Published online Dec 26, 2011. doi: 10.4252/wjsc.v3.i12.113
Revised: October 30, 2011
Accepted: November 7, 2011
Published online: December 26, 2011
AIM: To improve hepatic differentiation of human mesenchymal stem cell (MSC) using insulin growth factor 1 (IGF-I), which has important role in liver development, hepatocyte differentiation and function.
METHODS: Bone marrow of healthy donors was aspirated from the iliac crest. The adherent cells expanded rapidly and were maintained with periodic passages until a relatively homogeneous population was established. The identification of these cells was carried out by immunophenotype analysis and differentiation potential into osteocytes and adipocytes. To effectively induce hepatic differentiation, we designed a protocol based on a combination of IGF-I and liver specific factors (hepatocyte growth factor, oncostatin M and dexamethasone). Morphological features, hepatic functions and cytological staining were assessed to evaluate transdifferentiation of human marrow-derived MSCs.
RESULTS: Flow cytometric analysis and the differentiation potential into osteoblasts and adipocytes showed that more than 90% of human MSCs which were isolated and expanded were positive by specific markers and functional tests. Morphological assessment and evaluation of glycogen storage, albumin and α-feto protein expression, as well as albumin and urea secretion revealed a statistically significant difference between the experimental groups and control.
CONCLUSION: In vitro differentiated MSCs using IGF-I were able to display advanced liver metabolic functions, supporting the possibility of developing them as potential alternatives to primary hepatocytes.
- Citation: Ayatollahi M, Soleimani M, Tabei SZ, Kabir Salmani M. Hepatogenic differentiation of mesenchymal stem cells induced by insulin like growth factor-I. World J Stem Cells 2011; 3(12): 113-121
- URL: https://www.wjgnet.com/1948-0210/full/v3/i12/113.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v3.i12.113
Acute hepatic failure, despite recent therapeutic advances, remains associated with significant morbidity and mortality. The World Health Organization estimates that 20 million people worldwide have cirrhosis of the liver and/or liver cancer, arising predominantly among the estimated 500 million people (nearly 10% of the world population) who are afflicted with persistent hepatitis B (HBV) or hepatitis C (HCV) viral infection[1-2].
Liver transplantation has become the effective life-saving treatment for patients with end-stage chronic liver diseases. Due to the scarcity of donor organs, < 30% of patients on waiting lists receive a transplant. Furthermore, the majority of patients are not even placed on the transplant waiting list due to multi-organ failure, sepsis, psycho-social reasons or lack of adequate health care[3]. Since little can be done to access appropriate donors, new therapeutic approaches have gained importance in recent years.
It has been suggested that cell-based therapy is an alternative to whole organ transplantation. Cell transplantation is less invasive than whole-organ transplantation and can be performed repeatedly[4,5]. However, one major limitation of cell-based therapy for liver diseases is the availability of human hepatocytes. The storage of donor hepatocytes, the difficulties for large scale hepatocyte amplification, and function maintenance limit the clinical application of this cell-based therapy[6].
After MSCs were first described by Fridenstein et al[7], in 1976 as clonal, plastic adherent cells, interest in MSCs has rapidly grown with expanding knowledge about their exceptional characteristics and usefulness in the clinic by means of their differentiation potential under in vitro conditions[8,9].
MSCs can differentiate into cells of all mesodermal origins, including adipocytes, osteocytes, chondrocytes, myocytes and endothelial cells[10]. Besides these, MSCs are also capable of “transdifferentiation” into other cell lines, suggesting that MSCs belong to multipotent adult stem cells. Schwartz et al[11] isolated a non-hematopoietic stem cell subset (CD45−GlyA− in humans or CD45−Ter119− in mice) from bone marrow, termed multipotent adult progenitor cells (MAPC). Under appropriate conditions, MAPC were induced into cells with morphological, phenotypic and functional characteristics of hepatocytes in vitro.
Recently, in vitro hepatogenic differentiation of adult stem cells has been the subject of different reports[9,12,13]. Although there have been some protocols for hepatogenic differentiation of MSCs available up until now, it is essential to develop a well-defined protocol for cellular differentiation into hepatic lineage, followed by selective isolation and in vitro proliferation. This project was designed for transdifferentiation of human MSCs into liver cells using insulin-like growth factor 1 (IGF-I), which, despite its important role in liver development, has not been used for in vitro hepatic differentiation until now.
Bone marrow aspirates (5 mL) were obtained from the iliac crests of human donors ranging in age from 19 to 42 years at Bone Marrow Transplantation Center, Nemazi Hospital, Shiraz, Iran. They donated bone marrow to a related patient after obtaining approval of the Ethics Committee. Written informed consent was also obtained, allowing analysis of the clinical data and the testing mentioned in this study. The aspirates were diluted 1:1 with Dulbecco’s modified Eagle’s medium (DMEM)-low glucose (1000 mg/L) (Invitrogen, Merelbeke, Belgium) and layered over about 5 mL of ficoll (Lymphoprep; Oslo, Norway). The isolation method was according to a previously reported method[14] with some modifications which will be mentioned. After centrifugation at 2000 r/min for 20 min, the mononuclear cell layer was removed from the interface. The cells were suspended in DMEM and centrifuged at 1200 r/min for 15 min and then resuspended in basal DMEM medium containing 10% fetal calf serum (Invitrogen, Merelbeke, Belgium), 1% penicillin (Invitrogen, Merelbeke, Belgium), 1% streptomycin (Invitrogen, Merelbeke, Belgium) and 2 mmol/L glutamine (Invitrogen, Merelbeke, Belgium). The cells were seeded at a density of 80 000/cm2 in 25 cm2 T-flasks and maintained at 37 °C in an atmosphere of 5% CO2. Following 3 or 4 d of incubation, the non-adherent cells were removed and the media were changed every 3 d. In order to expand the MSC cells, the adhered monolayer was detached with trypsin-EDTA (Invitrogen, Merelbeke, Belgium) for 5 min at 37 °C, after 14 d for the first passage and every 3-4 d for successive passages. During in vitro passaging, the cells were seeded at a density of (5-10) × 103 cells/cm2 and expanded for several passages until they no longer reached confluence.
