Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.110190
Revised: July 1, 2025
Accepted: September 22, 2025
Published online: October 26, 2025
Processing time: 146 Days and 21.9 Hours
Steroid-induced avascular necrosis of the femoral head (SANFH) involves bone metabolism imbalance and lacks effective therapies. Mesenchymal stem cells (MSCs), particularly human umbilical cord MSCs (hUCMSCs), offer promise due to their osteogenic and immunomodulatory potential. Sclerostin (SOST) inhibits bone formation, so we developed a multi-target gene silencing strategy against SOST using RNA interference. We created hUCMSCs with SOST-silenced (sh-hUCMSCs) and compared their therapeutic efficacy with unmodified hUCMSCs in SANFH mice. This study explores a novel approach to enhance osteogenesis and mitigate SANFH progression.
To assess the effects of sh-hUCMSCs on bone metabolism in SANFH.
hUCMSCs were isolated from placental tissue and transfected with SOST-targeting short hairpin RNA plasmids. A SANFH mouse model was established through intraperitoneal injection of lipopolysaccharide (20 μg/kg) followed by intramuscular methylprednisolone administration (40 mg/kg). Mice were randomized into four experimental groups (n = 10/group): Sham control, SANFH (untreated), hUCMSCs-treated, and sh-hUCMSCs-treated. Micro-computed tomography was used to measure bone volume (BV), bone surface area, bone surface/BV ratio, tra
hUCMSCs and sh-hUCMSCs exhibited typical fibroblast-like morphology and high expression of MSC surface markers (CD90, CD73, CD105 > 98%). These cells demonstrated tri-lineage differentiation potential, confirmed by positive Alizarin Red S, Oil Red O, and Alcian Blue staining, and upregulation of lineage-specific genes. After SOST-RNA interference modification, sh-hUCMSCs showed enhanced inhibition of adipogenesis and improved bone formation in a rat model of SANFH. Histological analysis revealed reduced lipid infiltration and empty lacunae in the femoral head of the sh-hUCMSC group. Western blot showed decreased CCAAT/enhancer-binding protein and peroxisome proliferator-activated receptor gamma expression (P < 0.05). Masson staining and micro-computed tomography analysis confirmed significantly increased BV, trabecular number, trabecular thickness, and reduced trabecular separation in the sh-hUCMSC group compared to unmodified MSCs and SANFH groups (P < 0.05). Serum enzyme-linked immunosorbent assay showed higher OPG and lower RANK, RANKL, and tartrate-resistant acid phosphatase levels in the sh-hUCMSCs group. Western blot further confirmed upregulated alkaline phosphatase, OPG, β-catenin, and downregulated SOST expression in sh-hUCMSCs compared to controls (P < 0.05). These results suggest that SOST inhibition enhances the osteogenic potential and therapeutic efficacy of hUCMSCs in SANFH.
sh-hUCMSCs alleviate SANFH by activating the Wnt/β-catenin signaling pathway, thereby promoting osteogenic differentiation and suppressing adipogenesis to restore bone metabolic balance.
Core Tip: In this study, we developed a multi-target gene silencing strategy against sclerostin (SOST), a negative regulator of bone formation, using RNA interference technology. We established a cellular model of human umbilical cord mesenchymal stem cells (hUCMSCs) with SOST-silenced (sh-hUCMSCs) with SOST gene silencing and compared the therapeutic effects of hUCMSCs and sh-hUCMSCs in a mouse model of steroid-induced avascular necrosis of the femoral head. Compared with hUCMSCs, sh-hUCMSCs more effectively inhibited adipogenic differentiation, promoted new bone formation, and improved trabecular bone parameters such as bone volume, trabecular number, and thickness, while reducing trabecular separation. Additionally, sh-hUCMSCs modulated the expression of key bone metabolic factors, including increased levels of osteoprotegerin and decreased levels of receptor activator of nuclear factor kappa B ligand and peroxisome proliferator-activated receptor gamma. These results suggest that silencing SOST enhances the osteogenic capacity and regenerative efficacy of hUCMSCs.
