Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.104930
Revised: April 5, 2025
Accepted: July 1, 2025
Published online: August 26, 2025
Processing time: 226 Days and 21.2 Hours
Lupus nephritis (LN) is one of the most common and serious complications of systemic lupus erythematosus, which can lead to end-stage renal disease, and is an important cause of death in patients with systemic lupus erythematosus. Treatment options include glucocorticoids, immunosuppressive agents and the addition of biologics. Recently, the therapeutic role of mesenchymal stem cells (MSCs) in LN has received extensive attention worldwide. MSCs can suppress autoimmunity, alleviate proteinuria and restore renal function by modulating the functions of various immune cells and reducing the secretion of inflammatory cytokines. Several clinical trials have investigated MSC treatment in LN with promising but sometimes inconsistent outcomes. This review summarizes the sources of MSCs and mechanisms in immunoregulation. Furthermore, it examines clinical trials evaluating the efficacy, safety, and limitations of MSC therapy in LN. By highlighting advances and ongoing challenges, this review underscores the potential of MSCs for LN treatment. More large-scale randomized controlled trials are needed to support the effectiveness of this therapy and pave the way for personalized and combinatorial therapeutic approaches.
Core Tip: Lupus nephritis (LN) is one of the most common and serious complications of systemic lupus erythematosus. Mesenchymal stem cell (MSC) therapy has received extensive attention in LN. This article summarizes the sources of MSCs, the mechanisms of MSC therapy in LN treatment and the results of clinical trials. MSC therapy for LN shows great potential but still requires the support of large-scale clinical trials.
- Citation: Liu L, Behera TR, Wang QJ, Shen QQ. Advances in mesenchymal stem cell therapy for lupus nephritis. World J Stem Cells 2025; 17(8): 104930
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/104930.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.104930
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease that can affect multiple organs. It is characterized by the presence of autoantibodies and immune complex deposition. Lupus nephritis (LN) is one of the most common and severe complications of SLE, affecting approximately 25% to 50% of patients, depending on the population studied[1]. Kidney involvement in SLE has been linked to an increased risk of mortality, particularly among patients who progress to renal failure[2]. LN is classified into six pathological classifications based on kidney biopsy histopathology by a group of kidney pathologists, nephrologists, and rheumatologists in 2004[3] (the International Society of Nephrology/Renal Pathology Society classification) and revised in 2018[4] (Table 1). The aim of treating LN is to preserve kidney function, while reducing the associated morbidity and mortality stemming from kidney failure. Additionally, it is essential to minimize medication-associated toxicities.
| Classifications | Histopathology | Therapeutic approaches |
| Minimal mesangial LN (class I) | Only mesangial immune deposits; no light microscopic abnormalities | Hydroxychloroquine. Low-level proteinuria-immunosuppressive treatment guided by extrarenal manifestations of SLE. Nephrotic syndrome-maintenance combination therapy with low-dose glucocorticoid and another immunosuppressive agent |
| Mesangial proliferative LN (class II) | Mesangial hypercellularity or mesangial matrix expansion. A few isolated subepithelial or subendothelial deposits on IF or EM | |
| Focal LN (class III) | < 50% glomeruli affected. Active or inactive endocapillary or extracapillary glomerulonephritis, always segmental (< 50% glomerular tuft). Subendothelial deposits on EM | Hydroxychloroquine. Initial therapy-glucocorticoids plus MPAA/low-dose intravenous cyclophosphamide/belimumab and either MPAA or low-dose intravenous cyclophosphamide/MPAA and a CNI. Maintenance therapy-low-dose glucocorticoids plus MPAA |
| Diffuse LN (class IV) | ≥ 50% glomeruli affected. Endocapillary with or without extracapillary glomerulonephritis. Subendothelial deposits on EM | |
| Lupus membranous nephropathy (class V) | Diffuse thickening of the glomerular capillary wall. Mesangial involvement. Subepithelial immune deposits | Hydroxychloroquine. Low-level proteinuria-RAS blockade, immunosuppressive treatment guided by extrarenal manifestations of SLE. Nephrotic syndrome-RAS blockade, immunosuppressive treatment with glucocorticoid and one other agent |
| Advanced sclerosing LN (class VI) | Global sclerosis in > 90% glomeruli | General management |
Since the 20th century, several approaches have been employed to treat different classes of LN, including glucocorticoids, immunosuppressants such as calcineurin inhibitors, mycophenolic acid analogs, cyclophosphamide and azathioprine, as well as B-lymphocyte targeting biologics[5] (Table 1). Despite the utilization of diverse drugs, it has been reported that between 20% and 70% of patients with LN do not respond favorably to standard immunosuppressive therapy, thereby classified as refractory LN. Patients who suffer from refractory LN tend to experience poorer prognoses. A study of 86 individuals diagnosed with diffuse proliferative LN revealed that at the 10-year mark, the patient survival rate of those with complete remission, partial remission and no remission were 95%, 76%, and 46%, respectively[6]. Correspondingly, renal survival rates of those with complete remission, partial remission and no remission were 94%, 45%, and 19%, respectively. Even achieving partial remission significantly improved patient outcomes compared to no remission[6].
Long-term administration of nonspecific immunosuppressive regimens can elevate the risk of severe infections and secondary malignant tumors[7]. Consequently, there is an urgent need for safer and more effective therapeutic approaches for LN. Mesenchymal stem cells (MSCs) are multipotent cells with the ability to self-renew and differentiate into various cell types. In recent years, MSC transplantation has emerged as a treatment option for autoimmune diseases, particularly benefiting patients unresponsive to standard therapies. MSC transplantation has been used in the treatment of the following autoimmune diseases[8]: Rheumatoid arthritis, SLE, inflammatory bowel disease, ankylosing spondylitis, and multiple sclerosis. In clinical trials, MSC therapy showed great promise in the treatment of LN. A thorough comprehension of the underlying mechanisms of MSC therapy in LN is crucial for effectively understanding and conducting clinical trials.