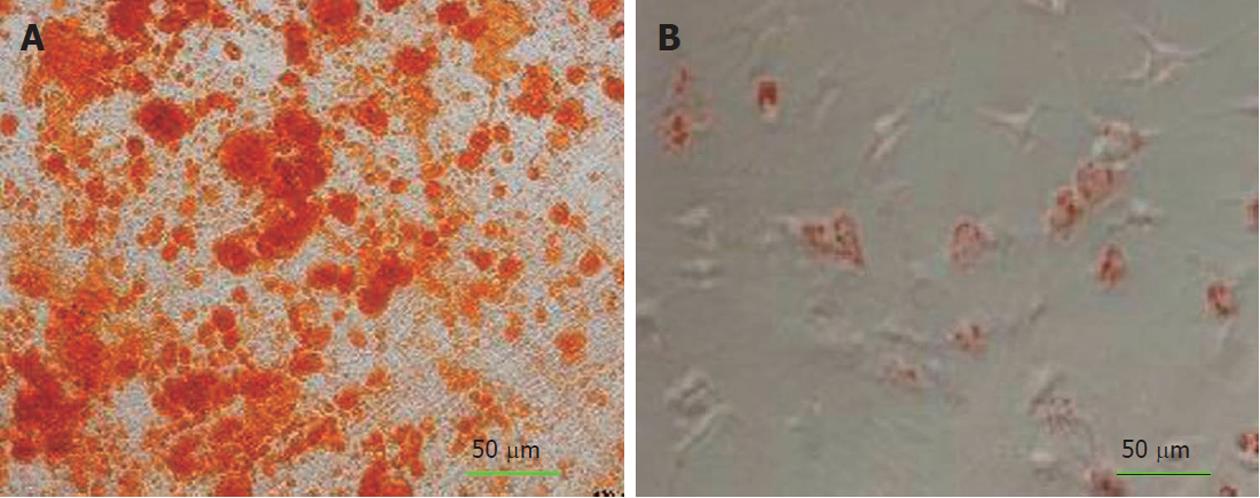
To induce adipogenic differentiation, the 4th passage cells were treated with adipogenic medium for 3 wk. Adipogenic medium consisted of DMEM supplemented with 1 M/L hydrocortisone (Sigma-Aldrich, St. Louis, United States), 0.05 g/L ascorbic acid, 0.05 g/L indomethacin (Sigma-Aldrich, St. Louis, United States) and 10-6 M/L dexamethasone, according to a previous method[15]. Adipogenesis was assessed by Oil Red O-staining. Medium changes were performed twice weekly for the two assays.
For osteogenic differentiation, the 4th passage cells were treated with osteogenic medium for three weeks. Osteogenic medium consisted of DMEM supplemented with 10-8 M/L dexamethasone (Sigma-Aldrich, St. Louis, United States), 10 mmol/L glycerol phosphate (Sigma-Aldrich, St. Louis, United States), 3.7 g/L sodium bicarbonate (Sigma-Aldrich, St. Louis, United States) and 0.05 g/L ascorbic acid (Sigma-Aldrich, St. Louis, United States)[15]. Osteogenesis was assessed by alizarin red staining.
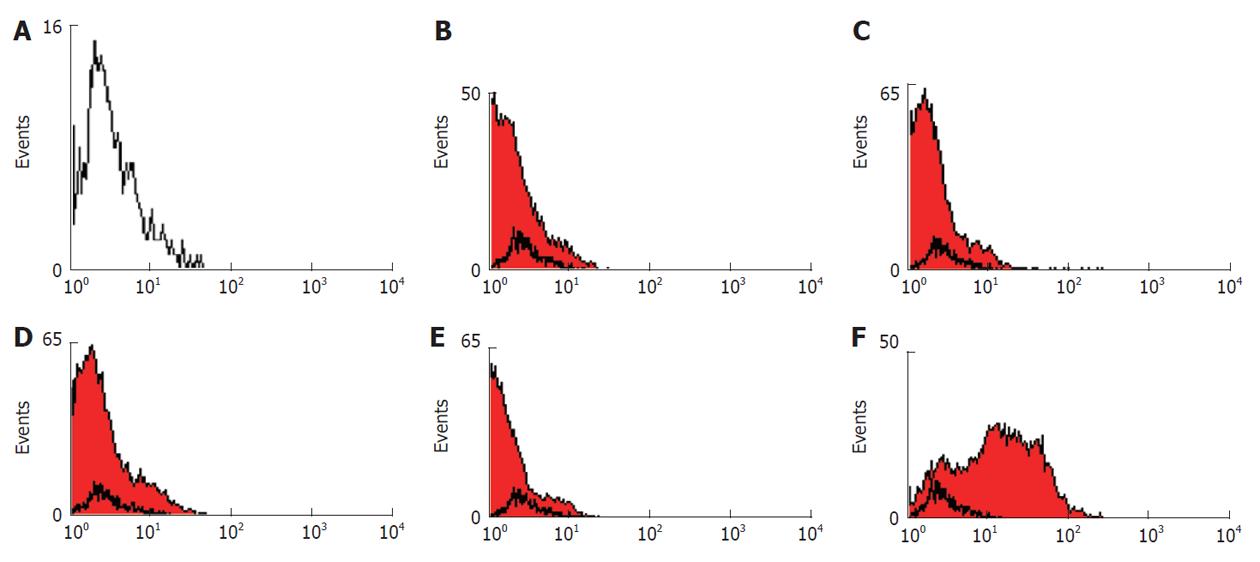
Flow cytometric analysis was according to a previously reported method[16], with some modifications which will be mentioned. The human bone marrow MSCs were detached from the tissue culture flasks at the third passage and counted. About 1 × 106 cells were incubated on ice for 30 min with goat serum, resuspended in phosophate-buffered saline (PBS) and pelleted by centrifugation for 4 min at 2000 r/min. Subsequently, the cells were stained for 30 min at 4 °C with fluorescent isothiocyanate (FITC)-conjugated CD40, CD45, CD80, CD90 and human leukocyte antigen class II (HLA-DR). An isotype control with FITC-labeled was included in each experiment and specific staining was measured from the cross point of the isotype with the specific antibody graph. The labeled cells were thoroughly washed with PBS and analyzed on a flow cytometer (FACS Calibur Becton, Dickinson, United States) using WinMidi software (Scripps Research Institute; San Diego, United States).
