Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.111497
Revised: July 31, 2025
Accepted: August 7, 2025
Published online: August 26, 2025
Processing time: 51 Days and 12.6 Hours
Bone marrow-derived mesenchymal stem cells (BMSCs) and adipose tissue-derived mesenchymal stem cells (ADSCs), two principal subtypes of mesen
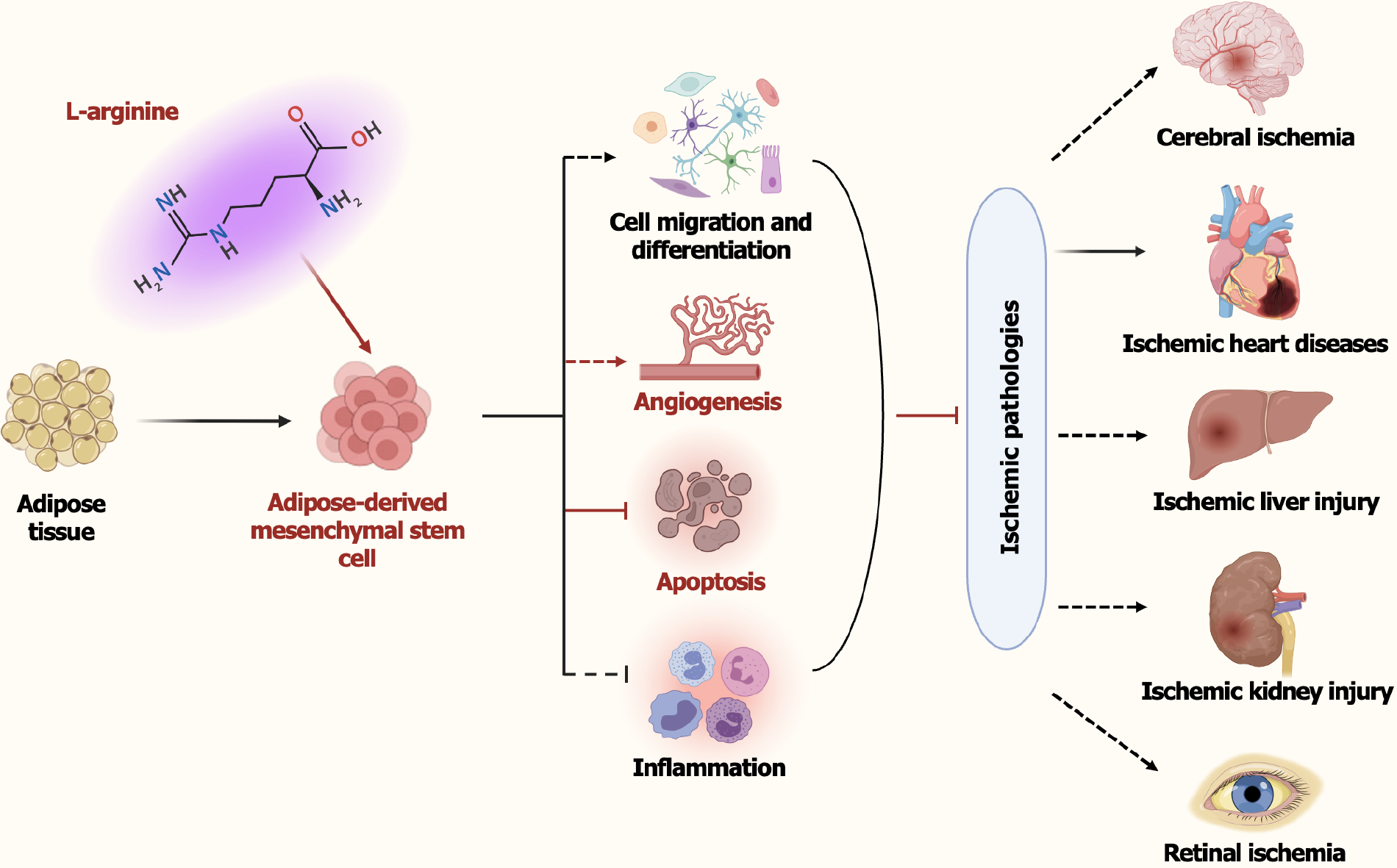
Core Tip: Ischemic pathologies impose global health challenges and economic burden due to limited therapeutic efficacy. Li et al revealed that the metabolite L-arginine, enriched in adipose tissue-derived mesenchymal stem cells (ADSCs) obtained from elderly patients, could enhance the therapeutic efficacy of ADSCs by promoting angiogenesis and inhibiting cell apoptosis in ischemic conditions. This research underscores the significance of L-arginine for ADSCs-based therapy from a metabolic perspective, highlighting the potential of combining metabolic modulation with ADSCs therapy to improve clinical outcomes in ischemic diseases, particularly in aging populations. However, additional clinical studies are essential to validate the clinical application of these findings.
