Published online Oct 26, 2010. doi: 10.4252/wjsc.v2.i5.114
Revised: September 22, 2010
Accepted: September 29, 2010
Published online: October 26, 2010
AIM: To establish and characterize a spontaneously immortalized human dermal microvascular endothelial cell line, iHDME1.
METHODS: We developed a spontaneous immortalization method. This approach is based on the application of optimized culture media and culture conditions without addition of any exogenous oncogenes or carcinogens. Using this approach, we have successfully established a microvascular endothelial cell line, iHDME1, from primary human dermal microvascular endothelial cells. iHDME1 cells have been maintained in culture dishes for more than 50 passages over a period of 6 mo. Using a GFP expressing retrovirus, we generated a GFP-stable cell line (iHDME1-GFP).
RESULTS: iHDME1 retain endothelial morphology and uniformly express endothelial markers such as VEGF receptor 2 and VE-cadherin but not α-smooth muscle actin (α-SM-actin) and cytokeratin 18, markers for smooth muscle cells and epithelial cells respectively. These cells retain endothelial properties, migrate in response to VEGF stimulation and form 3-D vascular structures in Matrigel, similar to the parental cells. There is no significant difference in cell cycle profile between the parental cells and iHDME1 cells. Further analysis indicates enhanced stemness in iHDME1 cells compared to parental cells. iHDME1 cells display elevated expression of CD133 and hTERT.
CONCLUSION: iHDME1 cells will be a valuable source for studying angiogenesis.
- Citation: Jiang M, Min Y, DeBusk L, Fernandez S, Strand DW, Hayward SW, Lin PC. Spontaneous immortalization of human dermal microvascular endothelial cells. World J Stem Cells 2010; 2(5): 114-120
- URL: https://www.wjgnet.com/1948-0210/full/v2/i5/114.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v2.i5.114
During angiogenesis, endothelial cells migrate, proliferate and assemble into vascular structures. To examine the molecular mechanisms of angiogenesis and vascular biology, appropriate cell lines are an important requirement. Currently, primary cells isolated from human tissues are the major source of endothelial cells. These cells are normal and, in principle, are suitable for angiogenic studies. However, under current cell culture conditions these cells lose their endothelial properties within a few passages. As with most mammalian somatic cells, these primary cells have a limited lifespan, stop dividing and become senescent after a predictable number of cell divisions[1,2]. Cellular senescence is triggered by two interdependent mechanisms; cell cycle arrest controlled by p19ARF/p53 and p16INKa/RB tumor suppressor pathways[3,4] and shortening of the telomeres due to the end-replication problem in chromosome replication[5]. These properties have limited the application of primary endothelial cells in experiments involving genetic manipulation.
As an alternative, viral oncogenes have commonly been used to immortalize primary cells. This approach prevents cells from senescence[6,7]. It also extends cell’s life span by binding and inactivating p53 and RB proteins[8]. Oncogene immortalized cells are generally stable and can be cultured for long periods. However, since the viruses used for this process are fundamentally oncogenic, concerns are raised as to whether these cells gain transformed properties[9,10].
A number of publications have reported spontaneous immortalization of human cell lines which include keratinocytes[11,12], breast epithelial cells MCF-10[13] and prostate epithelial cells[14,15]. The presence of nature elevated hTERT and CD133 in a rare population of stem/progenitor cells could be a factor to promote cell self-renewal and enrichment. However, no study has been performed on endothelial cells. In this report, we describe a novel approach to generate a spontaneously immortalized human dermal microvascular endothelial cell line without the use of oncogenes. These cells are stable for prolonged culture and make feasible stable genetic manipulation. They retain endothelial cell properties with progenitor/stem cell-like characteristics.
Primary human dermal microvascular endothelial cells (HDMECs) were purchased from Clonetics and cultured in EGM media (Clonetics). Antibodies against Ku70, CD133 and VEGF receptor 2 (VEGFR2) were purchased from AbCam, anti-hTERT from Santa Cruz and anti-α-SM-actin from Sigma. Anti-CK18 was a gift from Dr. EB Lane, University of Dundee, Scotland. FITC- or TRITC-conjugated secondary antibodies were purchased from Sigma.