The aim of this review is to evaluate the therapeutic potential of MSC therapy in LN. We introduce differences between MSCs from various sources and discuss their effects on circulating immunity, regional immunity, and renal innate cells. Additionally, we review clinical studies and identify current challenges in MSC implementation. By summarizing the latest developments in MSC-based research, this review presents an integrated examination of their clinical applications, limitations and potential future developments in LN management.
MSCs are derived from various sources including bone marrow (excluding the hematopoietic component), umbilical cord, adipose tissue, embryonic tissue and dental tissue. MSC-derived extracellular vesicles (EVs) have also demonstrated therapeutic potential in the treatment of LN. While MSCs are theoretically obtainable from almost any tissue, practical limitations - including the invasiveness of extraction and donor-specific variables - restrict their accessibility. Clinicians must carefully evaluate the feasibility of sample collection and potential adverse effects on donors when selecting a source. For example, harvest of bone marrow-derived MSCs (BM-MSCs) carries risks such as pain, bleeding, and infection, rendering it less favorable compared to minimally invasive alternatives like peripheral blood or discarded surgical tissues[9] (e.g., adipose tissue or birth-derived materials). Table 2 summarizes the advantages and disadvantages of current MSC sources used in LN treatment[10,11].
| Source | Method of procurement | Advantages | Disadvantages |
| BM-MSCs | Isolated from BM aspiration | Potential to differentiate into hepatocytes, much like AD-MSCs; short culture time | Invasive and painful procedure; use of anesthesia; cell yield, longevity and potential for differentiation diminishes with donor age |
| UC-MSCs | Isolated from the placenta, amnion and UC blood after birth | Avoidance of invasive procedures; abundance; reliability of sample collection; lack of transmission of herpes viruses; higher expansion and engraftment capacity than BM-MSCs | UC-MSCs may not have adipogenic potential; less osteogenesis potential |
| AD-MSCs | Isolated from liposuction, lipoplasty or lipectomy materials | Easy to access; abundant throughout the body; secrete cytokines and growth factors with anti-inflammatory, antiapoptotic and immunomodulatory properties; lower risk of immune rejection because of the absence of MHC-II antigen expression | Inferior osteogenic and chondrogenic potential in comparison to BM-MSCs |
| DT-MSCs | Isolated from tooth extraction or root canal surgery materials | Accessible source; higher frequency of colony forming cells from dental pulp compared to those from BM | Difficult and invasive procedure; ectomesenchymal and periodontal tissues affect MSC properties |
| MSC-Exos | Isolated from the cultural supernatant of MSCs using high speed centrifugation | Easy to obtain in large quantities from immortalized cells; lower immunogenicity; less possibility of graft rejection; higher safety profile due to the nanoscale size | Fast clearance of MSC-Exos limit their long-term therapeutic effects; heterogeneity of MSC-Exos due to different culture conditions and cell passages |
BM-MSCs were first isolated by Friedenstein in 1976[12] and have been extensively used to treat autoimmune disorders and evaluated in the management of biological engineering. The isolation and identification of BM-MSCs are based on their distinct biological characteristics, including plastic-adhesion, surface marker expression, and the capacity for multilineage differentiation. Ficoll density gradient centrifugation is used to isolate BM mononuclear cells. According to the characteristics of plastic-adhesion, non-adherent cells are removed, leaving only the adherent cells. Based on the criteria proposed by the International Society of Cellular Therapy, BM-MSCs are harvested after several passages and isolated by flow cytometry or magnetic-activated cell sorting technologies based on cell surface marker expression. BM-MSCs are negative for CD34 (endothelial cells), CD45 (hematopoietic cells), and HLA-DR (leukocytes) and positive for CD29, CD44, CD73, CD90, and CD105[13]. Additionally, CD271, CD49a, platelet-derived growth factor receptor α/β, epidermal growth factor receptor, insulin-like growth factors receptor, stromal precursor antigen 3, stromal precursor antigen 1, CD146, stage-specific embryonic antigen 4, and MSC antigen-1 may hold potential in future cell sorting following more evidence in preclinical studies[14,15].
Preclinical studies have employed various doses and administration routes to optimize therapeutic efficacy. In a preclinical study, (1-1.25) × 106 cells (human or allogeneic)/mouse 1-3 times weekly or (0.05-0.2) × 106 cells/10 g mouse body weight was injected through the tail vein or intraperitoneal route[16]. Assessment of the optimal infusion dose helps to obtain a balance between minimizing cellular utilization and maximizing treatment efficacy. For example, increasing the dose to 1.25 × 106 cells per animal did not exhibit a superior effect[17]. In the clinical study, allogeneic 1 × 106 BM-MSCs/kg body weight was intravenously infused once or twice (one week interval) to treat LN patients. In a randomized trial comparing single and double allogeneic BM-MSCs infusions, there were no statistically significant differences in terms of disease remission, relapse rates, or improvement in serum indices after one-year follow-up[18]. Notably, the short interval of one week between the first and second injections may have contributed to these comparable outcomes.
MSCs can be isolated from the umbilical cord through enzymatic digestion after blood is removed using phosphate-buffered saline (PBS). After seeding the cells in the culture dish, non-adherent cells are removed by washing. Once a population of fibroblast-like cells has been established after 10 days, the cultures are trypsinized and passaged into a new flask for continued expansion and harvesting. Immunophenotype analysis includes the same surface marker profile as BM-MSCs (positive for CD29, CD44, CD73, CD90, and CD105; negative for CD34, CD45, and HLA-DR). Umbilical cord-derived MSCs (UC-MSCs) possess the ability to differentiate into three germ layers and migrate to damaged tissue[19]. UC-MSCs display a primitive phenotype characterized by longer telomeres, giving them enhanced proliferative potential and reduced cellular senescence[20]. In animal studies, (0.5-1) × 106 human UC-MSCs/animal tail vein infusions were performed once or three times weekly [21]. In clinical studies, allogeneic 1 × 106 UC-MSCs/kg body weight were intravenously infused once or twice (one week interval)[22]. A multicenter clinical study demonstrated benefits from repeated infusions after 6 months, as relapse rates were 12.5% after 9 months and 16.7% after 12 months[22].