For hepatogenic differentiation, 3rd passage cells, 2 × 104 cells/cm2 were seeded on 24 well plastic cell culture plates. Hepatic differentiation was performed using a 2 step protocol. In the first step which lasted seven days, the cells were cultured in medium consisting of DMEM supplemented with 10% FBS, 20 ng/mL IGF-I (Peprotech, Paris, France), 20 ng/mL HGF (Peprotech, Paris, France) and 10-7 M/L dexamethasone. At the second phase, oncostatin M (Peprotech, Paris, France) was added to the mentioned media at a concentration of 10 ng/mL and continued until 21 d of differentiation. The culture media were changed twice weekly. Control cultures without the differentiation stimuli were maintained parallel to the differentiation experiments in the same manner.
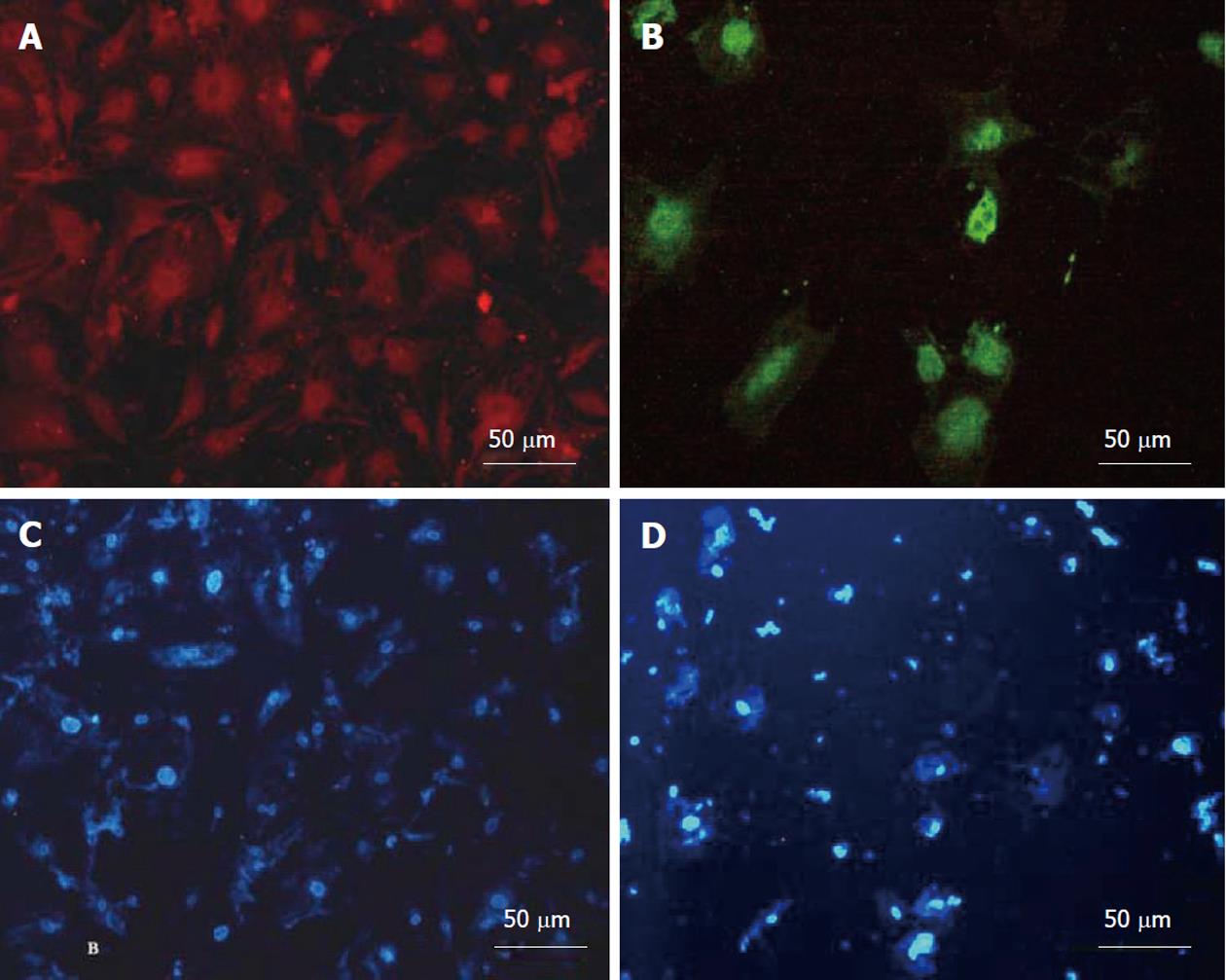
After 3 wk of differentiation, the cultured cells were washed twice with PBS and fixed with 4% paraformaldehyde for 30-45 min at room temperature and permeabilized with 0.4% Triton X-100 (Sigma-Aldrich, St. Louis, United States) for 10 min. After blocking with bovine serum albumin, the washed cells were incubated overnight at 4 °C with primary antibodies, including mouse anti-human albumin (1:1000) and mouse anti-human α-feto protein (AFP) (1:500) (Sigma-Aldrich, St. Louis, United States). Subsequently, the cells incubated with fluorescence labeled secondary goat anti-mouse IgG were marked with PE and FITC (Sigma-Aldrich, St. Louis, United States) at 37 °C for 3 h in a dark room. The