for neuron survival[14] and soluble intercellular adhesion molecule-1 (sICAM-1) for the induction of Aβ degrading enzyme and neprilysin on microglia when co-cultured with rat primary neuronal cells during exposure to amyloid-β42 (Aβ42)[15]. These actions reduce Aβ and improve memory deficit in an AD-transgenic mouse model[3].
In this study, we identify two additional soluble proteins, progranulin and decorin, which are released from hUCB-MSC under AD microenviroment. The data provide another example of the paracrine action of hUCB-MSCs for AD therapy.
This study was approved by the Institutional Review Board of MEDIPOST. Umbilical cord blood was collected from umbilical veins after neonatal delivery with informed consent of the pregnant mothers. hUCB-MSCs were isolated and expanded as described previously[15]. Pregnant Sprague-Dawley rats were purchased from Orientbio (Kyeonggi, South Korea). Brain tissue was dissected from embryonic-day-14 rat cortex and hippocampus, and cells were mechanically dissociated in Ca2+/Mg2+-free Hank’s balanced salt solution as we have described before[14,15]. Rat primary neuronal cultures were treated with Aβ42 (Sigma-Aldrich, St. Louis, MO, United States) for 12 h or 24 h. hUCB-MSCs (8 × 104 cells/cm2) were co-cultured in the upper chamber (pore size: 1 μm) of a Transwell apparatus (Falcon) with Aβ42-exposed rat primary neuronal cells or BV2 cells.
Enzyme-linked immunosorbant assay (ELISA) was performed according to the manufacturer’s instructions. A progranulin ELISA kit (R and D Systems, Minneapolis, MN, United States) and a kit for decorin (R and D Systems) were used in analyzing each conditioned medium. The capture antibody was diluted in phosphate-buffered saline and added to a 96-well plate for pre-coating. After overnight incubation, each plate was washed and blocked by the reagent. After aspiration, 100 μL of medium was incubated in wells coated with capture antibody for 2 h. After incubation of streptavidin-horseradish peroxidase, substrate solution was added. For the antibody array, the collected medium was dialyzed at 4 °C for 3 h then labeled with biotin reagent according to the recommended protocol. Biotin-labeled proteins in the medium were reacted with a glass chip assembly and further incubated with streptavidin-conjugated fluorescent dye. The glass chip was scanned and analyzed using Analysis Tool Software (Ray-Bio, Norcross, GA, United States).
Small interfering RNAs (siRNAs) for decorin and progranulin were purchased from Bioneer (Daejon, South Korea) and non-targeted control siRNA was from Thermo Scientific Dharmacon (Chicago, IL, United States). siRNA was treated with Lipofecamine-2000 (Invitrogen, Carlsbad, CA, United States) for 6 h without fetal bovine serum and antibiotics. siRNA treated cells were washed and cultured overnight in Dulbecco’s modified Eagle’s medium without antibiotics.
Fixed cells were stained with antibodies for microtubule associated protein 2 (MAP2; Millipore, Billerica, MA, United States) and fluorescein isothiocynate (FITC; Cy3) secondary antibodies (Jackson Immunoresearch Laboratories, Bar Harbor, ME, United States). Stained cells were photographed using a fluorescence microscope (Nikon, Tokyo, Japan).