Compared to other MSCs, the advantages of adipose tissue-derived MSCs (AD-MSCs) are mainly reflected in their abundant sources, simple isolation methods and low immunogenicity[23]. AD-MSCs express the same MSC surface markers as BM-MSCs and have been widely used in both basic research and clinical applications. In preclinical studies, (0.5-2) × 106 human AD-MSCs/mouse were intravenously injected multiple times biweekly[24]. AD-MSCs effectively decreased proteinuria, autoantibody levels, and blood urea nitrogen (BUN), while improving renal histopathology. Low doses but more prolonged therapy showed a significant increase in survival[25].
The first clonogenic, proliferative population of cells was isolated from adult human dental pulp in 2000[26]. Subsequently, researchers have isolated MSCs from other sites such as apical papilla, periodontal ligament and other dental tissues[27]. Dental tissue-derived MSCs are mostly used for dental tissue engineering and regenerative dental medicine. To date, only one group has assessed the abilities of dental pulp stem cells and periodontal ligament stem cells to treat SLE in mice[28]. The dental tissue-derived MSCs were intravenously infused at 2 × 105/10 g mouse body weight and could downregulate 24 hours proteinuria and anti-double-stranded DNA levels. Additionally, dental pulp stem cells could ameliorate renal pathogenic lesions in SLE mice, similar to UC-MSCs, suggesting that these cells may be another option for LN treatment.
MSCs secrete various types of EVs, including exosomes (30-150 nm), microvesicles (150-500 nm), and apoptotic bodies (800-5000 nm). Notably, MSC-derived exosomes (MSC-Exos) can mimic the therapeutic effects of MSCs, making them a promising cell-free alternative to traditional cell-based therapies. MSC-Exos are isolated from the culture supernatant of MSCs using high speed centrifugation[29]. For clinical trial applications, MSC-Exos should comply with the minimal characterization standards for EVs outlined in the MISEV2018 guidelines, encompassing both marker-based and physical assessments[30,31]. Briefly, MSC-Exos should have at least three EVs-associated proteins, including at least one transmembrane/lipid-bound protein, one cytosolic protein and negative expression of at least one non-EV protein like calnexin (endoplasmic reticulum marker) and cytochrome C (mitochondrial protein). In addition, electron microscopy (for morphology) and nanoparticle tracking analysis (for size distribution) were used to evaluate the physical characteristics[32].
MSC-Exos have been investigated as a therapeutic intervention in a range of diseases, including kidney diseases, graft-vs-host disease, osteoarthritis, stroke, Alzheimer’s disease and type 1 diabetes. The dose and frequency of MSC-Exos therapy varied widely, which has been reviewed in detailed previously[11]. Animal studies have demonstrated that MSC-Exos exhibit potent anti-inflammatory and immunomodulatory properties in SLE. By promoting M2 macrophage polarization and regulatory T (Treg) cell induction, MSC-Exos effectively mitigate nephritis[29]. Notably, MSC-EVs suppress renal T helper type 17 (Th17) cell differentiation via modulation of the interleukin (IL)-6/signal transducer and activator of transcription 3/IL-17 signaling axis, thus regulating Th1/Th17/Treg imbalance and inhibiting double negative T cells and plasma cells[33]. In the kidneys, MSC-EVs reduce proteinuria, serum creatinine (SCr) and renal pathological damage[33], thus representing a promising cell-free therapeutic approach for LN.
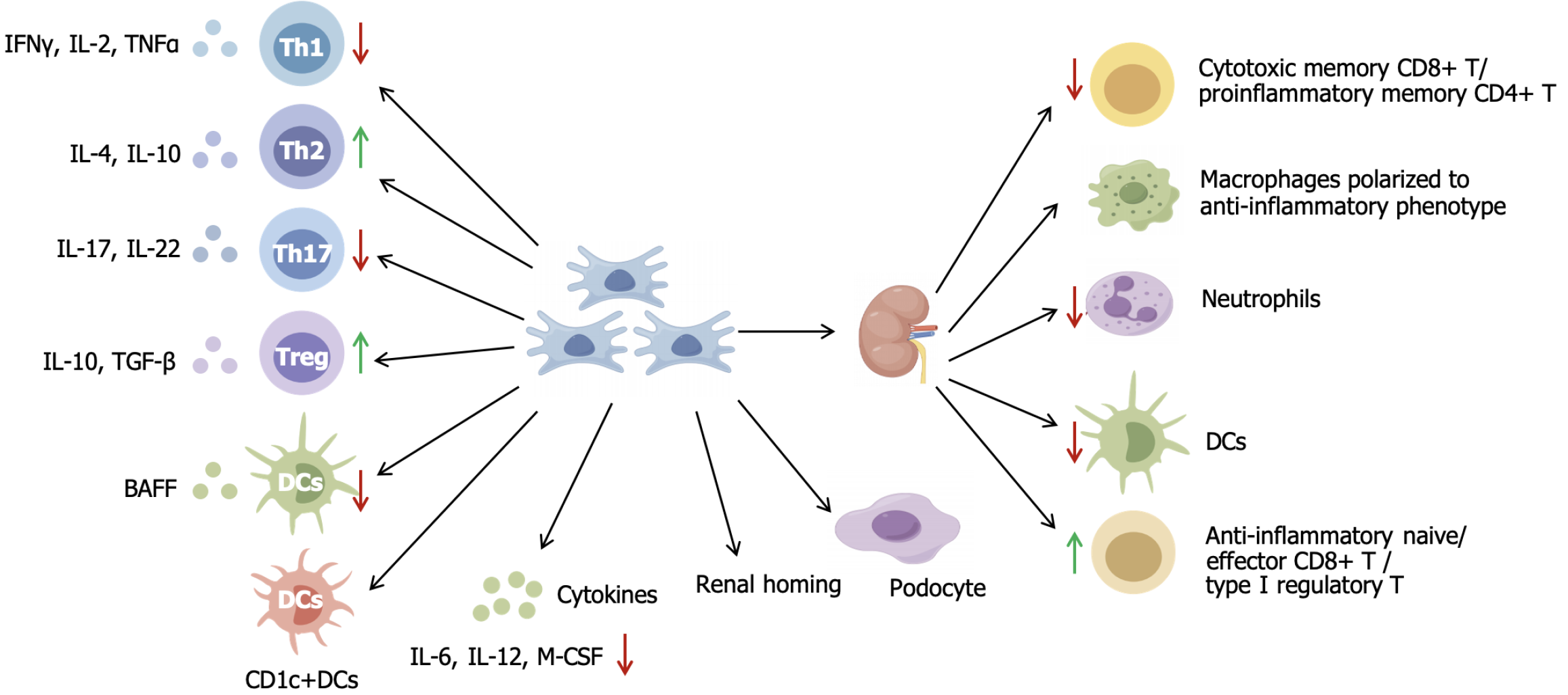
A meta-analysis including 28 studies concluded that MSC therapy resulted in improved immunological indicators, including lower levels of double-stranded DNA, antinuclear antibody, along with ameliorated renal function and pathology, as evidenced by decreased SCr, BUN, proteinuria and renal sclerosis score in LN mice[16]. Multiple preclinical studies have confirmed the effectiveness of MSCs in LN treatment[34], although this may be accompanied by various changes in different immune cells or cytokines. The underlying mechanisms include the regulation of circulating immune cells and cytokines, regulation of regional immunity and its direct therapeutic effects on renal innate cells (Figure 1).