cells were then incubated with DAPI (4’,6-diamidino-2-phenylindole; 1:1000) for nuclear staining. Between each incubation, the samples were washed with PBS-0.05% Tween. The ratio of immunopositive cells to the total number of cell nuclei labeled with DAPI was recorded.
Intracellular glycogen was analyzed by Periodic Acid-Schiff (PAS) staining. Culture dishes containing the cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 10 min. The samples were then oxidized in 1% periodic acid for 10 min, rinsed 3 times in deionized water (dH2O), treated with Schiff’s reagent for 20 min at room temperature and rinsed in dH2O for 5 to 10 min. The nuclei were stained with Mayer’s hematoxylin for 1 min, rinsed in dH2O and assessed under a light microscope.
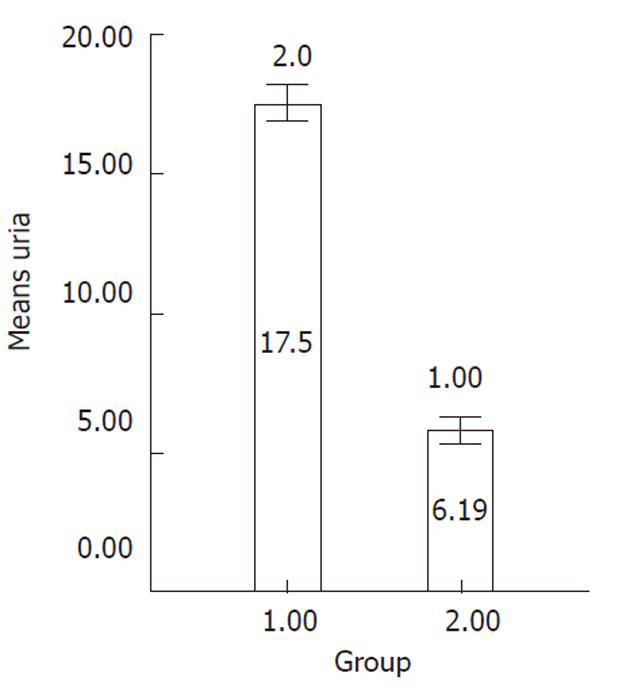
Differentiated cells were incubated with the medium containing 5 mmol/L NH4Cl for 24 h in 5% CO2 at 37 °C on day 21 of differentiation. Following incubation, the obtained supernatant was collected and urea concentration was measured by a colorimetric assay according to the manufacturer’s instructions (Quantichrom Urea assay kit, Bioassay Systems, Brussels, Belgium). This assay is based on the reduction of ammonia produced via urea hydrolysis. Undifferentiated MSCs were used as negative control.
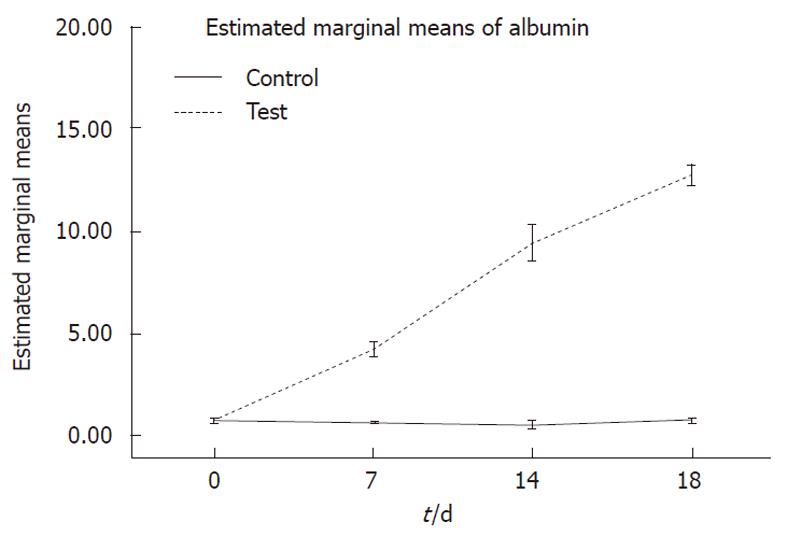
Albumin concentration, secreted into the culture media on days 0, 7, 14 and 18, was assayed by a quantitative enzyme-linked immunosorbent assay kit (ELISA)[17].
