Published online Apr 26, 2025. doi: 10.4252/wjsc.v17.i4.101891
Revised: February 17, 2025
Accepted: March 21, 2025
Published online: April 26, 2025
Processing time: 205 Days and 7.8 Hours
Dry eye disease (DED) is a multifactorial disorder that disturbs ocular surface equilibrium, considerably dimini
Core Tip: Stem cell treatment offers a potential regenerative strategy for addressing severe dry eye disease by restoring lacrimal gland function, regulating inflammation, and facilitating corneal restoration. This review incorporated current preclinical and clinical discoveries, contrasting various mesenchymal stem cell (MSC) sources and their modes of action. Notwithstanding promising outcomes, obstacles persist in enhancing MSC viability, standardizing administration techniques, and guaranteeing prolonged safety. More extensive randomized controlled studies are necessary to determine the effectiveness of MSC-based treatments in severe dry eye disease.
- Citation: Fu L, Pelosini L, Kopsachilis N, Foti R, D’Esposito F, Musa M, D’Amico A, Tognetto D, Gagliano C, Zeppieri M. Evaluating the efficacy of stem cells in treating severe dry eye disease. World J Stem Cells 2025; 17(4): 101891
- URL: https://www.wjgnet.com/1948-0210/full/v17/i4/101891.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i4.101891
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a chronic condition characterized by diminished tear film stability and elevated tear hyperosmolarity, which may result in peripheral nerve injury[1,2]. Patients experience various symptoms, including pain, blurred vision, and discomfort and in some cases neuropathic ocular pain[1,2]. Risk factors include environmental humidity, age, sex, use of computer screens, and ethnicity[1]. Globally, the incidence of DED is between 5.5% and 22.7%, and it is one of the most common causes of referrals to ophthalmology[3]. Given how common DED is, there is unfortunately no definitive cure available. Current management is focused on the symptoms and involves a step ladder approach, commencing with artificial lubricating tears, topical steroids, and nutritional supplements[4].
The primary function of the tear film is to protect and lubricate the ocular surface[1]. DED can be divided into evaporative, aqueous deficiency, and mixed types[5]. Evaporative DED results from an unstable lipid layer, leading to tear evaporation and hyperosmolarity due to meibomian gland dysfunction (MGD). Aqueous deficiency DED (ADDE) is due to lacrimal gland (LG) damage and can be exacerbated by thyroid disease, diabetes, and rosacea[5]. Other causes of LG damage are damage or radiation to the head and neck[6]. ADDE is further divided into Sjögren’s and non-Sjögren’s dry eye.
In the Western hemisphere, Sjögren’s syndrome (SS) is the most common cause of severe ADDE and is characterized by lymphocytic infiltration of the salivary gland and the LG, causing dry mouth and eyes[7]. Females are more likely to have SS, and the ocular sequelae can involve microbial keratitis, ulceration, vascularization, perforation, and scarring[8]. SS can be primary or associated with other autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus. The diagnosis of SS in patients with DED requires more than just clinical signs and symptoms. Primary SS is confirmed if a patient with clinical signs and symptoms of SS scores 4 or more points from Table 1. This review focused on the current research and development of clinical treatments for severe DED utilizing stem cells and their products.
| Diagnostic criteria | Points |
| Positive anti-SSA antibody from a peripheral blood sample | 3 |
| Focal lymphocytic sialadenitis | 3 |
| Abnormal ocular staining score 5 out of 7 | 1 |
| Schirmer test score ≤ 5 mm/5 minutes | 1 |
| Unstimulated salivary flow rate ≤ 0.1 mL/minute | 1 |
The Tear Film and Ocular Surface Dry Eye Workshop II redefined DED in 2017 as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability, hyperosmolarity, ocular surface inflammation, and neurosensory abnormalities play etiological roles”[5].
Recent research has shown that mesenchymal stem cells (MSCs) exhibit therapeutic benefits via many pathways, including the release of anti-inflammatory cytokines like interleukin (IL)-10 and transforming growth factor-beta (TGF-β), control of immune cell activity, and facilitation of epithelial regeneration[9]. Exosomes generated from MSCs include bioactive compounds that promote corneal epithelial repair and suppress apoptotic pathways. Furthermore, stem cells rejuvenate LG functionality by developing into glandular cells and releasing growth factors that facilitate tissue healing[10-14]. These strategies jointly mitigate the inflammatory and degenerative processes linked to DED.