SLE is a systemic autoimmune and inflammatory disease. Adaptive immune cells, such as B and T cells, play critical roles in SLE development and can be first-line targets for MSC therapy. Multiple preclinical studies have shown that MSC transplantation modulates T cell populations and function. MSCs suppress Th1, Th17 and T follicular helper cell lymphocyte function[35], thereby downregulating pro-inflammatory cytokines interferon-γ, IL-2, tumor necrosis factor (TNF)-α, IL-6 and IL-12[20]. Treatment with AD-MSCs (adipose-derived stem cells) decreases Th1/Th2 ratio, and increases Th2 cytokines (IL-4, IL-10)[35,36]. MSCs restore Treg (CD4+FoxP3+) cells with increased anti-inflammatory cytokine IL-4, IL-10, granulocyte-macrophage colony-stimulating factor and maintain immune balance[24]. Serum levels of B-cell activating factor decreased in MSC-treated mice, which can suppress excessive B-cell activation[37].
Innate immune cells, such as macrophages and dendritic cells (DCs), also participate in LN. DCs are the most functional and powerful antigen-presenting cells. MSCs reduce the surface expression of CD1-a, CD80, CD83, CD86, and HLA-DR, thereby inhibiting monocyte differentiation into DCs[38]. Furthermore, MSCs suppress B-cell activating factor production by DCs when BM-MSCs and DCs are incubated in vitro[37]. On the other hand, UC-MSCs promote proliferation and inhibit apoptosis of tolerogenic DC subsets (CD1c+ DCs) by expressing FMS-related tyrosine kinase 3 ligand binding to FMS-related tyrosine kinase 3 on CD1c+ DCs[39].
In addition to regulating circulating immune cells to improve overall condition, MSC therapy can also directly modulate renal regional immunity. Zhou et al[40] used single-cell sequencing and found that MSCs reduced cytotoxic memory CD8+ T cells, pro-inflammatory memory CD4+ T cells, infiltrating macrophages, neutrophils and DCs, and increased anti-inflammatory naive/effector CD8+ T cells and type 1 Treg cells in the kidney of LN mice. MSCs-treated macro
MSCs activate podocytes to produce C-C motif chemokine 8, establishing a positive feedback loop where C-C motif chemokine 8 subsequently stimulates MSCs to produce immunosuppressive factors (IL-10, indoleamine 2,3-dioxygenase, transforming growth factor-β1 and inducible nitric oxide synthase)[45]. MSCs unexpectedly promote the expression of podocyte-specific markers podocin and synaptopodin and decrease podocyte foot process effacement in LN mice, along with decreased levels of anti-double stranded DNA antibody and improvement in renal pathology[41]. In addition, MSCs can migrate to the kidney and integrate into tubular cells. This integration process aids in the repair of damaged tissue and leads to MSC differentiation into mesangial cells[46].
While MSC therapy has shown therapeutic potential in LN mouse models, improving renal function, reducing proteinuria and ameliorating histopathological changes, important exceptions have been observed. For instance, Youd et al[47] found that intraperitoneal BM-MSC transplantation in the NZB/W model worsened disease. This adverse outcome may be attributed to peritoneal sequestration of MSCs, wherein intraperitoneally delivered cells form multicellular aggregates with immune cells, subsequently adhering to the peritoneal wall and impairing systemic biodistribution[48]. Some research groups did not identify differences in IL-6, IL-10, IL-17, and monocyte chemoattractant protein-1 between MSCs and PBS-treated LN mice[28]. This null effect may be attributable to the use of dental pulp and periodontal ligament-derived MSCs, which exhibit distinct immunomodulatory profiles compared to conventional bone marrow sources[28]. BM-MSCs derived from MRL/Lpr mice failed to ameliorate renal immune complex deposition or suppress B lymphocyte activity, likely attributable to intrinsic functional impairments in MSCs isolated from diseased donors[49]. These divergent outcomes likely reflect how route of injection, source-dependent MSC heterogeneity, and donor immune function may affect MSC therapy efficacy. Optimization of standardized MSC isolation and expansion protocols, dosage, administration route and sources are needed to improve the efficacy of preclinical studies.