Triplicate samples obtained from the differentiated and undifferentiated cells were analyzed in duplicate for determination of albumin and urea. Statistical analysis were performed using two-way Anova test for albumin secretion and Kruskal-Wallis analysis for urea production. The significance level was set at 0.05.
Human bone marrow MSCs were cultivated from the mononuclear cell fraction of bone marrow samples. To ensure removal of contamination, the cells were selected based on plastic adherence and passaged 3 times prior to experiments.
To evaluate whether the culture-expanded cells were genuine MSCs, their mesodermal differentiation potential into osteogenic and/or adipogenic-specific agents and immunophenotyping profile were examined.
Alizarin red staining confirmed the deposition of a mineralized extracellular matrix in the culture plates that can be detected after osteogenic differentiation. Likewise, lipid droplets in differentiated adipocytes were located by Oil Red O-staining (Figure 1A-B) whereas undifferentiated MSCs were negative in staining procedure (data were not shown).
Flow cytometric analysis showed that the human bone marrow MSCs were negative for hematopoietic markers CD45 (95.49%), the human leukocyte antigen class II (HLA-DR) (98.15%) and the costimulatory molecules CD40 (97.62%) and CD80 (95.97%), whereas they were positive for CD90 (Figure 2). These results indicate that the isolated cells have the basic properties of the MSCs.
To evaluate transdifferentiation of MSCs to the hepatocyte-like cells, morphological features, cytological staining to detect specific biological markers, and hepatic functions were assessed.
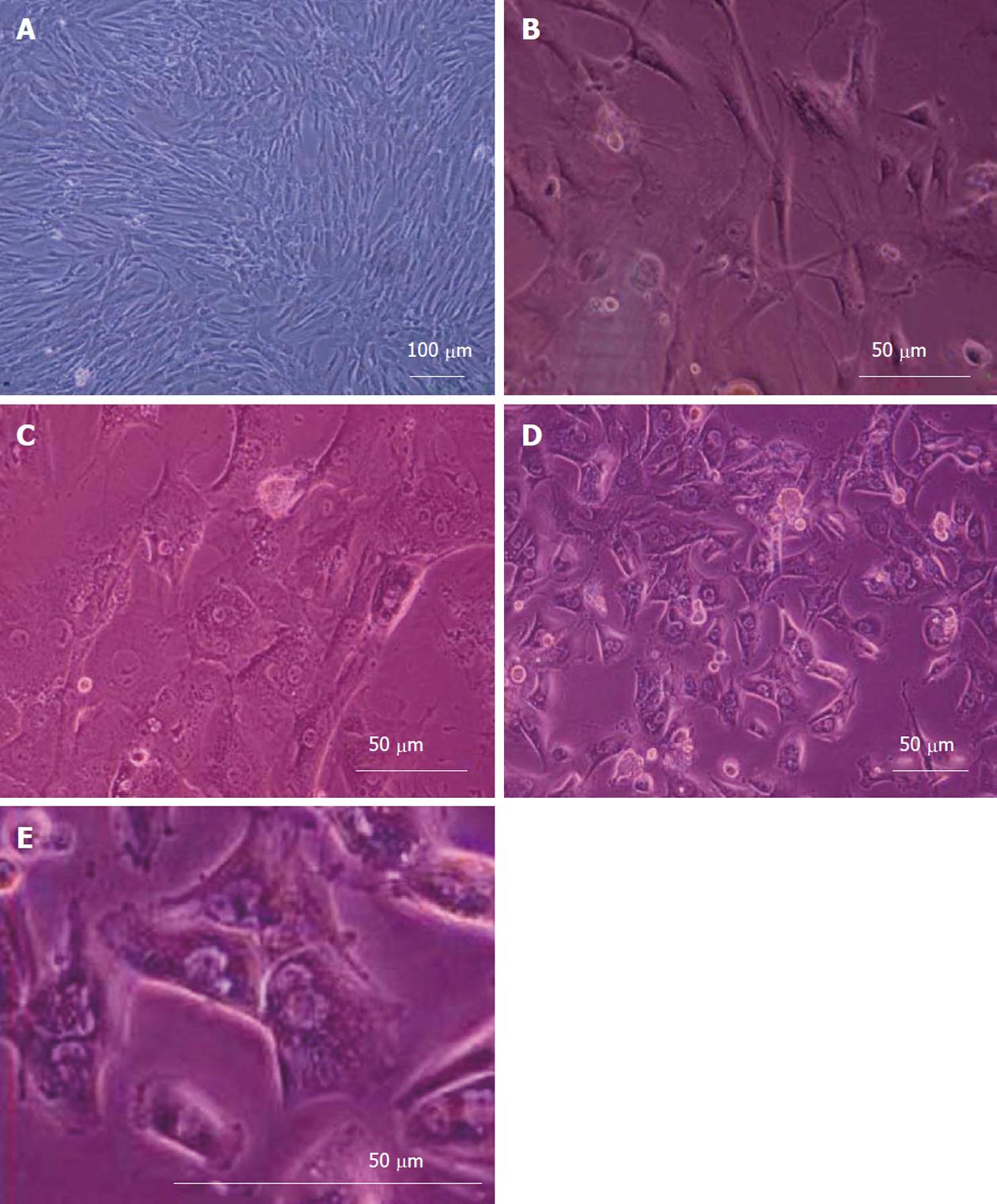
Figure 3 shows the morphological changes of bone marrow MSCs. The cells obtained from human culture before differentiation (day 0) exhibited a fibroblast-like morphology (Figure 3A). Under hepatogenic conditions, the fibroblastic morphology of MSCs gradually progressed toward the polygonal morphology of hepatocytes in a time-dependent manner. Although the epithelial cells appeared in the culture from day 6 on, these cells were still surrounded by spindle-shaped cells (Figure 3B). After 18 d, less fibroblastic cells were seen and binucleated cells appeared (Figure 3C). After 20 d, the mature cuboidal morphology with granulated structures fully developed in cultures and the cells exhibited a polygonal shape with cytoplasmic granules (Figure 3D and E).