- Citation: Liu N, Wan XX, Yan WT, Xiong K. L-arginine: A promising metabolite in enhancing the protective effects of adipose-derived stem cells against ischemic pathologies. World J Stem Cells 2025; 17(8): 111497
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/111497.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.111497
Ischemia, defined as the restriction or blockage of blood supply to specific organs, leads to a deficiency in oxygen and nutrients essential for cellular metabolism, ultimately contributing to a wide range of pathologies, including ischemic heart disease, cerebral ischemia, ischemic acute kidney injury, and peripheral artery disease[1-4]. The underlying mechanisms of ischemic injury are multifactorial, such as oxygen and nutrient deprivation, inflammatory responses, oxidative stress and endothelial dysfunction[5-7]. Ischemic pathologies have emerged as a significant public health challenge and economic burden worldwide, given their rising prevalence, high morbidity rates, and multiple severe complications[8-10]. Currently, available treatments for ischemia primarily involve pharmacological interventions and physical procedures aimed at restoring blood supply to ischemic tissues and minimizing subsequent tissue injury[11,12]. Despite significant advances in the development of ischemic therapy, current treatments remain invasive and ineffective, particularly in cases of severe ischemia or delayed hospital admission. Elucidating the pathophysiological mechanisms, as well as identifying new therapeutic strategies to improve ischemic control and mitigate complications, continues to be an ongoing effort with significant implications for global social and economic development.
Mesenchymal stem cells (MSCs), defined as multipotent progenitor cells with the capacity for self-renewal and differentiation, can be routinely harvested from various tissues, including dental pulp, adipose tissue, bone marrow, and umbilical cord blood[13]. MSCs have been demonstrated to play significant roles in tissue repair due to their pleiotropic functions, such as antiapoptotic, antifibrotic, proangiogenic, and neuroprotective effects[14-16]. This unique set of characteristics positions MSCs as promising candidates for therapeutic potential in the field of regenerative medicine[17-19]. Recent advances in cell therapy have established MSC-based treatments as safe and effective interventions that can be used to alleviate ischemic conditions[20,21]. For example, MSCs transplantation following acute myocardial infarction (MI) can inhibit ischemia-induced autophagy by the miR-125b-5p/p53/Bnip3 signaling pathway, ultimately exerting myocardial protective effects in the context of MI and ischemia/reperfusion injury[22]; MSC-derived exosomes alleviate cell cycle arrest and apoptosis in renal tubular epithelial cells through the miR-125b-5p/p53 signaling pathway, thereby mitigating ischemic acute kidney injury and promoting tubular repair[23]; gingival MSCs-derived exosomes confer neuroprotective effects in retinal ischemic injury via modulating the maternally expressed gene 3/miR-21a-5p/programmed cell death 4 pathway, ultimately suppressing retinal ganglion cell apoptosis and attenuating microglia-mediated neuroinflammation[24]. Additionally, both clinical trials and preclinical studies involving MSCs transplantation have shown significant improvement in patients with different types of ischemia[25,26]. The first-in-human PASSIoN trial (NCT03356821) demonstrated that intranasally administered bone marrow-derived MSCs (BMSCs) are feasible and safe in term neonates with perinatal arterial ischemic stroke[27]. A long-term follow-up of intravenously administrated MSCs in ischemic stroke patients revealed a favorable safety profile, along with enhanced functional outcomes and improved survival rates[28]. Collectively, these data support the feasibility and safety of MSCs for treating ischemic conditions in clinical trials, suggesting that MSCs transplantation may serve as a promising cell-based therapy in the future. However, additional investigation is required to comprehensively understand the underlying mechanisms of MSCs action in ischemic pathologies and to translate these insights into clinical treatments.