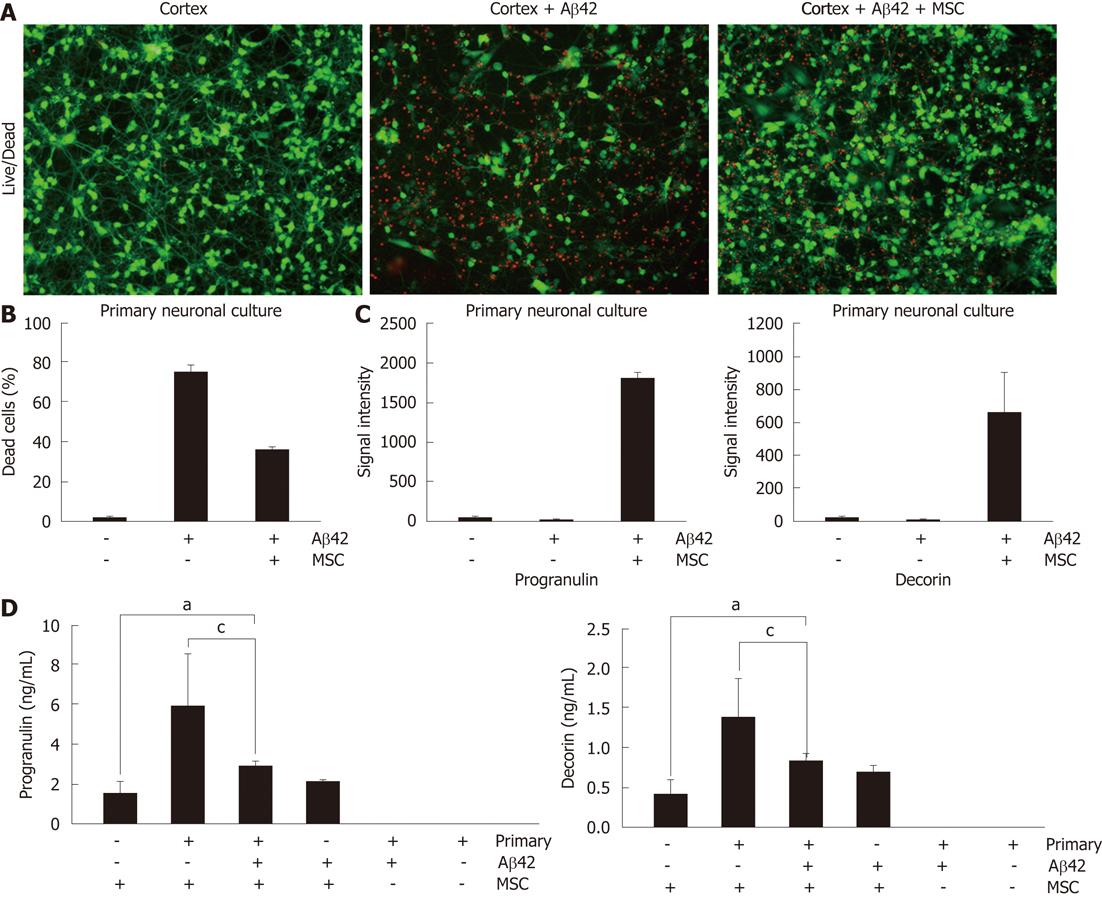
Since we already reported that co-culture of hUCB-MSCs protects from Aβ-mediated neurotoxicity in vitro[14], we sought to confirm these results in a co-cultured system using Live/Dead staining (Figure 1). hUCB-MSCs were co-cultured with Aβ42-exposed rat primary neuronal cells for 24 h in a Transwell chamber. Green and red fluorescence indicated lived and dead rat primary neuronal cells, respectively. The co-culture of hUCB-MSC protects Aβ42-mediated neurotoxicity in rat primary neuronal cells (Figure 1A and B). To identify soluble factors from hUCB-MSC during co-culture, we analyzed secreted proteins in the medium using an antibody-based array (unpublished data). Among the identified proteins, we focused on progranulin and decorin because their protein levels were highly elevated when hUCB-MSCs were co-cultured with rat primary neuronal cells in the presence of Aβ42 (Figure 1C). To confirm the accuracy of the antibody-based array, we performed ELISA to quantify the progranulin and decorin presence in the medium. Progranulin and decorin were also elevated during co-culture compare to hUCB-MSCs cultured alone (Figure 1D). However, Aβ42 exposure slightly inhibited secretion of decorin and progranulin (P < 0.05). Each ELISA was human specific because the medium used for rat primary neuronal cells did not react with decorin and progranulin of hUCB-MSCs. These data suggest that secretion of decorin and progranulin were induced in hUCB-MSCs by the co-culture of rat primary neuronal cells in the presence or absence of Aβ42.