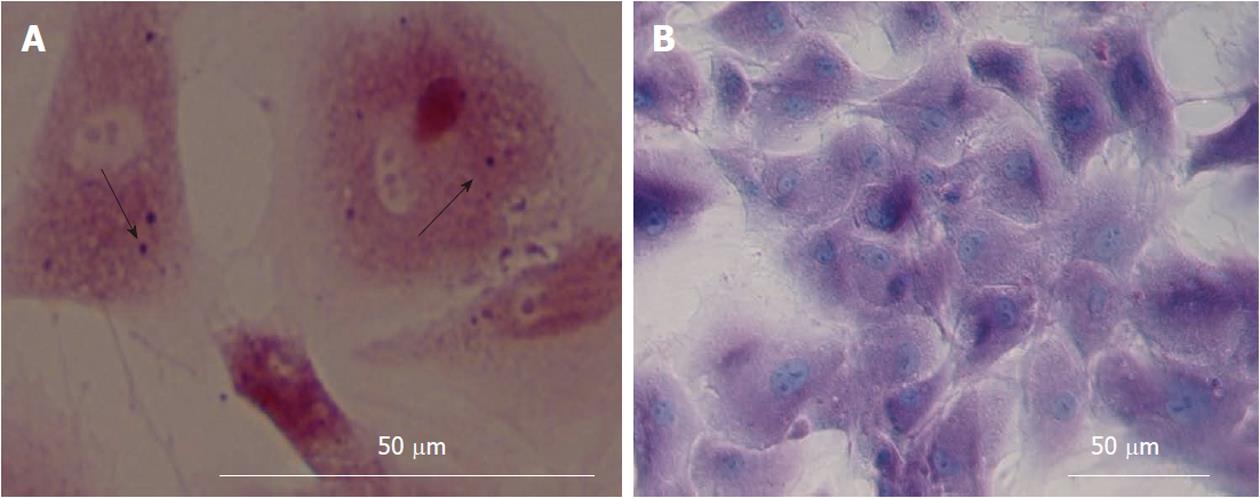
Hepatic differentiation from human bone marrow MSCs was confirmed by showing expression of albumin (the most abundant protein synthesized by functional hepatocytes) and AFP (a protein indicative of hepatocyte morphology) in our cell population. This analysis showed positive staining for albumin (Figure 4A) and AFP (Figure 4B). The nuclear staining is shown in (Figure 4C and D). The percentage of albumin and AFP positive cells were 52% ± 6% and 22% ± 4% in differentiated cells. These markers were not expressed in MSCs as the control group.
In order to assess whether these hepatocyte-like cells derived from the bone marrow also acquired typical functional hepatic features, albumin secretion, glycogen storage and urea production were evaluated.
Albumin secretion: Albumin was secreted in the culture media and was analyzed on days 0, 7, 14 and 18 of differentiation. Hepatogenic-treated MSCs produced significantly higher levels of albumin compared to undifferentiated cells (P < 0.001) (Figure 5).
Glycogen storage: Upon treatment with hepatogenic media, glycogen uptake was first seen after 21 d of the culture. Intracellular glycogen was analyzed by Periodic Acid-Schiff (PAS) staining (Figure 6A and B).
Ureogenesis: The urea production was significantly increased over the culture time compared to undifferentiated cells (P < 0.001) (Figure 7).
Liver development is accomplished by a sequential array of biological events. Each step of cell growth and differentiation is tightly regulated by cell autonomous mechanisms and extracellular signals, including cytokines and growth factors[18]. Previously, Schwartz et al[11] described a population of cells in postnatal rat bone marrow which was capable of differentiating into cells of endodermal (hepatocytes) origin upon exposure to defined-hepatogenic factors. Lee et al[13] have described hepatic differentiation of human MSCs upon exposure to hepatocyte growth factor (HGF), fibroblast growth factor (FGF) and basic fibroblast growth factor (bFGF). Snykers et al[12] have indicated hepatogenic differentiation of rat MSCs by using growth factors such as HGF, FGF, oncostatin M (OSM) and insulin-transferrin-selenium. Although such liver-specific cytokines and growth factors have been used for in vitro differentiation of stem cells into hepatocyte-like cells from adipose tissue[19], peripheral blood[20] and human bone marrow sources in further studies[21], attempts at using various approaches to enhance the effectiveness of cell therapy have become a challenge.
In the present study, an attempt was made to improve the hepatic differentiation process under exposure of human bone marrow MSCs in a novel culture composition media using HGF, OSM and dexamethasone in combination with IGF-I.