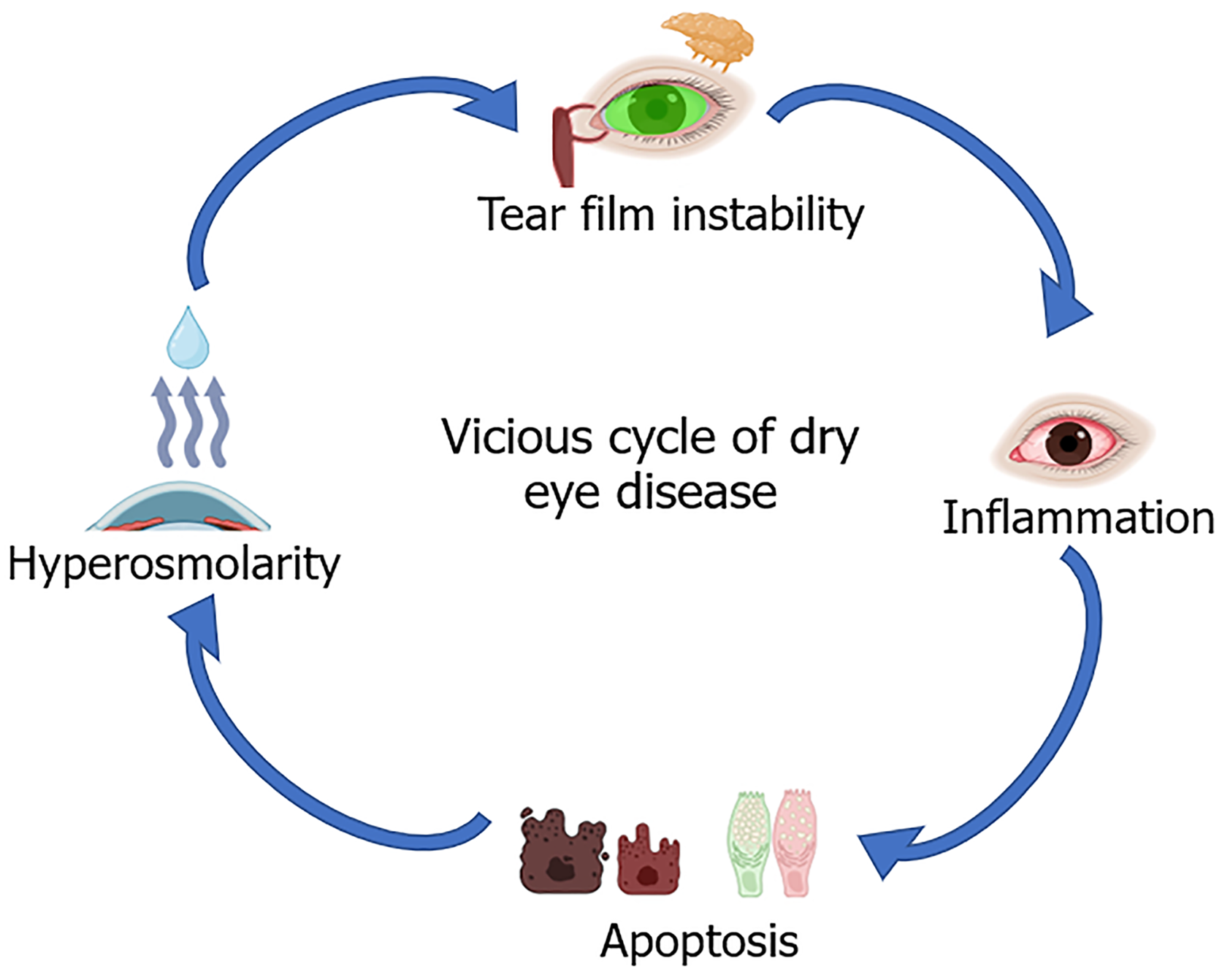
Chronic inflammation is a main contributing factor affecting LG dysfunction and secretion leading to ADDE[9]. SS, diabetes, chronic graft-vs-host disease, and aging are all risk factors for chronic inflammation. When tear cytokines in patients with DED were analysed, there were increased levels of IL-1, IL-4, IL-6, IL-8, IL-10, IL-17A, tumour necrosis factor-α, TGF-β, metalloproteinase (MMP)-3, and MMP-9 compared with controls[10-14]. The increased cytokine levels were also correlated with DED severity[10-14]. Levels of IL-6 were significantly higher in patients with DED with the release of inflammatory factors such as IL-17[15]. IL-17 also promoted MMP-3 and MMP-9 release, which are involved in wound healing and inflammation[16,17]. MMPs compromise the tight connections among epithelial cells, resulting in the deterioration of the corneal barrier. Chronic inflammation arises from the “vicious cycle” of dry eye illness (Figure 1): Several elements from the ocular and external environment contribute to tear film instability, subsequently resulting in tear hyperosmolarity. This leads to cellular death in the cornea and conjunctiva, inciting inflammation that subsequently activates the meibomian glands and LGs, negatively impacting the tear film. Thus, anti-inflammatory therapy is funda
The ocular surface in DED has elevated levels of C-C chemokine ligands (CCL) 3, CCL 5, CCL20, and T helper (Th) cell chemokines, which can promote proinflammatory Th cell migration to the ocular surface. CCL20 is responsible for the cellular homing of Th17 in DED. De Paiva et al[18] demonstrated that Th1 and Th17 cells were present in the conjunctiva with high expression of IL-17 and interferon (IFN)-γ. IFN-γ reduces the ability of goblet cells to secrete mucin, which is important for immune tolerance of the ocular surface[19,20]. Anti-inflammatory regulatory T cells (CD4+) are important modulators of the immune system, maintaining self-antigenic tolerance and preventing autoimmune disease locally and systemically (e.g., SS, chronic graft-vs-host disease) by suppressing autoreactive T cells[21]. Treatments that can target IL-17 or inhibit Th17 function can potentially reduce the progression and severity of DED[19].
In neurotrophic keratitis, impaired corneal innervation leads to corneal epithelial breakdown, tear film disruption due to decreased lacrimation reflex, and increased ocular surface inflammation. The corneal nerve plexus is amongst the densest in the human body and is populated with sensory nerves. The release of inflammatory factors in the tears or any other changes within the ocular surface environment will travel through afferent signalling and stimulate efferent innervation, gland secretion, and blink activity[21]. Disruption or dysfunction in the nerve conduction pathway can lead to DED by aggravating ocular surface injury and the persistence of inflammation[22]. The peripheral sympathetic and parasympathetic nerves regulate secretion by the conjunctival goblet cells[22].
Management of DED often begins with artificial tears to improve tear film stability, with some demonstrating clinical efficacy[23]. Patients test out different eye drop brands in a trial-and-error manner; although there are guidelines for ocular lubricant pathways, these vary depending on the locality. Patients with ADDE, especially due to SS, will have higher levels of ocular surface inflammation compared with patients with non-SS DED; artificial tears alone do not provide effective symptom relief[24]. Kim et al[25] have shown that topical steroids and/or cyclosporin A can signi
Surgical treatments such as punctal plugs and lid tarsorrhaphy (partial or complete) have been used as an adjuvant to topical therapy. More novel procedures, such as amniotic membrane transplant, have also been utilized. Amniotic membrane transplant does improve DED signs and symptoms, but the effect is temporary, with a mean relapse time of 25 days[28]. More experimental procedures, such as platelet-rich plasma (PRP) injections into the LG, have been attempted and show objective and subjective improvements in ADDE signs[29]. PRP production has no standardization. Therefore, studies may have different PRP compositions, making comparisons difficult[30]. There is a lack of randomized clinical trials comparing PRP injection with another interventional treatment and no long-term follow-up more than 3 months post-procedure. In summary, these treatments provide symptom relief only and do not target the underlying causes of DED. Hence, novel regenerative therapies are needed.