BMSCs and adiposederived MSCs (ADSCs) are considered to be primary sources of MSCs, offering significant potential in cell-based therapy for multiple diseases, including ischemic pathologies[29-31]. Currently, accumulating evidence suggests that ADSCs may offer superior therapeutic benefits over BMSCs in ischemic conditions due to their unique advantages (Table 1)[32]. For instance, ADSCs are widely distributed and can be relatively easily harvested in larger quantities via a minimally invasive procedure from diverse adipose tissue types in the body, including the abdomen, thigh, and arm, without being constrained by patient-specific conditions. Whereas BMSCs are typically isolated from the iliac crest and their extraction can be hindered by challenges related to the patient’s bone marrow quality, which may degrade with age or underlying conditions such as osteoporosis[33-35]; ADSCs have been shown to undergo senescence later than BMSCs and possess a higher proliferation capacity, making them a superior candidate for regenerative applications that require large numbers of cells[36-38]; compared with BMSCs, ADSCs also exhibit enhanced autocrine and paracrine production of proangiogenic factors, such as vascular endothelial growth factor-D (VEGF-D) and insulin-like growth factor 1, positioning them as optimal candidates for rescuing microvascular perfusion defects in ischemic tissues[39-43]; both ADSCs and BMSCs harbor immunomodulatory properties critical for controlling inflammation and facili
| Characteristics | Mechanism of action | Ref. |
| Higher availability and abundance | Widely distributed and richer adipose tissue harvest | [33-35] |
| Higher proliferation capacity and enhanced survival under hypoxic stress | Upregulated expression of a variety of cellular signaling molecules (such as IGF-3, FGF-2, HIF-1α), and activation anti-apoptotic signaling pathway, etc. | [36-38] |
| Superior angiogenic potential | Higher secretion of pro-angiogenic factors (e.g., L-arginine, VEGF, FGF-2), enhanced MMP-2/9-mediated extracellular matrix remodeling | [40,46] |
| Enhanced paracrine activity | Release a broader spectrum of functional molecules (such as growth factors, chemokines and cytokines) through paracrine manner | [39,42,43] |
| Stronger immunosuppressive and anti-inflammatory effects | Higher production of immunosuppressive factors (such as IL-10, TGF-β, and PGE2), and promoting M2 macrophage polarization | [30,44,45] |
| Improved homing and engraftment | Highly expression of chemokine receptors (e.g., CXCR4 and CXCL12), enhancing cells migration and engraftment to ischemic tissues | [47,48] |
Although numerous preclinical studies and clinical trials have been conducted to investigate the efficacy of ADSCs in ischemic conditions, phase III clinical studies have been impeded by some challenges. For instance, one study reported that in vitro-cultured unmodified MSCs derived from mice possess chromosomal abnormalities in early passages, and their local transplantation could induce malignant tumor formation in mouse models[49]; similarly, autologous adult human MSCs have been shown to acquire tumorigenic potential and contribute to the development of epithelial cancers[50,51]; studies have shown that ADSCs from obese subjects promote breast cancer proliferation and tumorigenicity both in vitro and in vivo via an estrogen-leptin pathway, potentially elevating the risk of tumor development[52]. Moreover, MSC immunogenicity is heightened during amplification processes as most MSC products are manufactured by amplifying a small number of cells obtained from donors. Accumulating evidence has also demonstrated that inflammatory cytokines (e.g., interferon-γ, tumor necrosis factor-α), interleukin 2-activated natural killer cells, and CD3/CD28-stimulated T cells could induce BMSCs apoptosis through the Fas-mediated pathway, thereby impairing the viability and differentiation capacity of MSCs[53,54]. It has also been reported that implanted human MSCs can activate the com
Emerging studies have highlighted that specific metabolites may enhance the therapeutic efficacy of MSCs-based therapies across multiple pathologies by improving cell survival, promoting angiogenesis, and modulating inflammation[58-60]. For example, lactate, as a critical intermediate and energy source, has been reported to enhance osteogenic differentiation of BMSCs through histone lactylation as well as the Olfr1440/Ca2+ signaling pathway, thereby ameliorating osteoporosis and improving bone defect repair[61,62]; similarly, lactate could enhance MSCs immunosuppressive activity through inhibiting the proinflammatory response of M1-like macrophages[58]. Pyruvate, the end-product of glycolysis and a primary source of the tricarboxylic acid cycle intermediates oxaloacetate and acetyl-CoA, has emerged as a critical regulator of MSCs functionality, potentially serving as a promising biomarker for optimizing large-scale MSC manufacturing processes in clinical trials[63]. L-arginine, a semi-essential amino acid derived from dietary intake, intracellular synthesis, and net proteolysis[64], plays crucial roles in various metabolic and physiological processes, including glycolysis, protein synthesis, vasodilation, mitochondrial homeostasis, immune modulation, and the production of ornithine, urea as well as nitric oxide (NO, a key intercellular messenger molecule)[65-67]. As a primary precursor of NO, L-arginine exhibits the capacities to regulate angiogenesis and hemodynamics[68]; improve endothelium-dependent blood flow and vasodilatation in hypercholesterolemic individuals[69]; inhibit platelet aggregation and thrombosis[70]; suppress macrophage infiltration and edema formation[71]; and promote the recovery of damaged tissues. Additionally, L-arginine supplementation has been extensively investigated in the treatment of numerous diseases[72]. Both acute and chronic administration of L-arginine has emerged as a promising therapeutic strategy for a range of diseases, such as cognitive impairment, endothelial dysfunction, atherosclerosis, and ischemic acute renal failure[73-75]. Nevertheless, its protective role in ADSCs, particularly in the context of ischemic injury, is still poorly understood.