HDMECs were cultured in an optimized culture medium made using 50% RPMI 1640 (Invitrogen, Grand Island, NY) and 50% Keratinocyte-SFM (K-SFM, Invitrogen) containing 5% fetal bovine serum, FBS (Atlanta Biologicals, Atlanta, GA) and 1% antibiotic-antimycotic mix (Ab/Am, Life Technologies, Grand Island, NY). The cells were continuously passaged in excess of 20 times at a suitable dilution ratio (1:2) every 3 d. After that, these cells were routinely maintained in RPMI 1640 with 5% FBS and 1% Ab/Am for more than 30 passages without senescence or crisis. At this point the cells were designated as an immortalized endothelial cell line, iHDME1. The iHDME1 cells are easily recovered from storage in liquid nitrogen.
iHDME1 cells were retrovirally infected using a pBird-CMV-EGFP vector[16]. GFP positive cells were sorted by FACS and stable cell lines were established (iHDME1-GFP). The cells were maintained in RPMI 1640 with 5% FBS and 1% Ab/Am.
HDMECs and iHDME1 cells grown on culture chamber slides were fixed in 50% methanol/ethanol and incubated with antibodies against VEGFR2, VE-cadherin, α-SM-actin, CK18, Ku70, hTERT and CD133, followed by incubation with fluorescence labeled 2nd antibodies. Observations were made under fluorescence microscopy (ZEISS, Axio imager M1).
For cell cycle analysis, HDMECs and iHDME1 cells were pulsed with BrdU (10 μmol/L) for 1 h. After incubation, the cells were washed with PBS and fixed with 75% ice-cold ethanol. Fixative was removed by centrifugation with PBS. DNA was partially denatured by incubation with 1 mol/L HCl for 20 min at room temperature, after which the cells were washed three times with PBS containing 0.05% Tween-20. Cells were incubated with 100 μL of FITC-conjugated anti-BrdU antibody (5 μg/mL; 1:100) in dark for one hour. The cells were further stained with PI staining solution (50 μg/mL PI, 10 μg/mL RNase, 0.01 mol/L Tris and 10 mmol/L NaCl) incubated in dark for 30 min and then analyzed using a FACScan flow cytometer (Becton Dickinson). The percentage of gated cells in G1/G0, G2/M or S-phase was calculated.
Angiogenesis assays were performed as described. For cell migration, HDMECs and iHDME1 cells were placed on the upper chamber of Transwells (Costar). The chamber was placed in medium containing 25 ng/mL of recombinant VEGF (R & D). Cells were allowed to migrate for 4 h, followed by fixation in 10% buffered formalin and staining with crystal violet. Cells that had migrated to the underside of the filter were counted in 10 randomly selected 200 × fields. For vascular tubule formation, HDMECs and iHDME1 cells were placed on top of Matrigel and incubated for 28 h at 37°C. Vascular branch crossings were counted in 10 randomly selected fields under microscopy. Each experiment was performed in triplicate and repeated three times.
To overcome the limitations of primary and viral oncogene-immortalized endothelial cell lines, we developed a novel approach to spontaneously immortalize primary HDMECs. HDMECs were purchased from Cambrex which were isolated from pooled human dermal tissues from multiple donors. Early passage cells (passage 3-5) were used in the study. We tested a number of commercial media including RPMI 1640, DMEM, F12 and K-SFM to replace the culture media specific for HDMEC endothelial cells supplied by the company. We found that the mixture of RPMI1640 and K-SFM (1:1) with total 2.5% FBS offered the best condition for cell growth and proliferation in vitro.