Stem cells are undifferentiated cells that have the potential to differentiate into a variety of specialized cells (differentiation) and can produce identical stem cells (self-renewal)[31]. Stem cells can be embryonic or adult, with embryonic stem cells derived from blastocysts having pluripotency (differentiating into three germ layers). This makes embryonic stem cells suitable for use in regenerative medicine. However, there are ethical issues and risks of tumorigenicity. Adult stem cells repair damage and maintain cellular turnover but have reduced differentiation ability ranging from unipotent (single specialized cell) to multipotency (differentiating in one germ layer). Adult stem cells exist in the LG and can differentiate into cells of the acinar, myoepithelium, and lacrimal ducts[32]. These can potentially be utilized to restore LG function. Alternative sources of adult stem cells in the human body include MSCs. MSCs have been studied for tissue and organ regeneration due to their wide availability and lack of cellular surface immune-stimulating markers. Allogenic use will avoid a graft-vs-host response[33].
Tissues within the body have some regeneration ability after injury, and adult stem cells have been found in specialized niches. Animal studies have identified stem cell niches within cultured in vitro LG organoids[34]. The LG stem cells (LGSCs) were isolated and identified with a variety of established techniques: Western blot; flow cytometry; immunostaining; and PCR[34-37]. Stem cell markers such as nestin and P63 were used to identify LGSCs[38,39]. These markers were found to be increased in number after leukin-1 injury, suggesting that stem cells were mobilized in response to the injury[34,40].
Different culture methods have been reported in these studies, and they can influence the characteristics and quality of identified stem cells and their phenotype. The explant culture method was utilized by many studies with cell strainers, flow cytometry, and Matrigel to isolate cells[35,39,41,42]. The cultured cells were classified by immunohistochemical staining and morphology[35,39,42]. Xiao and Zhang[39] used a serum-free medium and maintained LGSCs in a three-dimensional culture. Three-dimensional cell culture differs from traditional two-dimensional cell culture (cells grown in a monolayer) in that it allows cell growth and interaction with the surrounding extracellular framework in three dimensions. This technique has the potential to be used as an alternative to animal models of DED and transplantation.
The LG contributes to the aqueous component layer of the tear film and supplies numerous proteins, enzymes, antimicrobial factors, and immunoglobulins that are protective of the ocular surface[43-45]. Disruption of the LG would significantly affect the tear film and its protective role[45]. The LG acinar cells produce primary LG fluid that is subsequently modified by the ductal cells with electrolytes and water[46]. The myoepithelial cells are located around the acini and have a contractile function allowing regular fluid secretion to the ocular surface[47]. The ductal cells supply 30% of the LG fluid[43].
There are two main research areas within the field of LG regeneration. The first is to identify key signalling pathways and proteins for LG inflammation and promotion of regeneration[35,38]. The second is the development of treatments for direct in situ LG regeneration with cultured stem cells or stem cell products[35,36,48,49]. Studies in regenerative mechanisms of living tissues have demonstrated the importance of the epithelial-to-mesenchymal transition that allows cell proliferation and repair. You et al[34] found the mobilisation of nestin-positive MSCs within LG regeneration. Key regulatory proteins that coordinated the transition were snail and vimentin, which are potentially new therapeutic targets for LG repair mechanisms[34].
A signalling pathway that directs cells towards the epithelial-mesenchymal transition within the LG is the bone morphogenic protein-7 pathway[40]. This pathway has been shown to ameliorate tissue damage in an animal model of renal injury by reducing inflammation and fibrosis[50]. Therefore, the addition of exogenous bone morphogenic protein-7 to damaged LG or in combination with transplantation can potentially reduce the inflammatory process during LG regeneration.
Extracellular ATP is used as fuel during the inflammatory process. Basova et al[51] demonstrated that blocking Pannexin-1 reduced the import of extracellular ATP and led to reduced inflammation and improved donor cell survival during LG regeneration. Several stem cell types and products have been investigated for direct application in LG regeneration. The most promising are MSCs with human-subject clinical trials in progress (Table 2).
| Stem cell source | Differentiation potential | Anti-inflammatory properties | Clinical applications | Key findings |
| AD-MSCs[74,76,84] | Multipotent | High secretion of anti-inflammatory cytokines | Used in limbal stem cell deficiency and corneal wound healing | Show promising anti-inflammatory and regenerative effects |
| BM-MSCs[36,85,86] | Multipotent | Moderate secretion of anti-inflammatory cytokines | Used in severe DED and Sjögren’s syndrome-related dry eye | Effective in reducing inflammation and promoting epithelial proliferation |
| UC-MSCs[75,86-90] | Higher plasticity than AD-MSCs and BM-MSCs | High immunomodulatory potential | Investigated for corneal and lacrimal gland regeneration | Exhibits low immunogenicity and enhances corneal healing |
MSCs are adult stem cells with multipotency and self-renewal abilities, differentiating into osteoblasts, myocytes, adipocytes, and chondroblasts[52]. Bone marrow-derived MSCs (BM-MSCs), adipose-derived MSCs (AD-MSCs), umbilical cord-derived MSCs (UC-MSCs), and cornea-derived MSCs (C-MSCs) are frequently utilized in research. The MSCs are well-defined according to criteria set by the International Society for Cell and Gene Therapy[53]. Specific surface markers associated with MSCs include CD73, CD90, and CD105 as well as the ability to adhere to plastic surfaces[54]. Endothelial and hematopoietic markers are absent and a low expression of major histocompatibility complex molecules. This allows MSCs to have immune privilege and can be transplanted without immunosuppression.
MSCs communicate with the existing immune cells and release paracrine factors (secretomes) that maintain tissue homeostasis, immunomodulation, and regeneration[55]. When MSCs are exposed to proinflammatory factors (e.g., tumour necrosis factor-α), they will differentiate into an immunosuppressive phenotype. Liu et al[56] found that MSCs can activate CD4+ T cells and reduce IFN-γ production. Several studies have found dysregulation of the enzyme indoleamine 2,3-dioxygenase in autoimmune diseases such as SS that is induced by IFN-γ[57,58]. Indoleamine 2,3-dioxygenase modulates innate and adaptive immune responses resulting in suppression of effector T cells[59]. MSCs also can suppress the differentiation and maturation of dendritic cells, inhibit the action of natural killer cells, and neutrophil apoptosis via its secretome function[53]. Consequently, MSC transplantation has been used to treat SS and refractory rheumatoid arthritis[60,61].