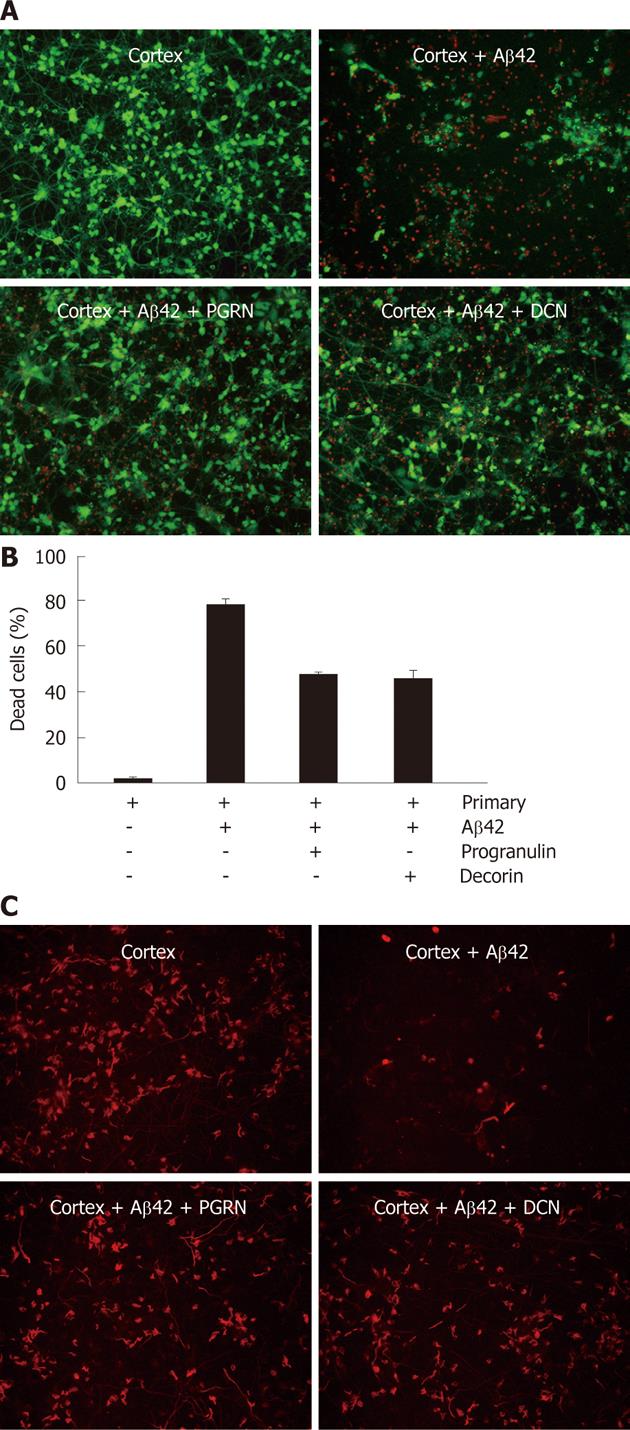
To test whether decorin and progranulin participate in the neuroprotection against Aβ42-neurotoxicity, recombinant decorin and progranulin were treated in Aβ42-exposed rat primary neurons at three doses (10 ng/mL, 20 ng/mL and 50 ng/mL). After treatment of recombinant decorin or progranulin for 36 h, rat primary neuronal cells were analyzed by Live/Dead staining. Almost the same neuroprotective effect of decorin and progranulin was apparent at each dose (data not shown). Representative percentage of dead cells using 10 ng of decorin or progranulin in Aβ42-exposed rat primary neuronal cells is shown in Figure 2B. Since neuron and glia cells were mixed in the rat primary neuronal cells, we tried to stain MAP2-positive neurons in recombinant decorin- and progranuin-treated cells exposed to Aβ42 (Figure 2C). Treatment of each protein reduced Aβ-mediated neurotoxicity because MAP2-positive cells were very apparent in Aβ-exposed neurons with decorin or progranulin, compared to controls. These data suggest that secreted decorin and progranulin from hUCB-MSCs have an anti-apoptotic effect against Aβ42-neurotoxicity in vitro.