- Citation: Lv H, Zheng CF, Chen XY, Wei JH, Tao YZ, Feng L, Feng Z, Lu SJ. Sclerostin-silenced human umbilical cord mesenchymal stem cells ameliorate bone metabolism in steroid-induced femoral head necrosis. World J Stem Cells 2025; 17(10): 110190
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/110190.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.110190
Steroid-induced avascular necrosis of the femoral head (SANFH) is a condition caused by long-term or high-dose hormone use. It is characterized by the collapse of trabecular bone and necrosis of bone tissue. The extensive clinical use of glucocorticoids for conditions such as systemic lupus erythematosus[1], organ transplantation[2], and acute inflammatory disorders[3] has contributed to the rising incidence of SANFH. In the United States, an estimated 300000 to 600000 individuals are affected, with 10000 to 20000 new cases diagnosed annually[4]. China bears one of the highest burdens globally, with over 8 million patients estimated to require treatment[5,6]. The widespread prevalence of SANFH leads to considerable medical, economic, and social challenges worldwide. Early-stage SANFH is mainly managed with pharmacological treatment, while late-stage cases often require total hip arthroplasty. As advanced SANFH is typically irreversible and has poor outcomes, early detection and intervention are essential[7]. However, these pharmacological treatments have limitations. The ability of mesenchymal stem cells (MSCs) to differentiate into osteoblasts has attracted considerable interest as a complementary therapy for osteonecrosis and related disorders[8-10]. Human umbilical cord MSCs (hUCMSCs) are totipotent stem cells derived from placental and umbilical cord tissues discarded after maternal delivery. Compared to other MSC sources, hUCMSCs offer multiple advantages, such as wide availability, easy collection, ethical acceptability, simple isolation, high yield, and low immunogenicity[11-14]. Recent studies indicate that adipocytes predominantly occupy the bone marrow cavity of the necrotic femoral head, causing severe damage to bone microstructures like trabeculae[15-17]. Therefore, we hypothesize that promoting stem cells’ osteogenic differentiation while inhibiting their lipogenic differentiation is a key strategy to address the complex pathology of femoral head necrosis.
Gene modification technology shows great potential in the treatment of osteonecrosis. Numerous studies have demonstrated that bone repair can be effectively promoted by targeting key regulatory factors[18,19]. One such factor is sclerostin (SOST), an osteoblast-secreted protein encoded by the SOST gene located on chromosome 17q12-q21. The Wnt/β-catenin signaling pathway plays a central role in bone formation by precisely regulating bone development and remodeling through osteoblast activation. SOST acts as a negative regulator of this pathway, thereby suppressing osteoblast proliferation and differentiation[20,21]. Furthermore, SOST suppresses osteoblast activity and delays bone formation. Notably, SOST-knockout mice display a high bone mass phenotype, with significantly increased bone density, volume, formation, and strength[22-24]. In our previous study, transplantation of SOST-silenced MC3T3-E1 cells (a mouse calvaria-derived osteoblastic cell line) effectively reversed osteoporosis in mice. These findings suggest that targeting SOST may offer a promising therapeutic approach for SANFH. Consequently, this study aims to evaluate the effects of hUCMSCs with SOST-silenced (sh-hUCMSCs) on bone metabolism in a SANFH mouse model. By comparing sh-hUCMSCs with unmodified hUCMSCs, we seek to elucidate their therapeutic potential, investigate the underlying mechanisms, and identify candidate genes that may serve as drug targets to promote bone regeneration in SANFH treatment.
Cells were isolated from the placenta of a newborn delivered at the Department of Obstetrics and Gynecology in Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine. After obtaining the placenta, blood vessels and umbilical cord epithelium were carefully removed, leaving the Wharton’s jelly. The tissue was thoroughly rinsed with phosphate-buffered saline (PBS) and cut into 1 mm × 1 mm fragments. These fragments were evenly placed on the bottom of a Petri dish and incubated for 0.5-1 hour to allow adherence. Subsequently, stem cell culture medium was added, ensuring that the medium level remained below the height of the tissue blocks. The cultures were maintained at 37 °C in a humidified incubator with 5% CO2. Cells were passaged upon reaching 90%-95% confluence and were characterized at the third passage (P3).