MSC therapy is well established for the treatment of autoimmune and autoinflammatory diseases, including inflammatory bowel disease[50], multiple sclerosis[51], type 1 diabetes mellitus[52], chronic kidney diseases[53] and so on. As of December 2024, there were 20 clinical trials registered on the NIH Clinical Trial Database (https://ClinicalTrials.gov/) using MSC therapy as treatment for SLE, of which 13 trials were for LN. Six of these trials are still recruiting. Here, we reviewed nine clinical trials published during 2010 to 2022 (Table 3).
| MSCs (sources, timing, dose) | Study design | Sample size (n), LN/SLE | Follow up (months) | Safety and adverse effects | Outcomes | Ref. |
| UC-MSCs (single i.v. injection 1 × 106 cells/kg) | Single arm | 15/16, refractory LN | 3-28 | Severe nausea (1/16). No treatment-related mortality or other adverse events | SLEDAI scores decreased gradually during 6 months follow-up (10/16). Anti-dsDNA antibody decreased significantly at 3 months (13/16) and gradually decreased after 6 months (3/16). Reduced proteinuria and improved eGFR during 6 months follow-up (8/16) and further improved during long-term follow-up (2/16) | Sun et al[58], 2010 |
| BM-MSCs (single i.v. injection 1 × 106 cells/kg) | Single arm | 15/15, refractory LN | 12-36 | No serious adverse events. No GVHD. Upper respiratory tract infections | Decreased SLEDAI score at 12 months follow-up (12/13). Lower titers of anti-dsDNA at 3 months follow-up (11/15). Reduced 24 hours proteinuria at 12 months follow-up (12/12) | Liang et al[57], 2010 |
| BM-MSCs/UC-MSCs (single or double i.v. injection 1 × 106 cells/kg) | Dose comparison trial | 50/58, refractory LN | 12-48 | One death in double transplantation group. Upper respiratory tract infection, intestinal infection, herpes zoster infection, pulmonary tuberculosis considered non transplantation related | Complete remission (16/30) in single transplantation group and (8/27) in multiple transplantation group during 4 years follow-up. Decreased SLEDAI score during 3 years follow-up. Decreased anti-dsDNA titer during 2 years follow-up. Reduced proteinuria and improved eGFR during 3 years follow-up | Wang et al[18], 2012 |
| BM-MSCs/UC-MSCs (single i.v. injection 1 × 106 cells/kg) | Single arm | 73/87, refractory LN | 48 | Death (5/87), diarrhea (2/87), herpesvirus infection (2/87), agranulocytosis and oral fungus infection (1/87), pulmonary tuberculosis (1/87), bone fracture (1/87), myocardial infarction (1/87) considered non transplantation related | Overall rate of survival was 94% (82/87). Complete clinical remission rate was 28% at 1 year (23/83), 31% at 2 years (12/39), 42% at 3 years (5/12), and 50% at 4 years (3/6). Rates of relapse were 12% (10/83) at 1 year, 18% (7/39) at 2 years, 17% (2/12) at 3 years, and 17% (1/6) at 4 years. The overall rate of relapse was 23% (20/87) | Wang et al[59], 2013 |
| UC-MSCs [double i.v. injection (1 week apart) 1 × 106 cells/kg] | Single arm | 38/40, active LN | 12 | Well tolerated. Herpesvirus infection, death, and tuberculosis infection with no relation to transplantation | Decreased SLEDAI score at 12 months follow-up. Decreased anti-dsDNA titer at 12 months follow-up. Reduced proteinuria and improved eGFR at 6 months follow-up. Four patients relapse at 12 months follow-up | Wang et al[22], 2014 |
| BM-MSCs/UC-MSCs (single i.v. injection 1 × 106 cells/kg) | Single arm | 81/81, active and refractory LN | 12 | Death (4/87), enteritis and diarrhea (2/87) with no relation to transplantation | Survival rate was 95 % (77/81) during the 12 months follow-up. Complete remission rate was 17.5% (14/80) at 3 months, 18.2% (14/77) at 6 months, and 23.4% (18/77) at 12 months. 60.5% renal remission, 22.4% renal flare. Decreased SLEDAI score and BILAG score, improved GFR at 12 months follow-up | Gu et al[54], 2014 |
| UC-MSCs [double i.v. injection (1 week apart) 2 × 108 cells/each] | Randomized double-blind, placebo-controlled trial | 18/18, WHO class III or IV LN | 12 | Leukopenia, pneumonia, subcutaneous abscess | Remission rate in the UC-MSCs group was 75% (9/12), compared to 83% (5/6) in the placebo group. No effect of UC-MSCs above standard immunosuppression | Deng et al[56], 2017 |
| UC-MSCs (single i.v. injection 1 × 106 cells/kg) | Single arm | 21/21, refractory LN | 6 | No treatment-related mortality or other adverse events | Decreased SLEDAI score at 6 months follow-up. Reduced proteinuria, maintained eGFR 6 months follow-up. Up-regulation of peripheral blood CD1c+ DCs and serum FLT3L | Yuan et al[39], 2019 |
| AD-MSCs (single i.v. injection, 2 × 106 cells/kg) | Single arm. Phase I clinical trial | 9/9, biopsy-proven refractory LN | 12 | No allergic reaction or other adverse effects | Decreased SLEDAI score. Decreased anti-dsDNA titer at the first month. Reduced proteinuria and improved eGFR at the first 3 months | Ranjbar et al[25], 2022 |
MSCs were primarily derived from umbilical cord and bone marrow, except in one study, where MSCs were derived from adipose tissue[25] (Table 3). Single-dose AD-MSC therapy showed significant short-term benefits, suggesting maintenance therapy with additional AD-MSC doses to sustain long-term remission in refractory LN cases[25]. However, Wang et al[18] compared the therapeutic effects of single vs double BM/UC-MSCs infusions in 58 refractory SLE patients, with 30 receiving a single dose and 28 receiving double doses. No remarkable differences were found between the two groups with more than 1 year follow up, including rates of survival, disease remission, and relapse, as well as transplantation-related adverse events[18]. The different conclusions of the two studies may be attributed to the different MSC sources and a lack of controlled study design with different doses. Given the high relapse rates in patients with refractory LN, the potential need for repeated MSC dosing to maintain therapeutic efficacy warrants systematic investigation. Gu et al[54] conducted Cox proportional hazards regression did not identify any significant association between survival rates or complete remission rates and MSC sources. More controlled, randomized clinical trials are necessary to evaluate the optimal sources and doses of MSC therapy.