The immunoregulatory and anti-inflammatory characteristics of MSCs render them suitable for severe dry eye illness associated with ocular surface inflammation, limbal stem cell deficiency (LSCD), and nerve injury. Adipose tissue has been a good source of MSCs and is used widely in research. Galindo et al[62] found good tolerance, anti-inflammatory, and anti-angiogenic effect when utilizing human AD-MSCs to treat LSCD in a rabbit model. The site of administration and concentration of AD-MSCs affect the efficacy, with Fuentes-Julián et al[63] giving local and intravenous AD-MSCs during keratoplasty surgery that resulted in neovascularization and inflammation. Indirect action by AD-MSCs through its paracrine signalling factors such as vascular endothelial growth factor, TGF-β, and insulin-like growth factor can improve wound healing in corneal tissues[64]. AD-MSCs were cultured with human corneal epithelial cells, and their secretomes were observed to inhibit the epithelial-mesenchymal transition in the cornea[65].
Bone marrow served as the initial source for the isolation of MSCs, and BM-MSCs have been delivered through subconjunctival and intravenous injections as well as corneal transplantation. Shukla et al[66] found that subconjunctival and intravenous MSC delivery had better therapeutic effects compared with other methods in a corneal injury murine model. In a proof-of-concept clinical trial, Calonge et al[67] found that BM-MSCs could promote corneal epithelium proliferation in LSCD.
UC-MSCs are harvested from umbilical cord blood, which is more abundant and easier to collect than other sources. Their expression profile more closely resembles embryonic stem cells with pluripotency and can differentiate into corneal epithelium, stromal, and endothelial cells. With demonstrated low immunogenicity and graft-vs-host disease, UC-MSCs have been used in corneal transplantation, with Coulson-Thomas et al[68] using human UC-MSCs to resolve corneal defects in a murine model of mucopolysaccharidosis VII. However, there is variation in the yield of harvested UC-MSCs and methods of isolation and culture can be complex, limiting clinical application in a real-world setting[69]. MSCs with multidirectional differentiation potential are also located in the anterior corneal stroma next to the limbal stem cells.
Jabbehdari et al[70] found that C-MSCs had greater differentiating potential to corneal cells than MSCs derived from other sources. Culture techniques that combined limbal epithelial cells with C-MSCs were superior compared with culture with only limbal epithelial cells[71]. C-MSCs from human cadaveric corneoscleral rims were successfully expanded and found to express stem cell genes[72]. Inhibition of corneal neovascularization has been demonstrated by Eslani et al[73] in mouse corneas that have been treated with C-MSCs. When directly engrafted into corneal wounds in mice, these autologous stem cells prevented the formation of fibrotic tissue and promoted regeneration of ablated stroma that was indistinguishable from native tissue[72].
Various sources of MSCs demonstrate distinct biological features, potentially affecting their effectiveness in the treatment of dry eye illness. BM-MSCs are well described and have significant immunomodulatory capabilities; nevertheless, their invasive extraction method restricts clinical use. AD-MSCs are readily accessible and have a high multiplication rate, rendering them appropriate for therapeutic use. UC-MSCs demonstrate less immunogenicity and enhanced pluripotency, providing benefits for tissue regeneration. C-MSCs are particularly advantageous for ocular applications, demonstrating enhanced differentiation potential into corneal epithelial cells. Future research should concentrate on direct comparison assessments to identify the most suitable MSC source for therapeutic use in DED.
The core mechanism underlying clinical DED is tear film instability. The tear film is maintained by tissues of the conjunctiva, cornea, LG, and eyelid. Numerous preclinical in vivo animal studies have demonstrated the potential of MSCs in DED (Table 2). MSCs have been studied in mice, canines, and rabbits[36,74-76]. Studies have used MSC-derived exosomes and other derivatives with promising results and no adverse events. Canines are used as DED models because they develop dry eyes naturally due to an immune-mediated inflammatory reaction affecting the LG, like humans[77]. Bittencourt et al[74] demonstrated transplantation of AD-MSCs around the LGs in 15 canines with ADDE to be safe and increased tear production significantly, resulting in clinical improvement during the 12-month follow-up. In another study, Villatoro et al[76] injected allogeneic adipose-derived stromal cells in 12 canines with refractory bilateral ADDE that resulted in improved tear production, and clinical signs were maintained up to 9 months after initial treatment.
The primary constituent of the lipid layer of the tear film is meibum, which is secreted by the meibomian gland. The quality and quantity of meibum will deteriorate in MGD and reduce tear film stability. MSCs have been used to treat a BAC-induced mouse model of DED resulting in improvement post-treatment[78]. The therapeutic mechanism remains unclear, despite the observable infiltration of MSCs into the meibomian gland cells. The main component of the mucoprotein layer in the tear film is mucosal protein, secreted by the conjunctival goblet cells. Diseases or processes resulting in the loss of goblet cell function will decrease tear film stability and activate the inflammatory cascade. The number of conjunctival goblet cells has been shown to increase after treatment with MSCs[78,79]. LG inflammation and atrophy can also lead to decreased tear secretion. MSCs have been shown to promote LG regeneration. Møller-Hansen et al[80] demonstrated the safety and feasibility of an AD-MSC injection treatment in 7 patients with ADDE with follow-up to 16 weeks in a phase I trial.
MSCs exercise their therapeutic benefits in dry eye illness by paracrine signalling, immune response regulation, and direct cellular differentiation. MSCs release trophic substances, including hepatocyte growth factor, insulin-like growth factor-1, and TGF-β, which enhance LG epithelial cell proliferation and diminish inflammatory cytokine production[56-58]. Furthermore, exosomes produced from MSCs have been demonstrated to suppress apoptosis in corneal epithelial cells by downregulating caspase-3 activation. Moreover, the treatment efficacy of MSCs may vary between evaporative DED and ADDE. In evaporative DED, MSCs predominantly demonstrate anti-inflammatory effects via regulating meibomian gland activity and diminishing lipid layer instability. In ADDE, MSCs facilitate LG regeneration and augment aqueous tear output. These particular functions underscore the necessity for customized MSC-based treatments that address the underlying pathophysiology of DED.