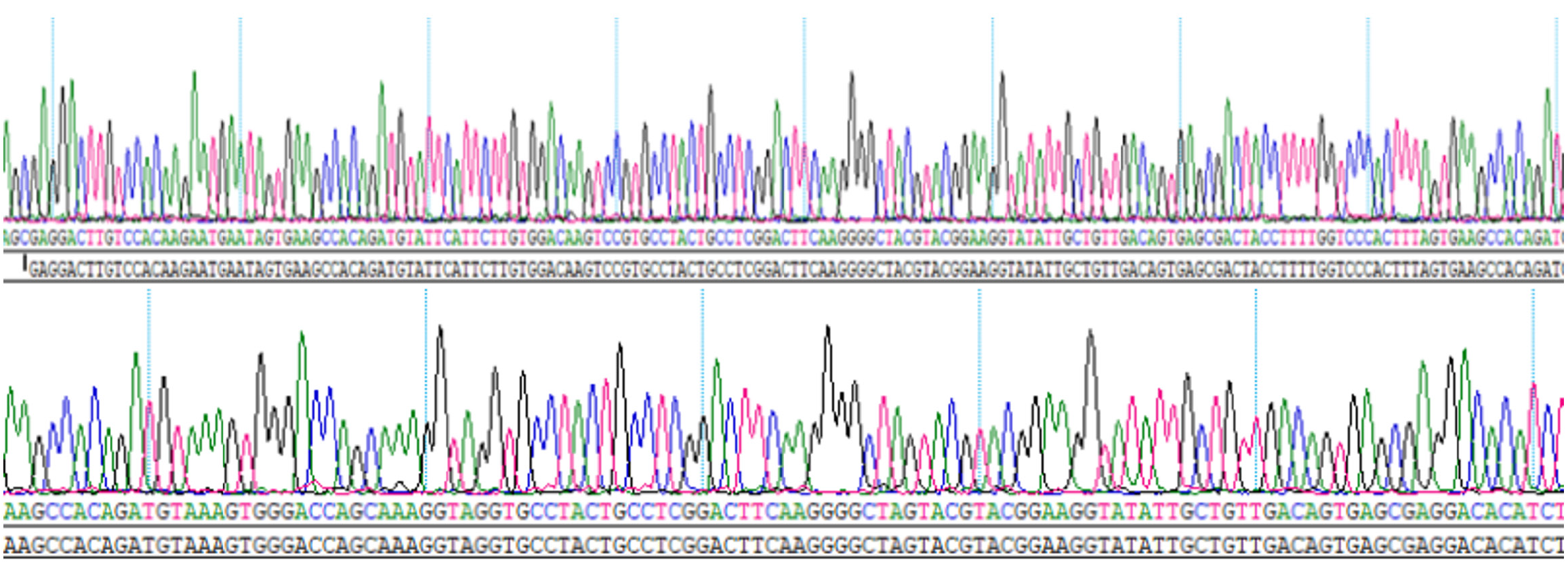
We designed two short hairpin RNA target sequences for SOST gene silencing and one negative control sequence. Based on the vector structure, single-stranded oligonucleotides (61-63 nucleotides in length) were synthesized and annealed to form double-stranded short hairpin RNAs (Table 1). The phosphorylation and annealing reaction was performed in a thermal cycler under the following conditions: 37 °C for 30 minutes, 95 °C for 5 minutes, followed by a gradual decrease to 25 °C at a rate of 5 °C/minute. Successful annealing of the oligonucleotides was confirmed. Sequencing results indicated correct insertion of the target duplex, confirming the successful construction of the SOST-silencing plasmid (Figure 1). The final plasmid concentration was 1343 ng/μL.
| Target | Sequence | |
| mSOST-shR1 | F | 5’-GATCCGCCAGTCGGAGCTCAAGGACTTTCAAGAGAGTCCTTGAGCTCCGACTGGCTTTTTACGCGTG-3’ |
| R | 5’-AATTCACGCGTAAAAAACCAGTCGGAGCTCAAGGACTCTCTTGAAAGTCCTTGAGCTCCGACTGGCG-3’ | |
| mSOST-shR2 | F | 5’-GATCCGGCCTTCAGGAATGATGCCACATTCAAGAGTGTGGCATCATTCCTGAAGGCTTTTTTACGCGTG-3’ |
| R | 5’-AATTCACGCGTAAAAAAGCCTTCAGGAATGATGCCACACTCTTGAATGTGGCATCATTCCTGAAGGCCG-3’ |
P3-generation hUCMSCs were harvested and resuspended in P3 Primary Cell Nucleofector™ Solution (Lonza, Switzerland) at a concentration of 2 × 106 cells. For electroporation, 10 μL of the SOST-RNA interference (RNAi) plasmid was added to the cell suspension and gently mixed. The plasmid-cell suspension mixture was transferred into a cuvette and placed in the electroporation apparatus. After electroporation, cells were immediately transferred to culture flasks containing prewarmed medium. After 48 hours, 30%-40% of the cells had adhered, as observed under bright-field microscopy, and approximately 10% of the cells exhibited green fluorescence under fluorescence microscopy. Selection medium containing 1 μg/mL puromycin (Biosharp, China) was added to eliminate cells without puromycin resistance. The selection process was continued for 4-5 days, during which cell morphology and fluorescence intensity were monitored daily. Selection was complete when all non-resistant hUCMSCs had been removed.
Cells were detached from culture flasks using digestive enzymes and adjusted to a concentration of 1 × 106 cells/mL. The following fluorochrome-conjugated antibodies (all from BD Biosciences, Germany) were sequentially added to flow cytometry tubes: APC Mouse Anti-Human CD34, PE Mouse Anti-Human CD73, FITC Mouse Anti-Human CD90, BV421 Mouse Anti-Human CD45, BV510 Mouse Anti-Human CD105, and APC-Cy7 Rat Anti-Human CD11b. The cell suspensions were gently mixed and incubated in the dark according to the manufacturer’s protocol. After staining, surface marker expression of hUCMSCs was analyzed using flow cytometry.