Recent clinical applications of MSCs have demonstrated their therapeutic potential in refractory LN (Table 3). Most of these trials evaluated the SLE disease activity index (SLEDAI) score for systemic manifestations and proteinuria and SCr for renal function. Unfortunately, none of the trials performed repeat renal biopsies for renal pathology assessment. A meta-analysis found that the SLEDAI score significantly improved after MSC injection from 1 month to 12 months follow up[55]. The BILAG score, an assessment of LN activity, significantly decreased after MSC therapy. The level of proteinuria, SCr and BUN showed a decreasing trend in most trials. However, the randomized double-blind, placebo-controlled trial demonstrated that a similar proportion of patients achieved complete remission following treatment with UC-MSC and placebo[56]. These contrasting outcomes may be attributed to key distinctions in patient populations between this randomized controlled trial and prior studies. Active LN patients receiving traditional immunosuppressive agents typically achieve relatively high remission rates. Whereas other studies have included patients with refractory LN failing conventional therapies, MSC-based combination regimens showed superior efficacy. The overall therapeutic efficacy of MSC-based interventions requires further optimization in LN patients. Subgroup comparisons between refractory and newly active LN patients and between different pathological types are needed.
While MSC therapy has shown efficacy in many cases, comprehensive long-term monitoring is essential to evaluate potential transplantation-related risks. In vivo studies generally support the safety profile of MSC therapy[8]. No transplantation-related mortality or severe adverse events were reported. The infusion reaction was rare, with no anaphylaxis or acute toxicity reported. There were no reports of graft-vs-host disease or alloimmunization in allogeneic MSC recipients. There was no evidence of malignant transformation or organ toxicity, but long-term studies are needed to confirm these findings. The common adverse events were as follows[57-59]: Diarrhea and infection (tuberculosis, herpes virus or fungal infection), but none were specifically associated with MSC transplantation. The incidence of pneumonia was similar between placebo and standard therapy groups. Higher doses[56] (e.g., 2 × 108 cells) or double doses[18] did not increase adverse events. MSC therapy was universally superimposed on existing immunosuppressive therapy in these studies, and reported adverse events may reflect cumulative pharmacological effects rather than MSC-specific toxicity.
Barkholt et al[60] evaluated the tumorigenic potential of MSCs through integrated scientific and regulatory perspectives. While no clinical cases of MSC-derived tumors have been reported, concerns exist regarding in vitro culture-induced genomic instability, as prolonged expansion and enzymatic dissociation may promote chromosomal abnormalities. Contamination risks exist, as early reports of MSC transformation were later attributed to co-cultured tumor cells, underscoring the need for good manufacturing practice compliance. In addition, given the theoretical risks of MSCs’ potential immunomodulatory effects on tumor surveillance, rigorously designed clinical trials must incorporate extended longitudinal monitoring. We recommend a minimum 5-year follow-up period for MSC therapies, aligned with the latency window observed in transplant-associated malignancies[61].
Although MSC therapy exhibits both promising efficacy and safety in LN treatment, limitations remain that warrant further optimization in subsequent investigations. Firstly, the sample sizes of the current trials were relatively small, which limits the ability to perform adequately powered stratified analyses across distinct LN histological classifications and reduces the capacity to identify high-responder populations for MSC therapy. This fundamental limitation underscores the necessity for larger multicenter cohorts to enable valid subclassification analyses. Secondly, most of the trials used single-arm design, with one study including two dose groups[18], and another including a placebo control[56]. More rigorously designed randomized controlled trials are required to further validate: (1) The efficacy of immunosuppressant monotherapy vs combination with MSC therapy; (2) The optimal dosing protocols and administration frequency; and (3) The comparative advantages or disadvantages of different MSC sources. Moreover, future investigations should integrate extended observation periods to properly evaluate the maintenance of initial therapeutic benefits, potential delayed treatment effects and long-term safety profiles.
SLEDAI score, proteinuria and SCr were most commonly used to evaluate the effectiveness of MSC therapy. However, renal biopsy was not performed in current trials. Given that renal biopsy serves as the gold standard for determining LN classification and evaluating renal therapeutic response or disease progression, repeat renal biopsy is strongly recommended, particularly in cases of refractory or relapsing LN, which will also contribute to a more comprehensive understanding of the underlying therapeutic mechanisms.
While sources of MSCs are abundant, standardized protocols for MSC isolation and in vitro expansion, evidence-based dose optimization and eligibility criteria are critical to ensure therapeutic reproducibility. MSCs can modulate circulating immunity and regional immunity, as well as renal innate cells. Preliminary studies suggest that MSC therapy may exhibit therapeutic potential without serious adverse effects, but current evidence remains heterogeneous. The mechanisms of MSC therapy for LN require further investigation. More large-scale and multicenter randomized controlled clinical trials with long-term follow-up are needed to support their effectiveness, as well as evaluate the sources, dosage, frequency and suitable LN populations for MSC therapy.