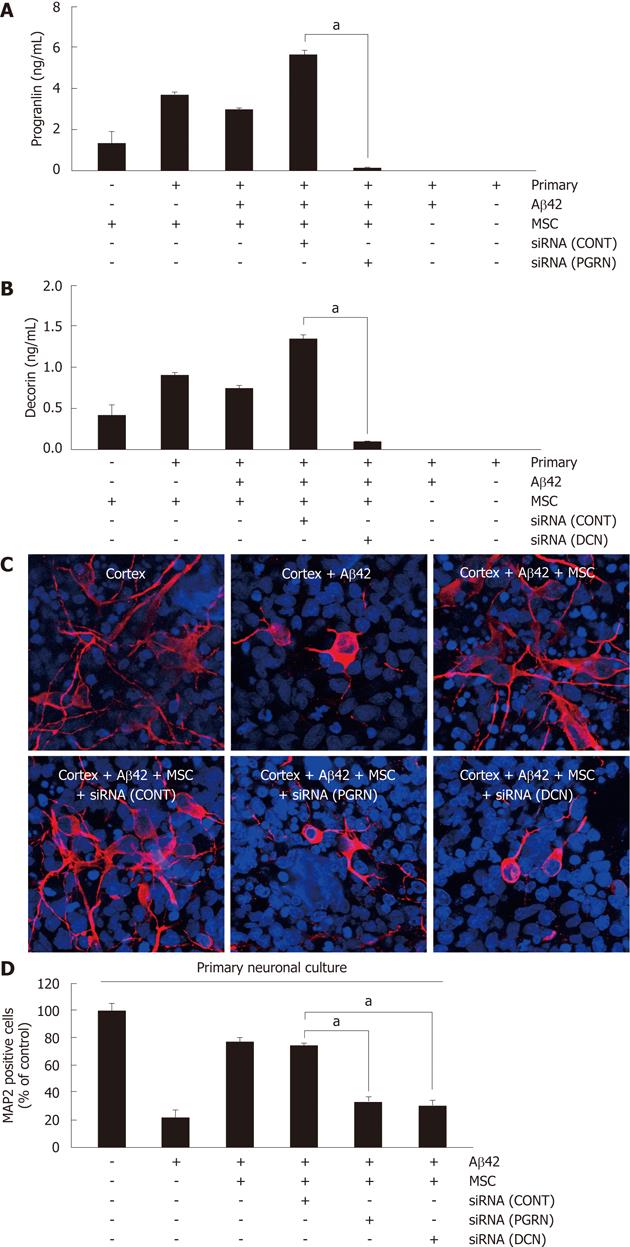
To assess whether the anti-apoptotic effect of hUCB-MSC against Aβ42 was mediated by the action of decorin and progranulin, each siRNA for decorin and progranulin were used for a 6-h pretreatment of hUCB-MSCs cocultured with Aβ42-exposed rat primary neurons for further 24 h. Pretreatment using either siRNA inhibited the secretion of decorin and progranulin (Figure 3A). To analyze the surviving neurons in rat primary neuronal culture by siRNA treated hUCB-MSCs, MAP2 was visualized by staining with anti-MAP2 antibody (Figure 3B). siRNA treated hUCB-MSCs were not protective against Aβ42 neurotoxicity. The percentage of surviving neurons is depicted in Figure 3C. The data suggested that decroin and progranulin released from hUCB-MSC mediate an anti-apoptotic effect of hUCB-MSCs against Aβ42 neurotoxicity in vitro.
MSC display paracrine action in pathological conditions[16]. Especially, we observed that hUCB-MSCs also secreted a variety of proteins by incubation with body fluid collected from patients (unpublished data). When we analyzed co-cultured media by various biochemical approaches such as antibody array, we identified several proteins including galectin-3 and sICAM-1. We have previously reported on the function of each identified protein in an AD model[14,15]. Here, we report on the role of decorin and progranulin in hUCB-MSC-action in an AD model. hUCB-MSCs seem to act simultaneously in an AD microenviroment because Aβ reduction and increased neuron survival were observed after application of the hUCB-MSCs.