Although numerous preclinical studies have shown the effectiveness of MSCs in DED models, there remains a lack of agreement regarding the ideal cell source and delivery method. Certain research implied that AD-MSCs demonstrate enhanced anti-inflammatory capabilities, whilst others indicate that UC-MSCs provide higher differentiating potential. Moreover, clinical studies are constrained, predominantly emphasizing safety over effectiveness. Future study must focus on direct comparisons of various MSC sources to determine optimal procedures for clinical use.
These preclinical animal studies have utilized comparatively brief follow-up durations, generally spanning only a few weeks to months. This is a substantial constraint as it precludes a thorough assessment of long-term effectiveness or possible hazards including cancer. Future investigations on MSC treatment must include prolonged follow-up periods of no less than 12 months, focusing specifically on any indications of atypical cellular proliferation or fibrosis in the treated tissues. Table 2 shows the comparison of different stem cell sources.
There are very few human clinical trials examining the use of MSCs in DED despite numerous preclinical studies (Table 3). While the phase I trial conducted by Møller-Hansen et al[80] indicated safety and feasibility, the limited sample size (n = 7) constrains the generalizability of the results. Limited sample numbers can result in statistical biases, unintended outcomes, and an inflation of treatment effects. Consequently, extensive randomized controlled trials with sufficient statistical power are essential to substantiate the clinical effectiveness of MSC treatment in dry eye illness. Minimum clinically important difference (MCID) has been proposed by the Tear Film and Ocular Surface Dry Eye Workshop II report as a primary endpoint in DED clinical trials. The MCID was the smallest amount of change that was significant for the patient[81,82]. OSDI score was recommended as one of the primary endpoints in DED trials for severe disease (score more than 33) with a MCID of 7.3 to 13.4 points[83].
| No. | Study | Diagnosis | Drug | Intervention | Patient number | Location(s) | Trial reference number (NCT) |
| 1 | Effect of UMSCs derived exosomes on dry eye in patients with cGVHD | Dry eye | UMSC-Exo | Transconjunctival injection | 27 | Guangzhou, Guangdong, China | 04213248 |
| 2 | Mesenchymal stem cell therapy of dry eye disease in patients with Sjögren’s syndrome | Keratoconjunctivitis sicca, in Sjögren’s syndrome | AD-MSCs | Transconjunctival injection | 40 | Copenhagen, DK, Denmark | 04615455 |
| 3 | Safety and efficacy of pluripotent stem cell-derived mesenchymal stem cell exosome (PSC-MSC-Exo) Eye drops treatment for dry eye disease post refractive surgery and associated with blepharospasm | Dry eye post refractive surgery | PSC-MSC-Exo | Topical eye drops | 12 | Hangzhou, Zhejiang, China | 05738629 |
| 4 | Treatment with allogeneic adipose-derived mesenchymal stem cells in patients with aqueous deficient dry eye disease | Aqueous deficiency dry eye disease, keratoconjunctivitis sicca | AD-MSCs | Lacrimal gland injection | 7 | Copenhagen, DK, Denmark | 03878628 |
| 5 | Allogeneic mesenchymal stem cells transplantation for primary Sjögren’s syndrome (pSS) | Keratoconjunctivitis sicca, in Sjögren’s syndrome | AlloMSC | Intravenous infusion (single dose) | 20 | Nanjing, Jiangsu, China | 00953485 |
| 6 | Therapeutic effect of stem cell eye drops on dry eye disease | Dry eye syndromes | MSC eye drops | Topical eye drops | 10 | Nanjing, Jiangsu, China | 05784519 |
Recently, a randomized controlled trial evaluating the safety and efficacy of AD-MSC injections into the LG of patients with ADDE was completed[80]. This double-blinded trial included 54 patients with severe ADDE secondary to SS and were randomized to AD-MSC injection (treatment; n = 20), placebo (n = 20), or an observation group (n = 14). The sample size was based on an 80% power (2-sided t-test), a significance level P < 0.05, and additional allowance for potential dropout for the duration of the trial. At the 12-month follow-up there was a significant reduction in primary endpoint measures (OSDI score) in the treatment group along with objective clinical signs[80]. Of interest, significant improvement in subjective OSDI scores was noted in the placebo group, who had an injection of 10% dimethyl sulfoxide (CryoStor10) with no AD-MSCs, possibly as a result of the anti-inflammatory and/or placebo effect. Clinically, only the AD-MSC treatment group showed objective clinical improvement at 4 weeks and 12 months of follow-up.
The assessment of MSC treatment in severe DED necessitates a comprehensive examination of clinical outcomes, comparative effectiveness, and constraints. Clinical investigations have indicated enhancements in corneal epithelial integrity, tear secretion, and a decrease in inflammatory markers subsequent to MSC treatment. Diversity in MSC sources, delivery methods, and follow-up lengths hinders direct comparisons. Future research must emphasize the standardization of MSC therapy techniques and the identification of appropriate MSC sources for sustained effectiveness. Table 3 shows the clinical trials in this sector.
MSC-based therapy for DED is feasible, and it has an exciting scope for the treatment of damaged tissue. Future research must concentrate on many critical domains to improve the practical use of MSC treatment for dry eye condition. Primarily, enhancing the survival rates of MSCs is a priority as transplanted MSCs frequently have restricted persistence in vivo. Exploration of strategies like genetic manipulation to augment MSC resilience or coadministration with biomaterials is warranted. MSC delivery strategies necessitate enhancement to optimize therapeutic effectiveness. Although topical and subconjunctival delivery have demonstrated potential, innovative biomaterial scaffolds and sustained-release formulations may enhance MSC retention at the ocular surface.