Recently, metabolomic analyses revealed that ADSCs and BMSCs isolated from elderly patients with coronary heart disease exhibit significant discrepancies in certain metabolites (e.g., α-ketoisovaleric acid, pyruvate) and their corresponding metabolic pathways, which may serve as the underlying mechanisms contributing to the varying efficacies of MSCs-based therapies in atherosclerosis-associated diseases[60]. However, the specific metabolite differences and underlying mechanisms responsible for superior therapeutic potential of ADSCs over BMSCs across multiple pathologies, especially in ischemic pathologies, remain poorly elucidated. The study entitled “L-arginine from elder human mesen
Although the data presented in this study are persuasive, several important issues must be resolved before L-arginine could be regarded as a viable therapeutic target for clinical application. As the sole substrate for endothelial NO synthase and a primary precursor of NO, L-arginine’s pro-angiogenesis has been well established. Specifically, NO and endothelial NO synthase have been demonstrated to enhance endothelial cell proliferation and migration, modulate cytoskeletal organization, as well as promote capillary tube formation via multiple signaling pathways, including the soluble guanylate cyclase/cyclic guanosine monophosphate/protein kinase G, protein kinase B/extracellular signal-regulated kinase 1/2, and activator protein-1/nuclear factor kappa B/matrix metalloproteinase 2 pathways[76,77]. It remains to be elucidated whether L-arginine potentiates ADSCs-mediated angiogenesis via these pathways. Furthermore, does L-arginine augment tissue repair in ischemic diseases through other mechanisms, like immune modulation? Given the pivotal roles of metabolites in modulating MSCs functionality, could other metabolites also enhance ADSCs functionality through distinct pathways? And the combination of L-arginine with other key metabolites (e.g., α-ketoglutarate, succinate) may exert synergistic effects in boosting the therapeutic efficacy of ADSCs for ischemic diseases, which warrants further investigation. Besides, could the therapeutic effects of L-arginine observed in elderly patients be generalized to younger populations? Importantly, sustained and comprehensive assessment of L-arginine’s specificity, safety, and potential adverse effects in ADSCs-based therapy for ischemic diseases is warranted. Therefore, further in depth and comprehensive studies are needed to fully elucidate the role and underlying mechanisms of L-arginine in ADSCs, as well as to facilitate its application as a key metabolite in enhancing MSCs-based therapies for ischemic pathologies.
Ischemic pathologies, including MI, ischemic stroke, and peripheral artery disease, continue to pose a substantial health burden worldwide. While stem cell therapy, particularly ADSCs, holds great promise in treating these conditions, enhancing their therapeutic efficacy is necessary for clinical translation. L-arginine, a key metabolite, plays critical roles in multiple physiological and pathological processes. Through systematically comparing the therapeutic efficacy of ADSCs and BMSCs derived from the same elderly patients, and exploring the underlying mechanism from a metabolic perspective, L-arginine was demonstrated to augment the protective effects of ADSCs against MI through inhibiting cell apoptosis and promoting angiogenesis. These findings imply that L-arginine is a key therapeutic metabolite in improving the efficacy of ADSCs for treating ischemic diseases. Moreover, considering the low cost of L-arginine compared to the significant therapeutic benefits it may offer, the combination of ADSCs and L-arginine presents a promising therapeutic strategy for ischemic diseases, potentially offering a new approach to enhance tissue repair and recovery, especially in elderly patients, within clinical settings. However, the potential risks associated with excessive L-arginine and NO should also be taken into consideration, such as vascular or inflammatory issues caused by imbalanced NO levels. Further exploration is essential to fully clarify their pharmacological effects, determine the optimal dosing range, and thoroughly assess their clinical applications.