During co-culture of hUCB-MSCs with rat primary neurons, secretion of decorin and progranulin were increased in the presence or absence of Aβ42 (Figure 1). These elevations were confirmed by two-different methods: antibody-based array and ELISA. Since hUCB-MSCs protected from Aβ42 neurotoxicity in vitro, we tested whether decorin and progranulin participated in the neuroprotection of hUCB-MSCs in an AD model. Human recombinant decorin and progranulin were used to treat Aβ42-exposed rat primary neurons for 24–36 h and then neuronal survival was tested in vitro. The two proteins protected against Aβ42 neurotoxicity in vitro. These data support the suggestion that decorin and progranulin are soluble factors which are released from hUCB-MSC to protect Aβ42 neurotoxicity in vitro.
The role of decorin in cell survival has been reported in various disease models. Decorin expressed in endothelial cells prevents apoptosis of the cells in a collagen lattice[17]. In a myocardial infarction model, decorin treatment significantly mitigated fibrosis compared to control[18]. In addition, decorin stimulated cell proliferation and reduced apoptosis in the infarct area. Especilly, decorin promotes robust axon growth on inhibitory CSPGs and myelin via a direct effect on neurons[19]. Decorin pretreatment of meningial fibroblasts in vitro resulted in a three-fold increase in neurite outgrowth from co-cultured adult sensory neurons[20]. The secretion of decorin has been reported[21,22]. Expression of decorin in adipose progenitor cells[23] supports the idea that hUCB-MSCs secrete decorin.
Recently, mutations in the progranulin (PGRN) gene were found to cause familial and apparently sporadic frontotemporal lobe dementia[24]. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. These data indicated that mutations in PGRN as a cause of neurodegenerative disease and indicate the importance of PGRN function for neuronal survival[25]. Interestingly, it has been demonstrated that progranulin neurotrophic factor enhances neuronal survival and axonal growth[26]. PGRN stimulates ribosomal S6 kinase (p90RSK) and phosphatidylinositol-3 kinase/Akt cell survival pathways, and rescues cortical neurons from cell death induced by glutamate or oxidative stress. Although progranulin is a well-known secreted protein[25], there is no report regarding secretion of progranulin in MSCs. Since we confirmed mRNA expression of progranulin in hUCB-MSCs (unpublished data) and since ELISA with rat-derived progranulin was negative (Figures 1 and 3), progranulin is expected to be secreted from hUCB-MSCs. Collectively, these reports support the view that decorin and progranulin are survival factor for cells in neurodegenerative diseases.
In previous reports, we have been shown that hUCB-MSCs exhibited neuroprotection in an AD model via paracrine action. Especially, sICAM-1 was released from hUCB-MSCs and stimulated microglia to produce the A degrading enzyme neprilysin[15]. Presently, we implicate progranulin and decorin as additional paracrine factors that exert an anti-apoptotic effect against Aβ42-neurotoxicity (Figure 4). Since hUCB-MSCs seem to act as part of a cocktail of several drugs, we expect the emergence of paradigm-shifting approaches such as stem cell therapeutics for AD in the near future.
Human umbilical cord blood-derived mesenchymal stem cell (hUCB-MSC) has been regarded as a fascinating candidate of stem cell therapy in alzheimer’s disease (AD). Recently, we reported that transplantation of hUCB-MSC reduced amyloid plaques via release of soluble intercellular adhesion molecule-1 in vitro and in vivo. The paracrine action has been spotlighted as a main mechanism of action for hUCB-MSC.
In this study, the authors identified paracrine factors of hUCB-MSC for Aβ42 neurotoxicity in vitro. This data will be additional example of paracrine action of hUCB-MSC in AD microenvironment.
Stem cells are important source for not only regeneration but also paracrine action. The data provided real protein factors to protect Aβ42 neurotoxicity in vitro.
The study results suggest that hUCB-MSC is a potential therapeutic material that could be used in treatment for AD.