Prolonged clinical studies with extensive follow-up periods are crucial for evaluating safety issues, including cancer and immunological rejection. Moreover, discrepancies in stem cell production techniques among research hinder direct comparisons. The establishment of standardized protocols for the isolation, characterization, and growth of MSCs will be crucial for their effective therapeutic use. Examining the function of MSC-derived exosomes in DED treatment is a potential approach since exosomes may provide a cell-free therapeutic option with comparable regeneration advantages. Finally, a uniform reporting structure for adverse events in MSC treatment must be established to enable accurate comparisons among trials. Resolving these fundamental difficulties would facilitate the effective incorporation of MSC-based treatments in clinical ophthalmology.
| 1. | Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, Yokoi N, Zoukhri D, Sullivan DA. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 1275] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 2. | Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, Dartt DA, Galor A, Hamrah P, Ivanusic JJ, Jacobs DS, McNamara NA, Rosenblatt MI, Stapleton F, Wolffsohn JS. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15:404-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 488] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 3. | Rabina G, Boguslavsky II, Mimouni M, Kaiserman I. The Association between Preoperative Dry Eye Symptoms and Postoperative Discomfort in Patients Underwent Photorefractive Keratectomy. J Ophthalmol. 2019;2019:7029858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Milner MS, Beckman KA, Luchs JI, Allen QB, Awdeh RM, Berdahl J, Boland TS, Buznego C, Gira JP, Goldberg DF, Goldman D, Goyal RK, Jackson MA, Katz J, Kim T, Majmudar PA, Malhotra RP, McDonald MB, Rajpal RK, Raviv T, Rowen S, Shamie N, Solomon JD, Stonecipher K, Tauber S, Trattler W, Walter KA, Waring GO 4th, Weinstock RJ, Wiley WF, Yeu E. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27 Suppl 1:3-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 5. | Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1187] [Cited by in RCA: 2258] [Article Influence: 250.9] [Reference Citation Analysis (0)] |
| 6. | Tiwari S, Bhatt A, Nagamodi J, Ali MJ, Ali H, Naik MN, Reddy VAP, Vemuganti GK. Aqueous Deficient Dry Eye Syndrome Post Orbital Radiotherapy: A 10-Year Retrospective Study. Transl Vis Sci Technol. 2017;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, Kim T, Mehta JS, Messmer EM, Pepose JS, Sangwan VS, Weiner AL, Wilson SE, Wolffsohn JS. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15:511-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 319] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 8. | Akpek EK, Bunya VY, Saldanha IJ. Sjögren's Syndrome: More Than Just Dry Eye. Cornea. 2019;38:658-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82:885-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Sugaya S, Sakimoto T, Shoji J, Sawa M. Regulation of soluble interleukin-6 (IL-6) receptor release from corneal epithelial cells and its role in the ocular surface. Jpn J Ophthalmol. 2011;55:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Choi M, Han SJ, Ji YW, Choi YJ, Jun I, Alotaibi MH, Ko BY, Kim EK, Kim TI, Nam SM, Seo KY. Meibum Expressibility Improvement as a Therapeutic Target of Intense Pulsed Light Treatment in Meibomian Gland Dysfunction and Its Association with Tear Inflammatory Cytokines. Sci Rep. 2019;9:7648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Wu X, Chen X, Ma Y, Lin X, Yu X, He S, Luo C, Xu W. Analysis of tear inflammatory molecules and clinical correlations in evaporative dry eye disease caused by meibomian gland dysfunction. Int Ophthalmol. 2020;40:3049-3058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Pinto-Fraga J, Enríquez-de-Salamanca A, Calonge M, González-García MJ, López-Miguel A, López-de la Rosa A, García-Vázquez C, Calder V, Stern ME, Fernández I. Severity, therapeutic, and activity tear biomarkers in dry eye disease: An analysis from a phase III clinical trial. Ocul Surf. 2018;16:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011;52:7725-7730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Fujimura T, Fujimoto T, Itaya-Hironaka A, Miyaoka T, Yoshimoto K, Sakuramoto-Tsuchida S, Yamauchi A, Takeda M, Tsujinaka H, Tanaka Y, Takasawa S. Significance of Interleukin-6/STAT Pathway for the Gene Expression of REG Iα, a New Autoantigen in Sjögren's Syndrome Patients, in Salivary Duct Epithelial Cells. Clin Rev Allergy Immunol. 2017;52:351-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Tan X, Sun S, Liu Y, Zhu T, Wang K, Ren T, Wu Z, Xu H, Zhu L. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye (Lond). 2014;28:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD 3rd, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 18. | De Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Pflugfelder SC. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Albertsmeyer AC, Kakkassery V, Spurr-Michaud S, Beeks O, Gipson IK. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp Eye Res. 2010;90:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Foulsham W, Marmalidou A, Amouzegar A, Coco G, Chen Y, Dana R. Review: The function of regulatory T cells at the ocular surface. Ocul Surf. 2017;15:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Dartt DA, McCarthy DM, Mercer HJ, Kessler TL, Chung EH, Zieske JD. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res. 1995;14:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, Dong PN, Geerling G, Hida RY, Liu Y, Seo KY, Tauber J, Wakamatsu TH, Xu J, Wolffsohn JS, Craig JP. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15:575-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 991] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 24. | Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, Kim EK, Seo KY. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients. Am J Ophthalmol. 2013;156:247-253.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Kim YJ, Ryu JS, Park SY, Lee HJ, Ko JH, Kim MK, Wee WR, Oh JY. Comparison of Topical Application of TSG-6, Cyclosporine, and Prednisolone for Treating Dry Eye. Cornea. 2016;35:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Noble BA, Loh RS, MacLennan S, Pesudovs K, Reynolds A, Bridges LR, Burr J, Stewart O, Quereshi S. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Tashbayev B, Yazdani M, Arita R, Fineide F, Utheim TP. Intense pulsed light treatment in meibomian gland dysfunction: A concise review. Ocul Surf. 2020;18:583-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Shafer B, Fuerst NM, Massaro-Giordano M, Palladino V, Givnish T, Macchi I, Sulewski ME, Orlin SE, Bunya VY. The use of self-retained, cryopreserved amniotic membrane for the treatment of Sjögren syndrome: a case series. Digit J Ophthalmol. 2019;25:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Mohammed MA, Allam IY, Shaheen MS, Lazreg S, Doheim MF. Lacrimal gland injection of platelet rich plasma for treatment of severe dry eye: a comparative clinical study. BMC Ophthalmol. 2022;22:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 30. | Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 1051] [Article Influence: 150.1] [Reference Citation Analysis (35)] |