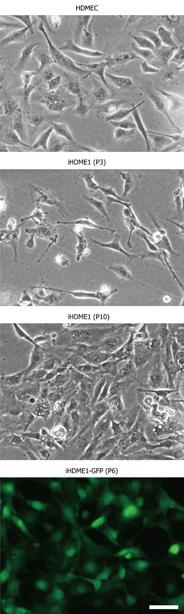
We observed that a small population of progenitor/stem-like microvascular endothelial cells was spontaneously immortalized in vitro using a set of optimized culture conditions including cell culture medium and serum. We named the cells iHDME1. These cells have been maintained in continuous culture in a simple RPMI 1640-based media for more than 50 passages over a period of 6 mo. In contrast, the parental primary cells require growth factor rich EGM media and only survive for a limited number of passages. iHDME1 cells maintain endothelial morphology in the culture dish (Figure 1). Next, we compared cell cycle profile between the parental cells and iHDME1 cells. We did not observe any significant difference in cell cycle profiles between the two cell populations (Table 1). Our protocol is based on modification of cell culture conditions and composition of culture media without using oncogenes; therefore it is unlikely that cell transformation occurs. Indeed, implantation of iHDME1 cells in nude mice did not yield any tumor formation (data not shown) which is consistent with spontaneous immortalization without using any oncogene. To demonstrate the ability to genetically manipulate these cells, we established a GFP-expressing derivative line, (iHDME1-GFP), using retroviral transduction (Figure 1). iHDME1 is phenotypically stable and is suitable for genetic manipulation.
| G1/G0 | S | G2/M | |
| HDMEC | 23.74 ± 5.9 | 27.77 ± 0.45 | 27.4 ± 3.75 |
| iHDME1 | 27.1 ± 4.12 | 28.83 ± 2.7 | 34.82 ± 1.34 |
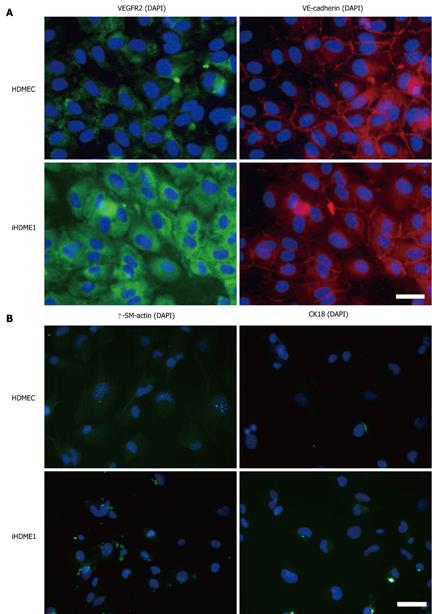
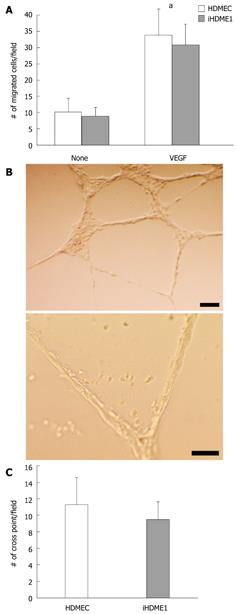
iHDME1 cells maintain uniform expression of VEGFR2 and VE-cadherin, specific markers for endothelial cells, by immunofluorescence staining. The staining is specific as the isotype controls are totally negative (data not shown). The expression of VE-cadherin is comparable to the parental cells but VEGFR2 expression is higher in iHDME1 cells than the parental cells (Figure 2A). They are negative of α-SM-actin and CK18 (Figure 2B), markers for smooth muscle and epithelial cells respectively. Further analysis confirmed that iHDME1 cells responded to VEGF stimulation and migrated along a VEGF gradient (Figure 3A). iHDME1 cells form tubule structures in 3-D Matrigel (Figure 3B). There are no significant differences in these angiogenic functions between the parental cells and iHMDE1 cells (Figure 3C). Collectively, these data demonstrate that iHDME1 cells are immortal but retain typical endothelial cell properties.