This is a good descriptive study in which authors analyze the therapeutic effect of hUCB-MSC on AD Induced by toxix amyloid beta proteins. The results are interesting and suggest that hUCB-MSC is a potential therapeutic source of stem cells that could be used in treatment of AD.
| 1. | Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3429] [Cited by in RCA: 3737] [Article Influence: 233.6] [Reference Citation Analysis (10)] |
| 2. | Miller G. Pharmacology. The puzzling rise and fall of a dark-horse Alzheimer's drug. Science. 2010;327:1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh JG, Lee BH, Jin HK. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 4. | Lee HJ, Lee JK, Lee H, Shin JW, Carter JE, Sakamoto T, Jin HK, Bae JS. The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer's disease. Neurosci Lett. 2010;481:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Lee H, Lee JK, Min WK, Bae JH, He X, Schuchman EH, Bae JS, Jin HK. Bone marrow-derived mesenchymal stem cells prevent the loss of Niemann-Pick type C mouse Purkinje neurons by correcting sphingolipid metabolism and increasing sphingosine-1-phosphate. Stem Cells. 2010;28:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton's Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells. 2010;2:34-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 658] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 10. | Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, Dilley RJ. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21:2189-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A. 2012;18:1479-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 12. | Wang Y, Yao HL, Cui CB, Wauthier E, Barbier C, Costello MJ, Moss N, Yamauchi M, Sricholpech M, Gerber D. Paracrine signals from mesenchymal cell populations govern the expansion and differentiation of human hepatic stem cells to adult liver fates. Hepatology. 2010;52:1443-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211-26222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Kim JY, Kim DH, Kim DS, Kim JH, Jeong SY, Jeon HB, Lee EH, Yang YS, Oh W, Chang JW. Galectin-3 secreted by human umbilical cord blood-derived mesenchymal stem cells reduces amyloid-beta42 neurotoxicity in vitro. FEBS Lett. 2010;584:3601-3608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ. 2012;19:680-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1574] [Cited by in RCA: 1481] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 17. | Schönherr E, O'Connell BC, Schittny J, Robenek H, Fastermann D, Fisher LW, Plenz G, Vischer P, Young MF, Kresse H. Paracrine or virus-mediated induction of decorin expression by endothelial cells contributes to tube formation and prevention of apoptosis in collagen lattices. Eur J Cell Biol. 1999;78:44-55. [PubMed] |
| 18. | Li L, Okada H, Takemura G, Kosai K, Kanamori H, Esaki M, Takahashi T, Goto K, Tsujimoto A, Maruyama R. Postinfarction gene therapy with adenoviral vector expressing decorin mitigates cardiac remodeling and dysfunction. Am J Physiol Heart Circ Physiol. 2009;297:H1504-H1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Minor K, Tang X, Kahrilas G, Archibald SJ, Davies JE, Davies SJ. Decorin promotes robust axon growth on inhibitory CSPGs and myelin via a direct effect on neurons. Neurobiol Dis. 2008;32:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004;19:1226-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Brandan E, Fuentes ME, Andrade W. The proteoglycan decorin is synthesized and secreted by differentiated myotubes. Eur J Cell Biol. 1991;55:209-216. [PubMed] |
| 22. | Seo NS, Hocking AM, Höök M, McQuillan DJ. Decorin core protein secretion is regulated by N-linked oligosaccharide and glycosaminoglycan additions. J Biol Chem. 2005;280:42774-42784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011;9:74-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1663] [Cited by in RCA: 1582] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 25. | Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, van Swieten J, Carmeliet P, Van Den Bosch L, Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 26. | Xu J, Xilouri M, Bruban J, Shioi J, Shao Z, Papazoglou I, Vekrellis K, Robakis NK. Extracellular progranulin protects cortical neurons from toxic insults by activating survival signaling. Neurobiol Aging. 2011;32:2326.e5-2326.16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Peer reviewers: Juan Antonio Marchal Corrales, MD, PhD, Professor, Department of Human Anatomy and Embriology, School of Medicine, Biopathology and Regenerative Medicine Institute, Centre for Biomedical Research, University of Granada, Avenida del Conocimiento s/n, 18100 Granada, Spain; Shihori Tanabe, PhD, Senior Researcher, Division of Safety Information on Drug, Food and Chemicals, National Institute of Health Sciences, 1-18-1, Kami-yoga, Setagaya-ku, Tokyo 158-8501, Japan
S- Editor Jiang L L- Editor A E- Editor Xiong L