| 32. | You S, Tariq A, Kublin CL, Zoukhri D. Detection of BrdU-label retaining cells in the lacrimal gland: implications for tissue repair. Cell Tissue Res. 2011;346:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 780] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 34. | You S, Avidan O, Tariq A, Ahluwalia I, Stark PC, Kublin CL, Zoukhri D. Role of epithelial-mesenchymal transition in repair of the lacrimal gland after experimentally induced injury. Invest Ophthalmol Vis Sci. 2012;53:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Dietrich J, Ott L, Roth M, Witt J, Geerling G, Mertsch S, Schrader S. MSC Transplantation Improves Lacrimal Gland Regeneration after Surgically Induced Dry Eye Disease in Mice. Sci Rep. 2019;9:18299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Abughanam G, Elkashty OA, Liu Y, Bakkar MO, Tran SD. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren's Syndrome-Like Disease. Int J Mol Sci. 2019;20:4750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (7)] |
| 37. | Jeong SY, Choi WH, Jeon SG, Lee S, Park JM, Park M, Lee H, Lew H, Yoo J. Establishment of functional epithelial organoids from human lacrimal glands. Stem Cell Res Ther. 2021;12:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Ali M, Shah D, Pasha Z, Jassim SH, Jassim Jaboori A, Setabutr P, Aakalu VK. Evaluation of Accessory Lacrimal Gland in Muller's Muscle Conjunctival Resection Specimens for Precursor Cell Markers and Biological Markers of Dry Eye Disease. Curr Eye Res. 2017;42:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Xiao S, Zhang Y. Establishment of long-term serum-free culture for lacrimal gland stem cells aiming at lacrimal gland repair. Stem Cell Res Ther. 2020;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. 2008;49:4399-4406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Lu Q, Yin H, Grant MP, Elisseeff JH. An In Vitro Model for the Ocular Surface and Tear Film System. Sci Rep. 2017;7:6163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Xie C, Li XY, Cui HG. Potential candidate cells for constructing tissue-engineered lacrimal duct epithelium: a histological and cytological study in rabbits. J Zhejiang Univ Sci B. 2015;16:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Dartt DA, Willcox MD. Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp Eye Res. 2013;117:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Shatos MA, Ríos JD, Horikawa Y, Hodges RR, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2477-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Pflugfelder SC, de Paiva CS. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology. 2017;124:S4-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 378] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 46. | Shatos MA, Haugaard-Kedstrom L, Hodges RR, Dartt DA. Isolation and characterization of progenitor cells in uninjured, adult rat lacrimal gland. Invest Ophthalmol Vis Sci. 2012;53:2749-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Makarenkova HP, Dartt DA. Myoepithelial Cells: Their Origin and Function in Lacrimal Gland Morphogenesis, Homeostasis, and Repair. Curr Mol Biol Rep. 2015;1:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Møller-Hansen M, Larsen AC, Toft PB, Lynggaard CD, Schwartz C, Bruunsgaard H, Haack-Sørensen M, Ekblond A, Kastrup J, Heegaard S. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul Surf. 2021;19:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Yu C, Chen P, Xu J, Liu Y, Li H, Wang L, Di G. hADSCs derived extracellular vesicles inhibit NLRP3inflammasome activation and dry eye. Sci Rep. 2020;10:14521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 50. | Simic P, Vukicevic S. Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev. 2005;16:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Basova LV, Tang X, Umasume T, Gromova A, Zyrianova T, Shmushkovich T, Wolfson A, Hawley D, Zoukhri D, Shestopalov VI, Makarenkova HP. Manipulation of Panx1 Activity Increases the Engraftment of Transplanted Lacrimal Gland Epithelial Progenitor Cells. Invest Ophthalmol Vis Sci. 2017;58:5654-5665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1066] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 53. | Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L; MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 54. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13052] [Article Influence: 686.9] [Reference Citation Analysis (12)] |
| 55. | Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol. 2019;10:1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 505] [Article Influence: 72.1] [Reference Citation Analysis (15)] |
| 56. | Liu Q, Zheng H, Chen X, Peng Y, Huang W, Li X, Li G, Xia W, Sun Q, Xiang AP. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8(+)CD28(-) regulatory T cells. Cell Mol Immunol. 2015;12:708-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Bengtsson AA, Trygg J, Wuttge DM, Sturfelt G, Theander E, Donten M, Moritz T, Sennbro CJ, Torell F, Lood C, Surowiec I, Rännar S, Lundstedt T. Metabolic Profiling of Systemic Lupus Erythematosus and Comparison with Primary Sjögren's Syndrome and Systemic Sclerosis. PLoS One. 2016;11:e0159384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 58. | Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, Han Y, Rabson AB, Wang Y, Shi Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25:1209-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 59. | Selvan SR, Dowling JP, Kelly WK, Lin J. Indoleamine 2,3-dioxygenase (IDO): Biology and Target in Cancer Immunotherapies. Curr Cancer Drug Targets. 2016;16:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 60. | Gowhari Shabgah A, Shariati-Sarabi Z, Tavakkol-Afshari J, Ghasemi A, Ghoryani M, Mohammadi M. A significant decrease of BAFF, APRIL, and BAFF receptors following mesenchymal stem cell transplantation in patients with refractory rheumatoid arthritis. Gene. 2020;732:144336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Shi B, Qi J, Yao G, Feng R, Zhang Z, Wang D, Chen C, Tang X, Lu L, Chen W, Sun L. Mesenchymal stem cell transplantation ameliorates Sjögren's syndrome via suppressing IL-12 production by dendritic cells. Stem Cell Res Ther. 2018;9:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Galindo S, Herreras JM, López-Paniagua M, Rey E, de la Mata A, Plata-Cordero M, Calonge M, Nieto-Miguel T. Therapeutic Effect of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Experimental Corneal Failure Due to Limbal Stem Cell Niche Damage. Stem Cells. 