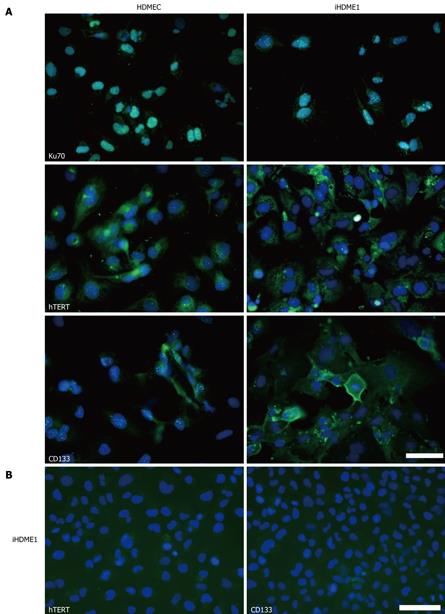
We compared protein expression profiles between parental HDMECs and immortalized iHDME1 cells by immunofluorescence staining. Both types of cells showed 100% nuclear positive staining for Ku70 protein which confirmed the human origin of the cells (Figure 4A). Interestingly, there was elevated hTERT protein expression in iHDME1 compared to parental cells (Figure 4A). TERT synthesizes and maintains the telomeres, thus preventing cellular senescence caused by the shortening of telomeres[17]. We also detected an increase of CD133 (AC133 and prominin-1), a human stem cell-associated marker, in iHDME1 compared to parental cells (Figure 4A). CD133 is also detected in endothelial progenitor cells[18]. The staining is specific as the isotype controls of hTERT and CD133 are totally negative (Figure 4B).
Angiogenesis is defined as capillary sprouting process which is essential for accommodating the metabolic demand of growing tissues. As tissue growth, tissue repair and regeneration are tightly linked to disease conditions, a study of the molecular mechanism of angiogenesis offers great promise for therapy for a variety of diseases such as cancer and cardiovascular conditions. Endothelium is a major component of blood vessel wall which plays critical roles in angiogenesis and vascular homeostasis. Therefore, establishment of proper endothelial cell lines are essential for studying the mechanisms of these processes.
Primary cells isolated directly from tissues are the major source of endothelial cells. These cells are normal and are suitable for angiogenic studies. However, these primary cells gradually lose their endothelial properties and become senescence within a few passages. Thus the cells are not suitable for genetic manipulations for mechanistic analysis. To overcome this limitation, attempts were made to immortalize the cells with viral oncogenes. The oncogene immortalized cells are stable and have an unlimited life span but concerns are raised as to whether these cells gain transformed properties.
Spontaneous immortalization has been used to establish stable cell lines through changing cell culture media and culture conditions. It does not involve utilizing viral oncogenes, thus it presents a significant advantage over oncogene-mediated process. This approach has been used to generate several epithelial cell lines, often tumor cell lines. There is no report on endothelial cells.
In this report, we developed a spontaneous immortalization method without using oncogene and established an immortalized human dermal microvascular endothelial cell line, iHDME1. iHDME1 cells are immortal and maintain endothelial properties. They did not form tumor in vivo. They display progenitor/stem cell characteristics. Based on elevated expression of stem/progenitor related genes in iHDME1, we suggest that spontaneous immortalization is a process that enriches or preferentially selects progenitor cells existing in heterogeneous primary cell population. Current effort is engaged to elucidate the mechanism. Considering the importance of endothelial cells, this method and cell line has great potential in angiogenesis and vascular biology research.
Endothelial cells are invaluable in angiogenesis and vascular biology research. Currently, there are two choices for the cell: freshly isolated primary cells from tissues and oncogene immortalized cell lines. Primary cells have a limited life span and are unstable, thereby limiting experimental genetic manipulation; while oncogene immortalized cells are stable, even if not transformed, contain significant genetic initiation.
Development of proper endothelial cell lines is essential to study angiogenesis and vascular biology. Spontaneous immortalization offers the potential to generate immortalized normal cells without introducing any oncogene.