| 1. | Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1886] [Cited by in RCA: 2626] [Article Influence: 175.1] [Reference Citation Analysis (0)] |
| 2. | Van Nguyen TT, Vu NB, Van Pham P. Mesenchymal Stem Cell Transplantation for Ischemic Diseases: Mechanisms and Challenges. Tissue Eng Regen Med. 2021;18:587-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Gu J, Huang W, Duanmu Z, Zhuang R, Yang X. Cuproptosis and copper deficiency in ischemic vascular injury and repair. Apoptosis. 2024;29:1007-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Wan H, Ban X, He Y, Yang Y, Hu X, Shang L, Wan X, Zhang Q, Xiong K. Voltage-dependent anion channel 1 oligomerization regulates PANoptosis in retinal ischemia-reperfusion injury. Neural Regen Res. 2026;21:1652-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 5. | Garcia-Bonilla L, Shahanoor Z, Sciortino R, Nazarzoda O, Racchumi G, Iadecola C, Anrather J. Analysis of brain and blood single-cell transcriptomics in acute and subacute phases after experimental stroke. Nat Immunol. 2024;25:357-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 106] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 6. | Zhang K, Li R, Chen X, Yan H, Li H, Zhao X, Huang H, Chen S, Liu Y, Wang K, Han Z, Han ZC, Kong D, Chen XM, Li Z. Renal Endothelial Cell-Targeted Extracellular Vesicles Protect the Kidney from Ischemic Injury. Adv Sci (Weinh). 2023;10:e2204626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Yan WT, Zhao WJ, Hu XM, Ban XX, Ning WY, Wan H, Zhang Q, Xiong K. PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons. Neural Regen Res. 2023;18:357-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 8. | Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 454] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 9. | Avan A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G, Amiri A, Tabrizi R, Mokhber N, Spence JD, Azarpazhooh MR. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 319] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 10. | Ferdinandy P, Andreadou I, Baxter GF, Bøtker HE, Davidson SM, Dobrev D, Gersh BJ, Heusch G, Lecour S, Ruiz-Meana M, Zuurbier CJ, Hausenloy DJ, Schulz R. Interaction of Cardiovascular Nonmodifiable Risk Factors, Comorbidities and Comedications With Ischemia/Reperfusion Injury and Cardioprotection by Pharmacological Treatments and Ischemic Conditioning. Pharmacol Rev. 2023;75:159-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 11. | Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Riccardi M, Pagnesi M, Chioncel O, Mebazaa A, Cotter G, Gustafsson F, Tomasoni D, Latronico N, Adamo M, Metra M. Medical therapy of cardiogenic shock: Contemporary use of inotropes and vasopressors. Eur J Heart Fail. 2024;26:411-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 13. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 671] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Fang J, Liu B, Shao C, Shi Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell. 2022;29:1515-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 207] [Reference Citation Analysis (0)] |
| 15. | Zhang Q, Wan XX, Hu XM, Zhao WJ, Ban XX, Huang YX, Yan WT, Xiong K. Targeting Programmed Cell Death to Improve Stem Cell Therapy: Implications for Treating Diabetes and Diabetes-Related Diseases. Front Cell Dev Biol. 2021;9:809656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Qin B, Zhang Q, Chen D, Yu HY, Luo AX, Suo LP, Cai Y, Cai DY, Luo J, Huang JF, Xiong K. Extracellular vesicles derived from mesenchymal stem cells: A platform that can be engineered. Histol Histopathol. 2021;36:615-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Lu W, Allickson J. Mesenchymal stromal cell therapy: Progress to date and future outlook. Mol Ther. 2025;33:2679-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 18. | Fernández-Garza LE, Barrera-Barrera SA, Barrera-Saldaña HA. Mesenchymal Stem Cell Therapies Approved by Regulatory Agencies around the World. Pharmaceuticals (Basel). 2023;16:1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 19. | Wan XX, Zhang DY, Khan MA, Zheng SY, Hu XM, Zhang Q, Yang RH, Xiong K. Stem Cell Transplantation in the Treatment of Type 1 Diabetes Mellitus: From Insulin Replacement to Beta-Cell Replacement. Front Endocrinol (Lausanne). 2022;13:859638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Barrère-Lemaire S, Vincent A, Jorgensen C, Piot C, Nargeot J, Djouad F. Mesenchymal stromal cells for improvement of cardiac function following acute myocardial infarction: a matter of timing. Physiol Rev. 2024;104:659-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 21. | Lu T, Zhang J, Cai J, Xiao J, Sui X, Yuan X, Li R, Li Y, Yao J, Lv G, Chen X, Chen H, Zeng K, Liu Y, Chen W, Chen G, Yang Y, Zheng J, Zhang Y. Extracellular vesicles derived from mesenchymal stromal cells as nanotherapeutics for liver ischaemia-reperfusion injury by transferring mitochondria to modulate the formation of neutrophil extracellular traps. Biomaterials. 