2017;35:2160-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 63. | Fuentes-Julián S, Arnalich-Montiel F, Jaumandreu L, Leal M, Casado A, García-Tuñon I, Hernández-Jiménez E, López-Collazo E, De Miguel MP. Adipose-derived mesenchymal stem cell administration does not improve corneal graft survival outcome. PLoS One. 2015;10:e0117945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Yao Y, Huang J, Geng Y, Qian H, Wang F, Liu X, Shang M, Nie S, Liu N, Du X, Dong J, Ma C. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS One. 2015;10:e0129164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 65. | Shibata S, Hayashi R, Okubo T, Kudo Y, Baba K, Honma Y, Nishida K. The secretome of adipose-derived mesenchymal stem cells attenuates epithelial-mesenchymal transition in human corneal epithelium. Regen Ther. 2019;11:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Shukla S, Mittal SK, Foulsham W, Elbasiony E, Singhania D, Sahu SK, Chauhan SK. Therapeutic efficacy of different routes of mesenchymal stem cell administration in corneal injury. Ocul Surf. 2019;17:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 67. | Calonge M, Pérez I, Galindo S, Nieto-Miguel T, López-Paniagua M, Fernández I, Alberca M, García-Sancho J, Sánchez A, Herreras JM. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl Res. 2019;206:18-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 68. | Coulson-Thomas VJ, Caterson B, Kao WW. Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells. 2013;31:2116-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Ziaei M, Zhang J, Patel DV, McGhee CNJ. Umbilical cord stem cells in the treatment of corneal disease. Surv Ophthalmol. 2017;62:803-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Jabbehdari S, Yazdanpanah G, Kanu LN, Anwar KN, Shen X, Rabiee B, Putra I, Eslani M, Rosenblatt MI, Hematti P, Djalilian AR. Reproducible Derivation and Expansion of Corneal Mesenchymal Stromal Cells for Therapeutic Applications. Transl Vis Sci Technol. 2020;9:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Zhang J, Huang C, Feng Y, Li Y, Wang W. Comparison of beneficial factors for corneal wound-healing of rat mesenchymal stem cells and corneal limbal stem cells on the xenogeneic acellular corneal matrix in vitro. Mol Vis. 2012;18:161-173. [PubMed] |
| 72. | Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE, Funderburgh JL. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6:266ra172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 73. | Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S, Rosenblatt MI, Dana R, Hematti P, Djalilian AR. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Invest Ophthalmol Vis Sci. 2017;58:5507-5517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 74. | Bittencourt MK, Barros MA, Martins JF, Vasconcellos JP, Morais BP, Pompeia C, Bittencourt MD, Evangelho KD, Kerkis I, Wenceslau CV. Allogeneic Mesenchymal Stem Cell Transplantation in Dogs With Keratoconjunctivitis Sicca. Cell Med. 2016;8:63-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Li N, Gao Z, Zhao L, Du B, Ma B, Nian H, Wei R. MSC-Derived Small Extracellular Vesicles Attenuate Autoimmune Dacryoadenitis by Promoting M2 Macrophage Polarization and Inducing Tregs via miR-100-5p. Front Immunol. 2022;13:888949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 76. | Villatoro AJ, Fernández V, Claros S, Rico-Llanos GA, Becerra J, Andrades JA. Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. Biomed Res Int. 2015;2015:527926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 77. | Quimby FW, Schwartz RS, Poskitt T, Lewis RM. A disorder of dogs resembling Sjögren's syndrome. Clin Immunol Immunopathol. 1979;12:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Beyazyıldız E, Pınarlı FA, Beyazyıldız O, Hekimoğlu ER, Acar U, Demir MN, Albayrak A, Kaymaz F, Sobacı G, Delibaşı T. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. 2014;2014:250230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, Khwarg SI, Oh JY. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. 2015;23:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 80. | Møller-Hansen M, Larsen AC, Wiencke AK, Terslev L, Siersma V, Andersen TT, Hansen AE, Bruunsgaard H, Haack-Sørensen M, Ekblond A, Kastrup J, Utheim TP, Heegaard S. Allogeneic mesenchymal stem cell therapy for dry eye disease in patients with Sjögren's syndrome: A randomized clinical trial. Ocul Surf. 2024;31:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 81. | O'Neill RT. FDA's critical path initiative: a perspective on contributions of biostatistics. Biom J. 2006;48:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Stratford PW, Binkley JM, Riddle DL, Guyatt GH. Sensitivity to change of the Roland-Morris Back Pain Questionnaire: part 1. Phys Ther. 1998;78:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 315] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 83. | Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, Sullivan BD, Tomlinson A, Tong L, Villani E, Yoon KC, Jones L, Craig JP. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 1489] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 84. | Wang G, Li H, Long H, Gong X, Hu S, Gong C. Exosomes Derived from Mouse Adipose-Derived Mesenchymal Stem Cells Alleviate Benzalkonium Chloride-Induced Mouse Dry Eye Model via Inhibiting NLRP3 Inflammasome. Ophthalmic Res. 2022;65:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 85. | Aluri HS, Samizadeh M, Edman MC, Hawley DR, Armaos HL, Janga SR, Meng Z, Sendra VG, Hamrah P, Kublin CL, Hamm-Alvarez SF, Zoukhri D. Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Tear Production in a Mouse Model of Sjögren's Syndrome. Stem Cells Int. 2017;2017:3134543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Rui K, Shen Z, Peng N, Zhao F, Tang Y, Liu S, Xu X, Liu C, Wu L, Tian J, Lu L. Olfactory Ecto-mesenchymal Stem Cell-derived Exosomes Ameliorate Murine Sjögren's Syndrome via Suppressing Tfh Cell Response. Rheumatol Immunol Res. 2022;3:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 87. | Guo R, Liang Q, He Y, Wang C, Jiang J, Chen T, Zhang D, Hu K. Mesenchymal Stromal Cells-Derived Extracellular Vesicles Regulate Dendritic Cell Functions in Dry Eye Disease. Cells. 2022;12:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 88. | Lu X, Li N, Zhao L, Guo D, Yi H, Yang L, Liu X, Sun D, Nian H, Wei R. Human umbilical cord mesenchymal stem cells alleviate ongoing autoimmune dacryoadenitis in rabbits via polarizing macrophages into an anti-inflammatory phenotype. Exp Eye Res. 2020;191:107905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 89. | Sun T, Liu S, Yang G, Zhu R, Li Z, Yao G, Chen H, Sun L. Mesenchymal stem cell transplantation alleviates Sjögren's syndrome symptoms by modulating Tim-3 expression. Int Immunopharmacol. 2022;111:109152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 90. | Wang L, Wang X, Chen Q, Wei Z, Xu X, Han D, Zhang Y, Chen Z, Liang Q. MicroRNAs of extracellular vesicles derived from mesenchymal stromal cells alleviate inflammation in dry eye disease by targeting the IRAK1/TAB2/NF-κB pathway. Ocul Surf. 2023;28:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/