In this study, the authors developed a spontaneous immortalization method to establish a microvascular endothelial cell line, iHDME1, from primary human dermal microvascular endothelial cells. This approach is based on the application of optimized culture media and culture conditions without addition of any exogenous oncogenes or carcinogens. The cells retain immortal status but less likely have oncogenic potential.
iHDME1 cells are invaluable in angiogenesis and vascular biology research. Spontaneous immortalization method allows us to develop endothelial cell lines from any tissue or organ.
Endothelial cells: The endothelium is the thin layer of cells that lines the interior surface of blood vessels, forming an interface between circulating blood in the lumen and the rest of the vessel wall. These cells are called endothelial cells. Endothelial cells line the entire circulatory system from the heart to the smallest capillary. Primary cells are normal cells directly isolated from tissues. They have a limited life span, either undergoing apoptosis or senescent after a predictable number of cell divisions in vitro. Spontaneous immortalization is a process in which primary cells are spontaneously induced to become immortal without using any oncogenes.
The authors have demonstrated a novel culture technique to establish an immortalized but functional human vascular endothelial cell line without introducing any oncogenes. Due to its high feasibility, the method will prevail among the researchers in this field. This work is worth reporting and will make a great contribution to an advanced understanding on human angiogenesis.
| 1. | Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585-621. |
| 3. | Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436-1447. |
| 4. | Munro J, Stott FJ, Vousden KH, Peters G, Parkinson EK. Role of the alternative INK4A proteins in human keratinocyte senescence: evidence for the specific inactivation of p16INK4A upon immortalization. Cancer Res. 1999;59:2516-2521. |
| 5. | Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349-352. |
| 6. | Gimbrone MA Jr, Fareed GC. Transformation of cultured human vascular endothelium by SV40 DNA. Cell. 1976;9:685-693. |
| 7. | Rhim JS, Tsai WP, Chen ZQ, Chen Z, Van Waes C, Burger AM, Lautenberger JA. A human vascular endothelial cell model to study angiogenesis and tumorigenesis. Carcinogenesis. 1998;19:673-681. |
| 8. | Vaziri H, Benchimol S. Alternative pathways for the extension of cellular life span: inactivation of p53/pRb and expression of telomerase. Oncogene. 1999;18:7676-7680. |
| 9. | Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683-690. |
| 10. | Matsumura T, Takesue M, Westerman KA, Okitsu T, Sakaguchi M, Fukazawa T, Totsugawa T, Noguchi H, Yamamoto S, Stolz DB. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation. 2004;77:1357-1365. |
| 11. | Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761-771. |
| 12. | Allen-Hoffmann BL, Schlosser SJ, Ivarie CA, Sattler CA, Meisner LF, O'Connor SL. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J Invest Dermatol. 2000;114:444-455. |
| 13. | Soule HD, Maloney TM, Wolman SR, Peterson WD Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075-6086. |
| 14. | Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells. 2010;28:344-356. |
| 15. | Sinisi AA, Chieffi P, Pasquali D, Kisslinger A, Staibano S, Bellastella A, Tramontano D. EPN: a novel epithelial cell line derived from human prostate tissue. In Vitro Cell Dev Biol Anim. 2002;38:165-172. |
| 16. | Tang DG, Tokumoto YM, Apperly JA, Lloyd AC, Raff MC. Lack of replicative senescence in cultured rat oligodendrocyte precursor cells. Science. 2001;291:868-871. |
| 17. | Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407-410. |
| 18. | Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592-2599. |
Peer reviewers: Ursula Margarethe Gehling, MD, Paul-Ehrlich-Institute, Paul-Ehrlich-Strasse 51-59, 63225 Langen, Germany; Tong-Chuan He, MD, PhD, Associate Professor, Molecular Oncology Lab, University of Chicago Medical Center, 5841 South Maryland Avenue, MC 3079, Chicago, IL 60637, United States; Kumiko Saeki, MD, PhD, Department of Hematology, Research Institute, International Medical Center of Japan, 1-21-1, Toyama, Shinjuku-ku, Tokyo 162-8655, Japan
S- Editor Wang JL L- Editor Roemmele A E- Editor Yang C