| 1. | Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 678] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 2. | Moroni G, Vercelloni PG, Quaglini S, Gatto M, Gianfreda D, Sacchi L, Raffiotta F, Zen M, Costantini G, Urban ML, Pieruzzi F, Messa P, Vaglio A, Sinico RA, Doria A. Changing patterns in clinical-histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis. 2018;77:1318-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 3. | Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M; International Society of Nephrology Working Group on the Classification of Lupus Nephritis; Renal Pathology Society Working Group on the Classification of Lupus Nephritis. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 1133] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 4. | Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D'Agati VD, Ferrario F, Haas M, Jennette JC, Joh K, Nast CC, Noël LH, Rijnink EC, Roberts ISD, Seshan SV, Sethi S, Fogo AB. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 719] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 5. | Kidney Disease: Improving Global Outcomes (KDIGO) Lupus Nephritis Work Group. KDIGO 2024 Clinical Practice Guideline for the management of LUPUS NEPHRITIS. Kidney Int. 2024;105:S1-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 242] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 6. | Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ; Collaborative Study Group. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. 2008;3:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Cervera R, Mosca M, Ríos-Garcés R, Espinosa G, Trujillo H, Bada T, Praga M. Treatment for refractory lupus nephritis: Rituximab vs triple target therapy. Autoimmun Rev. 2019;18:102406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Zeng L, Yu G, Yang K, Xiang W, Li J, Chen H. Efficacy and Safety of Mesenchymal Stem Cell Transplantation in the Treatment of Autoimmune Diseases (Rheumatoid Arthritis, Systemic Lupus Erythematosus, Inflammatory Bowel Disease, Multiple Sclerosis, and Ankylosing Spondylitis): A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Stem Cells Int. 2022;2022:9463314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Berebichez-Fridman R, Gómez-García R, Granados-Montiel J, Berebichez-Fastlicht E, Olivos-Meza A, Granados J, Velasquillo C, Ibarra C. The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells-Their Current Uses and Potential Applications. Stem Cells Int. 2017;2017:2638305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 308] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 11. | Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 294] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 12. | Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 480] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 13. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13072] [Article Influence: 688.0] [Reference Citation Analysis (12)] |
| 14. | Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6:2173-2185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 549] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 15. | Chu DT, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, Truong DT, Pham VH, Ngoc VTN, Chu-Dinh T, Kushekhar K. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int J Mol Sci. 2020;21:708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 16. | Zhou T, Liao C, Li HY, Lin W, Lin S, Zhong H. Efficacy of mesenchymal stem cells in animal models of lupus nephritis: a meta-analysis. Stem Cell Res Ther. 2020;11:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, Traggiai E. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62:2776-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Wang D, Akiyama K, Zhang H, Yamaza T, Li X, Feng X, Wang H, Hua B, Liu B, Xu H, Chen W, Shi S, Sun L. Double allogenic mesenchymal stem cells transplantations could not enhance therapeutic effect compared with single transplantation in systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:273291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 322] [Article Influence: 26.8] [Reference Citation Analysis (5)] |
| 20. | Mennan C, Garcia J, Roberts S, Hulme C, Wright K. A comprehensive characterisation of large-scale expanded human bone marrow and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2019;10:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Chang JW, Hung SP, Wu HH, Wu WM, Yang AH, Tsai HL, Yang LY, Lee OK. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplant. 2011;20:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Chu DT, Nguyen Thi Phuong T, Tien NLB, Tran DK, Minh LB, Thanh VV, Gia Anh P, Pham VH, Thi Nga V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J Clin Med. 2019;8:917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Choi EW, Shin IS, Park SY, Park JH, Kim JS, Yoon EJ, Kang SK, Ra JC, Hong SH. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum. 2012;64:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Ranjbar A, Hassanzadeh H, Jahandoust F, Miri R, Bidkhori HR, Monzavi SM, Sanjar-Moussavi N, Matin MM, Shariati-Sarabi Z. Allogeneic adipose-derived mesenchymal stromal cell transplantation for refractory lupus nephritis: Results of a phase I clinical trial. Curr Res Transl Med. 2022;70:103324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3448] [Article Influence: 132.6] [Reference Citation Analysis (1)] |
| 27. | Gan L, Liu Y, Cui D, Pan Y, Zheng L, Wan M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020;2020:8864572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Tang X, Li W, Wen X, Zhang Z, Chen W, Yao G, Chen H, Wang D, Shi S, Sun L. Transplantation of dental tissue-derived mesenchymal stem cells ameliorates nephritis in lupus mice. Ann Transl Med. 2019;7:132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Sun W, Yan S, Yang C, Yang J, Wang H, Li C, Zhang L, Zhao L, Zhang J, Cheng M, Li X, Xu D. Mesenchymal Stem Cells-derived Exosomes Ameliorate Lupus by Inducing M2 Macrophage Polarization and Regulatory T Cell Expansion in MRL/lpr Mice. Immunol Invest. 2022;51:1785-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T, Sahoo S, Torrecilhas AC, Zheng L, Zijlstra A, Abuelreich S, Bagabas R, Bergese P, Bridges EM, Brucale M, Burger D, Carney RP, Cocucci E, Crescitelli R, Hanser E, Harris AL, Haughey NJ, Hendrix A, Ivanov AR, Jovanovic-Talisman T, Kruh-Garcia NA, Ku'ulei-Lyn Faustino V, Kyburz D, Lässer C, Lennon KM, Lötvall J, Maddox AL, Martens-Uzunova ES, Mizenko RR, Newman LA, Ridolfi A, Rohde E, Rojalin T, Rowland A, Saftics A, Sandau US, Saugstad JA, Shekari F, Swift S, Ter-Ovanesyan D, Tosar JP, Useckaite Z, Valle F, Varga Z, van der Pol E, van Herwijnen MJC, Wauben MHM, Wehman AM, Williams S, Zendrini A, Zimmerman AJ; MISEV Consortium, Théry C, Witwer KW. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2852] [Cited by in RCA: 2326] [Article Influence: 1163.0] [Reference Citation Analysis (0)] |