HGF is a multifunctional factor, can promote cell survival and regeneration, inhibit the apoptosis of stem cells and increase the survival rate of the transplanted cells[22]. OSM is a member of the interleukin-6 family cytokines which demonstrates the progression of hepatocyte development toward maturation[23]. Dexamethasone is a synthetic glucocorticoid hormone active in induction of enzymes concerned with gluconeogenesis in the liver[12]. IGF-I is a potent cytoprotective and anabolic hormone, synthesized mainly in the liver. Once IGF-I binds to IGF-I receptor, the receptor autophosphorylates and activates phosphatidyl-inositol (PI) 3 kinase or mitogen-activated protein (MAP) kinase cascades that regulate genes involved in cell survival, growth and differentiation of stem cells[24]. In rat, the expression of IGF-I appears in the mesenchyme of the septum transversum where hepatoblasts originate on the 9th day of gestation and diminish at 11 d after birth[25]. In human liver, the expression of IGF-I is more abundant in the fetus than in adults, with the peak from around birth until the age of 9 mo[26].
Interestingly, the expression of IGF-I appears slightly earlier than CAAT/Enhancer-Binding Protein (C/EBP) in the proximity of hepatoblasts, suggesting that IGF-I might influence C/EBP[27]. The liver-enriched transcription factor of C/EBP belongs to a family of “Leucine Zipper” DNA-binding proteins. The six members of the C/EBP family (C/EBPα to C/EBPδ) all play central roles in the growth and differentiation of hepatocytes and regulating the expression of liver-specific genes[28]. Furthermore, it was reported that in liver cirrhosis, as the result of hepatocellular insufficiency, there is a marked reduction in the levels of IGF-I[29] and IGF-I gene transfer to the cirrhotic liver improves liver function and causes a marked reduction of liver fibrosis[30]. This indicates that IGF-I is deeply involved in hepatocyte differentiation and function, but little is known about IGF-I effect on in vitro hepatic differentiation.
Here, we report our experience in the development of a novel strategy which allows hepatic differentiation of human bone marrow derived MSCs to functional hepatocytes, based on a protocol with sequential addition of IGF-I in combination with the growth factors HGF, OSM and dexamethasone. Under these culture conditions, human MSCs were successful in all examinations related to hepatogenic differentiation, including morphology, albumin and α-feto protein expression, glycogen storage, urea production and albumin secretion. Furthermore, more than 50% of these epithelioid cells expressed liver-associated protein albumin. Indeed, functional characterization of the differentiated cells at different time points were consistent with the morphological changes, with the exception of albumin which is strongly detectable by the protein level secretion and immunocytochemical analysis at all time points (days 7, 14, 18 and 21).
It is well documented that cytokines and growth factors act cooperatively and synergistically to promote liver-specific gene transcription in differentiated cells[15]. It was previously shown that HGF combined with IGF improves expression of GATA-4 in MSCs[31]. GATA-4 is the earliest known transcription factor to bind the albumin gene enhancer in liver precursor cells, reflecting liver-specific functions[32]. The functional assays for hepatocytes are mainly urea and albumin synthesis; the synthesis of urea is performed only by hepatocytes and not by yolk sac and should be fit as a test for hepatocytes only[21]. In this regard, differentiated cells in our study acquired hepatocyte functions, including urea secretion and glycogen storage after 4 wk, and were sustained to 6 wk postinduction or later. Furthermore, exposure of the cells under in vitro conditions featured highly differentiated hepatic morphological changes. According to these findings, the mature cuboidal morphology with cytoplasmic-granulated structure was fully developed before 4 wk of postinduction. It has been indicated that MSCs acquired morphological features (polygonal-shaped and binucleated cells) similar to those of primary hepatocytes. Indeed, the cells shared typical characteristics with hepatocytes including albumin secretion and urea production which was statistically significant, as well as cytoplasmic storage of glycogen.
In this study, we have reported a novel strategy which allows hepatic differentiation of human bone marrow derived MSCs to functional hepatocytes. This model opens new perspectives: it may be applicable not only to the study of cell fate and trans-differentiate uncommitted cells towards endodermal lineages, but also indicates that the growth factors may have a great potential in clinical cell therapy.
The authors would like to thank Mr. HR Zare for his assistance in flow cytometric analysis at Immunology Department; and also Dr. N Shokrpour at Research and Consultation Center in regard to editing the manuscript.
Mesenchymal stem cell (MSC) therapies have gained importance as a suitable potential substitution for liver transplantation. Although there have been some protocols for hepatogenic differentiaion of MSCs available up till now, it is essential to develop well-defined protocols for cellular differentiation into hepatic lineage. This project was designed to transdifferentiation of human MSCs into the liver cells using insulin-like growth factor 1 (IGF-I) which despite of its important role in liver development, has not been used for in vitro hepatic differentiation till now.
The research hotspot is to improve hepatic differentiation of human MSC using IGF-I which has important role in liver development, hepatocyte differentiation and function.
In the present study, the authors report a novel strategy for the hepatogenic differentiation of bone marrow-derived human MSCs, which allows the hepatic differentiation from progenitor cells to functional hepatocytes, based on using a combination of IGF-Iand cytokines. Morphological assessment and evaluation of glycogen storage, albumin and α-feto protein expression as well as albumin and urea secretion revealed a statistically significant difference between the experimental groups and control. The current study demonstrates that the in vitro differentiated MSCs using IGF-I are able to display advanced liver metabolic functions supporting the possibility to develop them as potential alternatives to primary hepatocytes.
The present study may not only be helpful for the clinical application of hepatocyte transplantation, but also it is indicating that the growth factors may have a great potential in the clinical cell therapy.