2022;284:121486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 22. | Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang Y, Zhong Z, Zhao J, Li Q, Zhu D, Ke C, Zhong S, Wu X, Yu H, Zhu W, Chen J, Zhang J, Wang J, Hu X. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of miR-125b. Circ Res. 2018;123:564-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 23. | Cao JY, Wang B, Tang TT, Wen Y, Li ZL, Feng ST, Wu M, Liu D, Yin D, Ma KL, Tang RN, Wu QL, Lan HY, Lv LL, Liu BC. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11:5248-5266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 24. | Yu Z, Wen Y, Jiang N, Li Z, Guan J, Zhang Y, Deng C, Zhao L, Zheng SG, Zhu Y, Su W, Zhuo Y. TNF-α stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials. 2022;284:121484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 25. | Ala M. The beneficial effects of mesenchymal stem cells and their exosomes on myocardial infarction and critical considerations for enhancing their efficacy. Ageing Res Rev. 2023;89:101980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 26. | Zhang Q, Li S, Chen H, Yin J, Chen Y, Liu L, He W, Min Z, Gong Y, Xu J, Song K, Lv W, Xin H. Reduction of Oxidative Stress and Excitotoxicity by Mesenchymal Stem Cell Biomimetic Co-Delivery System for Cerebral Ischemia-Reperfusion Injury Treatment. Small. 2024;20:e2401045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 27. | Baak LM, Wagenaar N, van der Aa NE, Groenendaal F, Dudink J, Tataranno ML, Mahamuud U, Verhage CH, Eijsermans RMJC, Smit LS, Jellema RK, de Haan TR, Ter Horst HJ, de Boode WP, Steggerda SJ, Prins HJ, de Haar CG, de Vries LS, van Bel F, Heijnen CJ, Nijboer CH, Benders MJNL. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): a first-in-human, open-label intervention study. Lancet Neurol. 2022;21:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 28. | Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY; STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 29. | Afshar Hezarkhani L, Veysi K, Rahmani A, Salari N, Hasheminezhad R, Nasr V, Mohammadi M. Safety and Efficacy of Bone Marrow and Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Ischemic Stroke: A Systematic Review. Cardiol Rev. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 30. | Yoshida M, Nakashima A, Ishiuchi N, Miyasako K, Morimoto K, Tanaka Y, Sasaki K, Maeda S, Masaki T. Comparison of the Therapeutic Effects of Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells on Renal Fibrosis. Int J Mol Sci. 2023;24:16920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 292] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 32. | Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, Feng G, Cai Y, Xia C, Liu H, Shen W, Hu X, Ouyang H. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am J Sports Med. 2019;47:1722-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 33. | Qin Y, Ge G, Yang P, Wang L, Qiao Y, Pan G, Yang H, Bai J, Cui W, Geng D. An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity. Adv Sci (Weinh). 2023;10:e2207334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 143] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 34. | Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, Hui Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed Pharmacother. 2019;114:108765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 35. | Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 902] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 36. | Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 37. | Qi Z, Zhang Y, Liu L, Guo X, Qin J, Cui G. Mesenchymal stem cells derived from different origins have unique sensitivities to different chemotherapeutic agents. Cell Biol Int. 2012;36:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Zhu D, Wang Y, Thomas M, McLaughlin K, Oguljahan B, Henderson J, Yang Q, Chen YE, Liu D. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1α pathway. J Mol Cell Cardiol. 2022;162:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 39. | Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, Dilley RJ. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21:2189-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 40. | Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 41. | Wan X, Wang L, Khan MA, Peng L, Sun X, Yi X, Wang Z, Chen K. NAT10-mediated N4-acetylcytidine modification in KLF9 mRNA promotes adipogenesis. Cell Death Differ. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Hu J, Jiang Y, Wu X, Wu Z, Qin J, Zhao Z, Li B, Xu Z, Lu X, Wang X, Liu X. Exosomal miR-17-5p from adipose-derived mesenchymal stem cells inhibits abdominal aortic aneurysm by suppressing TXNIP-NLRP3 inflammasome. Stem Cell Res Ther. 2022;13:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 43. | Ma T, Sun J, Zhao Z, Lei W, Chen Y, Wang X, Yang J, Shen Z. A brief review: adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res Ther. 2017;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 44. | Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36:1111-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 415] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 45. | Li JZ, Cao TH, Han JC, Qu H, Jiang SQ, Xie BD, Yan XL, Wu H, Liu XL, Zhang F, Leng XP, Kang K, Jiang SL. Comparison of adipose and bone marrowderived stem cells in protecting against oxLDLinduced inflammation in M1macrophagederived foam cells. Mol Med Rep. 2019;19:2660-2670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Li JZ, Zhan X, Sun HB, Chi C, Zhang GF, Liu DH, Zhang WX, Sun LH, Kang K. L-arginine from elder human mesenchymal stem cells induces angiogenesis and enhances therapeutic effects on ischemic heart diseases. World J Stem Cells. 2025;17:103314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 47. | Yuan Z, Cai C, Qin Y, Yan K, Wang J. CXCR4(+) Sorted Adipose-Derived Stem Cells Enhance Their Functional Benefits and Improve Cardiac Function after Myocardial Infarction. Stem Cells Int. 2022;2022:6714765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Chen Y, Li Y, Li B, Hu D, Dong Z, Lu F. Migrasomes from adipose derived stem cells enrich CXCL12 to recruit stem cells via CXCR4/RhoA for a positive feedback loop mediating soft tissue regeneration. J Nanobiotechnology. 2024;22:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 49. | Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (2)] |
| 50. | Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrødder H, Jensen T, Kassem M. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23:5095-5098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 51. | Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 844] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 52. | Strong AL, Strong TA, Rhodes LV, Semon JA, Zhang X, Shi Z, Zhang S, Gimble JM, Burow ME, Bunnell BA. Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res. 2013;15:R102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 53. | Barrachina L, Remacha AR, Romero A, Vázquez FJ, Albareda J, Prades M, Gosálvez J, Roy R, Zaragoza P, Martín-Burriel I, Rodellar C. Priming Equine Bone Marrow-Derived Mesenchymal Stem Cells with Proinflammatory Cytokines: Implications in Immunomodulation-Immunogenicity Balance, Cell Viability, and Differentiation Potential. Stem Cells Dev. 2017;26:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 54. | Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 55. | Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120:3436-3443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 56. | Rangasami VK, Asawa K, Teramura Y, Le Blanc K, Nilsson B, Hilborn J, Varghese OP, Oommen OP. Biomimetic polyelectrolyte coating of stem cells suppresses thrombotic activation and enhances its survival and function. Biomater Adv. 2023;147:213331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 57. | Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk HD, Geissler S, Reinke P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med. 2019;25:149-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 58. | Horie T, Hirata H, Sakamoto T, Kitajima H, Fuku A, Nakamura Y, Sunatani Y, Tanida I, Sunami H, Tachi Y, Ishigaki Y, Yamamoto N, Shimizu Y, Ichiseki T, Kaneuji A, Iwabuchi K, Osawa S, Kawahara N. Multiomics analyses reveal adipose-derived stem cells inhibit the inflammatory response of M1-like macrophages through secreting lactate. Stem Cell Res Ther. 2024;15:485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | González-Cruz RD, Fonseca VC, Darling EM. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci U S A. 2012;109:E1523-E1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 60. | Li JZ, Qu H, Wu J, Zhang F, Jia ZB, Sun JY, Lv B, Kang Y, Jiang SL, Kang K. Metabolic profiles of adipose-derived and bone marrow-derived stromal cells from elderly coronary heart disease patients by capillary liquid chromatography quadrupole time-of-flight mass spectrometry. Int J Mol Med. 2018;41:184-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Wu J, Hu M, Jiang H, Ma J, Xie C, Zhang Z, Zhou X, Zhao J, Tao Z, Meng Y, Cai Z, Song T, Zhang C, Gao R, Cai C, Song H, Gao Y, Lin T, Wang C, Zhou X. Endothelial Cell-Derived Lactate Triggers Bone Mesenchymal Stem Cell Histone Lactylation to Attenuate Osteoporosis. Adv Sci (Weinh). 