| 31. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 8327] [Article Influence: 1040.9] [Reference Citation Analysis (1)] |
| 32. | Maumus M, Rozier P, Boulestreau J, Jorgensen C, Noël D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front Bioeng Biotechnol. 2020;8:997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 33. | Li C, Wu F, Mao J, Wang Y, Zhu J, Hong K, Xie H, Zhou X, Tian J, Wen C. Mesenchymal stem cells-derived extracellular vesicles ameliorate lupus nephritis by regulating T and B cell responses. Stem Cell Res Ther. 2024;15:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 34. | Li J, Luo M, Li B, Lou Y, Zhu Y, Bai X, Sun B, Lu X, Luo P. Immunomodulatory Activity of Mesenchymal Stem Cells in Lupus Nephritis: Advances and Applications. Front Immunol. 2022;13:843192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Hoseinzadeh A, Rezaieyazdi Z, Mahmoudi M, Tavakol Afshari J, Lavi Arab F, Esmaeili SA, Faridzadeh A, Rezaeian A, Hoseini S, Barati M, Mahmoudi A, Sadat Tabasi N. Dysregulated balance in Th17/Treg axis of Pristane-induced lupus mouse model, are mesenchymal stem cells therapeutic? Int Immunopharmacol. 2023;117:109699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 36. | Choi EW, Lee M, Song JW, Shin IS, Kim SJ. Mesenchymal stem cell transplantation can restore lupus disease-associated miRNA expression and Th1/Th2 ratios in a murine model of SLE. Sci Rep. 2016;6:38237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Ma X, Che N, Gu Z, Huang J, Wang D, Liang J, Hou Y, Gilkeson G, Lu L, Sun L. Allogenic mesenchymal stem cell transplantation ameliorates nephritis in lupus mice via inhibition of B-cell activation. Cell Transplant. 2013;22:2279-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 992] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 39. | Yuan X, Qin X, Wang D, Zhang Z, Tang X, Gao X, Chen W, Sun L. Mesenchymal stem cell therapy induces FLT3L and CD1c(+) dendritic cells in systemic lupus erythematosus patients. Nat Commun. 2019;10:2498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 40. | Zhou C, Bai X, Yang Y, Shi M, Bai XY. Single-Cell Sequencing Informs That Mesenchymal Stem Cell Alleviates Renal Injury Through Regulating Kidney Regional Immunity in Lupus Nephritis. Stem Cells Dev. 2023;32:465-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 41. | Zhang Z, Niu L, Tang X, Feng R, Yao G, Chen W, Li W, Feng X, Chen H, Sun L. Mesenchymal stem cells prevent podocyte injury in lupus-prone B6.MRL-Faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrol Dial Transplant. 2019;34:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 42. | Ma H, Liu C, Shi B, Zhang Z, Feng R, Guo M, Lu L, Shi S, Gao X, Chen W, Sun L. Mesenchymal Stem Cells Control Complement C5 Activation by Factor H in Lupus Nephritis. EBioMedicine. 2018;32:21-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Liu J, Lu X, Lou Y, Cai Y, Cui W, Wang J, Nie P, Chen L, Li B, Luo P. Xenogeneic Transplantation of Human Placenta-Derived Mesenchymal Stem Cells Alleviates Renal Injury and Reduces Inflammation in a Mouse Model of Lupus Nephritis. Biomed Res Int. 2019;2019:9370919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Huang C, Meng M, Li S, Liu S, Li L, Su Y, Gao H, He S, Zhao Y, Zhang M, Hou Z, Wang W, Wang X. Umbilical Cord Mesenchymal Stem Cells Ameliorate Kidney Injury in MRL/Ipr Mice Through the TGF-β1 Pathway. Front Cell Dev Biol. 2022;10:876054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Kim HS, Lee HK, Kim K, Ahn GB, Kim MS, Lee TY, Son DJ, Kim Y, Hong JT, Han SB. Mesenchymal stem cells enhance CCL8 expression by podocytes in lupus-prone MRL.Fas(lpr) mice. Sci Rep. 2023;13:13074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 46. | Li W, Chen W, Sun L. An Update for Mesenchymal Stem Cell Therapy in Lupus Nephritis. Kidney Dis (Basel). 2021;7:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Youd M, Blickarz C, Woodworth L, Touzjian T, Edling A, Tedstone J, Ruzek M, Tubo R, Kaplan J, Lodie T. Allogeneic mesenchymal stem cells do not protect NZBxNZW F1 mice from developing lupus disease. Clin Exp Immunol. 2010;161:176-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Bazhanov N, Ylostalo JH, Bartosh TJ, Tiblow A, Mohammadipoor A, Foskett A, Prockop DJ. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Res Ther. 2016;7:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Che N, Li X, Zhang L, Liu R, Chen H, Gao X, Shi S, Chen W, Sun L. Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol. 2014;193:5306-5314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Tian CM, Zhang Y, Yang MF, Xu HM, Zhu MZ, Yao J, Wang LS, Liang YJ, Li DF. Stem Cell Therapy in Inflammatory Bowel Disease: A Review of Achievements and Challenges. J Inflamm Res. 2023;16:2089-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 51. | Petrou P, Kassis I, Levin N, Paul F, Backner Y, Benoliel T, Oertel FC, Scheel M, Hallimi M, Yaghmour N, Hur TB, Ginzberg A, Levy Y, Abramsky O, Karussis D. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020;143:3574-3588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 52. | Izadi M, Sadr Hashemi Nejad A, Moazenchi M, Masoumi S, Rabbani A, Kompani F, Hedayati Asl AA, Abbasi Kakroodi F, Jaroughi N, Mohseni Meybodi MA, Setoodeh A, Abbasi F, Hosseini SE, Moeini Nia F, Salman Yazdi R, Navabi R, Hajizadeh-Saffar E, Baharvand H. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther. 2022;13:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 53. | Li J, Wu M, He L. Immunomodulatory effects of mesenchymal stem cell therapy in chronic kidney disease: a literature review. BMC Nephrol. 2025;26:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 54. | Gu F, Wang D, Zhang H, Feng X, Gilkeson GS, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin Rheumatol. 2014;33:1611-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 55. | Xia Y, Ye H, Li K, Shi B, Sun X, Wu J. Efficacy of Mesenchymal Stem Cell Therapy on Lupus Nephritis and Renal Function in Systemic Lupus Erythematosus: A Meta-Analysis. Clin Invest Med. 2023;46:E24-E35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 56. | Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76:1436-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 57. | Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 333] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 58. | Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 59. | Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22:2267-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 60. | Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, Büscher D, Fibbe W, Foussat A, Kwa M, Lantz O, Mačiulaitis R, Palomäki T, Schneider CK, Sensebé L, Tachdjian G, Tarte K, Tosca L, Salmikangas P. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 61. | Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1140] [Article Influence: 76.0] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/