The IGF-I is a potent cytoprotective and anabolic hormone, synthesized mainly in the liver. According to the data, IGF-I is deeply involved in hepatocyte differentiation and function, but little is known about IGF-I effect on in vitro hepatic differentiation.
To improve the protocols for hepatogenic differentiation is an important area for research as these cells are potential alternatives to primary hepatocytes for future clinical application.
| 1. | Dietreich D. Strategies for management of HCV/HIV coinfection. 3rd ed. PDR. 2005;101-113. |
| 2. | Rozga J. Liver support technology--an update. Xenotransplantation. 2006;13:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Jalan R. Acute liver failure: current management and future prospects. J Hepatol. 2005;42 Suppl:S115-S123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Nussler A, Konig S, Ott M, Sokal E, Christ B, Thasler W, Brulport M, Gabelein G, Schormann W, Schulze M. Present status and perspectives of cell-based therapies for liver diseases. J Hepatol. 2006;45:144-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Selden C, Hodgson H. Cellular therapies for liver replacement. Transpl Immunol. 2004;12:273-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 7. | Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267-274. [PubMed] |
| 8. | Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 830] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 9. | Dong XJ, Zhang GR, Zhou QJ, Pan RL, Chen Y, Xiang LX, Shao JZ. Direct hepatic differentiation of mouse embryonic stem cells induced by valproic acid and cytokines. World J Gastroenterol. 2009;15:5165-5175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 666] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 11. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [PubMed] |
| 12. | Snykers S, Vanhaecke T, Papeleu P, Luttun A, Jiang Y, Vander Heyden Y, Verfaillie C, Rogiers V. Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol Sci. 2006;94:330-341; discussion 235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 664] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 14. | Ayatollahi M, Kabir Salmani M, Soleimani M, Geramizade B, Sanati MH, Gardaneh M, Tabei SZ. Expansion of human marrow derived mesenchymal stem cells and their transdifferentiation potential. IRCMJ. 2010;12:446-452. |
| 15. | Nardi BN, Meirelles LS. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249-282. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Kazemnejad S, Allameh A, Soleimani M, Gharehbaghian A, Mohammadi Y, Amirizadeh N, Jazayery M. Biochemical and molecular characterization of hepatocyte-like cells derived from human bone marrow mesenchymal stem cells on a novel three-dimensional biocompatible nanofibrous scaffold. J Gastroenterol Hepatol. 2009;24:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Koebe HG, Wick M, Cramer U, Lange V, Schildberg FW. Collagen gel immobilisation provides a suitable cell matrix for long term human hepatocyte cultures in hybrid reactors. Int J Artif Organs. 1994;17:95-106. [PubMed] |
| 18. | Kinoshita T, Miyajima A. cytokine regulation for liver development. Biochim Biophys Acta. 2002;1592:303-331. [RCA] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 398] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 20. | Ruhnke M, Ungefroren H, Nussler A, Martin F, Brulport M, Schormann W, Hengstler JG, Klapper W, Ulrichs K, Hutchinson JA. Differentiation of in vitro-modified human peripheral blood monocytes into hepatocyte-like and pancreatic islet-like cells. Gastroenterology. 2005;128:1774-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Lavon N, Benvenisty N. Study of hepatocyte differentiation using embryonic stem cells. J Cell Biochem. 2005;96:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1039] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 23. | Miyajim A, Kinoshita T, Tanaka M, Kamiya A, Mukouyama Y, Hara T. Role of oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev. 2000;11:177-183. [RCA] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13 Suppl 1:S33-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Streck RD, Pintar JE. The embryonic pattern of rat insulin-like growth factor-I gene expression suggests a role in induction and early growth of the liver. Endocrinology. 1992;131:2030-2032. [PubMed] [DOI] [Full Text] |
| 26. | Li X, Cui H, Sandstedt B, Nordlinder H, Larsson E, Ekström TJ. Expression levels of the insulin-like growth factor-II gene (IGF2) in the human liver: developmental relationships of the four promoters. J Endocrinol. 1996;149:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Tomizawa M, Saisho H. Insulin-like growth factor (IGF)-II regulates CCAAT/enhancer binding protein alpha expression via phosphatidyl-inositol 3 kinase in human hepatoblastoma cell lines. J Cell Biochem. 2007;102:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part II: the C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol Rev. 2004;56:291-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, Castilla-Cortazar I, Frystyk J, Flyvbjerg A, Yoshizawa C. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. 2005;43:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Sobrevals L, Rodriguez C, Romero-Trevejo JL, Gondi G, Monreal I, Pañeda A, Juanarena N, Arcelus S, Razquin N, Guembe L. Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology. 2010;51:912-921. [PubMed] |
| 31. | Li Z, Gu TX, Zhang YH. Hepatocyte growth factor combined with insulin like growth factor-1 improves expression of GATA-4 in mesenchymal stem cells cocultured with cardiomyocytes. Chin Med J (Engl). 2008;121:336-340. [PubMed] |
| 32. | Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret K. S. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Molecular Cell. 2002;9:279-289. [RCA] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 939] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
Peer reviewers: Mark A LaBarge, PhD, Life Science Division, Lawrence Berkeley National Laboratoy, Berkeley, CA 94720, United States; Ling-Ling Hou, PhD, College of Life Sciences and Bioengineering, School of Sciences, Beijing Jiaotong University, Beijing 100044, China
S- Editor Wang JL L- Editor Roemmele A E- Editor Zhang DN