2023;10:e2301300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 62. | Wei T, Ma D, Liu L, Huang Y, Zhang X, Xu M, Wei Y, Wei J, Deng X. Lactate promotes bone healing by regulating the osteogenesis of bone marrow mesenchymal stem cells through activating Olfr1440. Transl Res. 2024;273:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Van Grouw A, Colonna MB, Maughon TS, Shen X, Larey AM, Moore SG, Yeago C, Fernández FM, Edison AS, Stice SL, Bowles-Welch AC, Marklein RA. Development of a Robust Consensus Modeling Approach for Identifying Cellular and Media Metabolites Predictive of Mesenchymal Stromal Cell Potency. Stem Cells. 2023;41:792-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 64. | Cao X, Wu VWY, Han Y, Hong H, Wu Y, Kong APS, Lui KO, Tian XY. Role of Argininosuccinate Synthase 1 -Dependent L-Arginine Biosynthesis in the Protective Effect of Endothelial Sirtuin 3 Against Atherosclerosis. Adv Sci (Weinh). 2024;11:e2307256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Xu M, Guo Y, Wang M, Luo X, Shen X, Li Z, Wang L, Guo W. L-arginine homeostasis governs adult neural stem cell activation by modulating energy metabolism in vivo. EMBO J. 2023;42:e112647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 66. | Bode-Böger SM, Scalera F, Ignarro LJ. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther. 2007;114:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 67. | Forzano I, Avvisato R, Varzideh F, Jankauskas SS, Cioppa A, Mone P, Salemme L, Kansakar U, Tesorio T, Trimarco V, Santulli G. L-Arginine in diabetes: clinical and preclinical evidence. Cardiovasc Diabetol. 2023;22:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 68. | Piñeiro V, Ortiz-Moreno A, Mora-Escobedo R, Hernández-Navarro MD, Ceballos-Reyes G, Chamorro-Cevallos G. Effect of L-arginine oral supplementation on response to myocardial infarction in hypercholesterolemic and hypertensive rats. Plant Foods Hum Nutr. 2010;65:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Kurhaluk N, Tkaczenko H. L-Arginine and Nitric Oxide in Vascular Regulation-Experimental Findings in the Context of Blood Donation. Nutrients. 2025;17:665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 70. | Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 413] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 71. | Vos IHC, Rabelink TJ, Dorland B, Loos R, Middelaar BV, Gröne HJ, Joles JA. L-arginine supplementation improves function and reduces inflammation in renal allografts. J Am Soc Nephrol. 2001;12:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Fiordelisi A, Cerasuolo FA, Avvisato R, Buonaiuto A, Maisto M, Bianco A, D'Argenio V, Mone P, Perrino C, D'Apice S, Paolillo R, Pezone A, Varzideh F, Santulli G, Sorriento D, Iaccarino G, Gambardella J. L-Arginine supplementation as mitochondrial therapy in diabetic cardiomyopathy. Cardiovasc Diabetol. 2024;23:450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 73. | Hou Q, Dong Y, Huang J, Liao C, Lei J, Wang Y, Lai Y, Bian Y, He Y, Sun J, Sun M, Jiang Q, Wang B, Yu Z, Guo Y, Zhang B. Exogenous L-arginine increases intestinal stem cell function through CD90+ stromal cells producing mTORC1-induced Wnt2b. Commun Biol. 2020;3:611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Schneider R, Raff U, Vornberger N, Schmidt M, Freund R, Reber M, Schramm L, Gambaryan S, Wanner C, Schmidt HH, Galle J. L-Arginine counteracts nitric oxide deficiency and improves the recovery phase of ischemic acute renal failure in rats. Kidney Int. 2003;64:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | He J, Hou T, Wang Q, Wang Q, Jiang Y, Chen L, Xu J, Qi Y, Jia D, Gu Y, Gao L, Yu Y, Wang L, Kang L, Si J, Wang L, Chen S. L-arginine metabolism ameliorates age-related cognitive impairment by Amuc_1100-mediated gut homeostasis maintaining. Aging Cell. 2024;23:e14081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 76. | Song JH, Hwang B, Lyea Park S, Kim H, Jung S, Choi C, Myung Lee H, Yun SJ, Hyun Choi Y, Cha EJ, Patterson C, Kim WJ, Moon SK. IL-28A/IL-10Rβ axis promotes angiogenesis via eNOS/AKT signaling and AP-1/NF-κB/MMP-2 network by regulating HSP70-1 expression. J Adv Res. 2025;73:247-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 77. | Dhahri W, Dussault S, Raguema N, Desjarlais M, Rivard A. Stimulation of soluble guanylate cyclase activity with riociguat promotes angiogenesis and improves neovascularization after limb ischemia. Atherosclerosis. 2023;372:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/