To verify the multipotent differentiation capacity of the isolated MSCs, osteogenic, adipogenic, and chondrogenic induction were performed. For osteogenic differentiation, P3 cells were cultured in osteogenic induction medium (TBD, China) for 21 days. After incubation, cells were washed twice with PBS, fixed in 4% paraformaldehyde (Biosharp, China) for 15 minutes at room temperature, and stained with 0.5% Alizarin Red S for 30 minutes. Mineralized matrix formation was evaluated under an inverted fluorescence microscope (Leica DMi8, Germany). For adipogenic differentiation, cells were seeded in 6-well plates and cultured in adipogenic induction medium (TBD, China) for 21 days. The cells were then fixed with 4% paraformaldehyde, stained with Oil Red O working solution (prepared as a 60% aqueous solution of 0.5% Oil Red O in isopropanol) for 1 hour at room temperature, and observed under a light microscope. For chondrogenic differentiation, cells were pelleted and resuspended in 0.5 mL chondrogenic induction medium (TBD, China), with medium changes every 3 days. After 21 days, cell pellets were fixed in 4% paraformaldehyde for 1 hour at room temperature, embedded in paraffin, sectioned at 5 μm thickness, and stained with Alcian Blue for 30 minutes. Sections were evaluated using a light microscope.
P5 hUCMSCs and sh-hUCMSCs were digested, washed with PBS, resuspended in complete stem cell medium, and seeded into four 96-well plates at 100 μL per well, with four replicates per group. The plates were cultured in a 37 °C incubator with 5% CO2. On days 1, 3, 5, and 7 post-seeding, 10 μL of Cell Counting Kit-8 solution was added to each well and gently mixed to avoid bubbles. Plates were then incubated at 37 °C for 2 hours. Absorbance at 450 nm was measured using a microplate reader. Cell proliferation curves were generated based on viability data from the four time points.
The animal protocol was approved by the Animal Ethics Committee of Guangxi University of Traditional Chinese Medicine (Ethics No. DW20220408-053). A total of 40 Sprague-Dawley (SD) mice (both male and female) were randomly divided into four groups (n = 10 per group): Sham group, SANFH (SAN) group, MSC-treated group, and sh-MSC-treated group. Body weight was recorded before modeling. All groups except the sham group underwent SANFH induction over a period of 7 days. From days 1 to 2, mice received intraperitoneal injections of lipopolysaccharide (20 μg/kg) once daily. From days 3 to 7, methylprednisolone sodium succinate (40 mg/kg) was injected bilaterally into the posterior thigh muscles once daily. Sham group mice received equivalent volumes of saline at the same time points via the same routes. Four weeks after modeling, one mouse from each non-sham group was randomly selected for histological validation. Femoral heads were harvested bilaterally, fixed, sectioned, and stained with hematoxylin and eosin (HE). Successful induction of osteonecrosis was indicated by the presence of empty lacunae, pyknotic nuclei, bone marrow necrosis, or trabecular fractures. The appearance of any one of these pathological features confirmed the validity of the model.
Following successful model establishment, the sham and SAN groups received daily saline gavage (10 mL/kg) for four weeks. The MSC group was administered P5 hUCMSCs (2.5 × 107/mL) via tail vein injection once weekly for four consecutive weeks. Similarly, the sh-MSC group received tail vein injections of P5 sh-hUCMSCs (2.5 × 107/mL) following the same schedule. At the end of treatment, bilateral proximal femurs were collected. The entire proximal segment of the right femur was fixed in 4% paraformaldehyde and stored at 4 °C for histological analysis. The left femoral segment was snap-frozen and stored at -80 °C for molecular assays.
Enzyme-linked immunosorbent assay (ELISA) kits were purchased from RUIXIN BIOTECH, China. Blood samples were collected from the orbital vein and immediately centrifuged at 3000 rpm for 10 minutes at 4 °C to obtain serum. ELISA was conducted strictly following the manufacturer’s instructions.
Femoral samples for HE and Masson’s trichrome staining were fixed in 10% neutral formalin for 24 hours and decalcified using 10% ethylenediaminetetraacetic acid (Biosharp, China) at a volume at least five times that of the bone tissue for 2 weeks at room temperature with gentle agitation. After decalcification, samples were embedded in paraffin, sectioned, and stained with HE and Masson’s trichrome to evaluate morphological changes.
Tissue specimens fixed and preserved in 4% paraformaldehyde were clearly labeled by group and scanned using micro-computed tomography (micro-CT; SCANCO Medical AG, Switzerland) after fixation. The scanning region extended from the proximal femur, including the femoral head, to the mid-shaft. Primary parameters analyzed included bone volume (BV), BV fraction (BV/TV), bone surface area (BS), BS/BV, trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp).
Total RNA was extracted from cells using the TRIzol reagent (Magen) according to the manufacturer’s protocol. Complementary DNA was synthesized from 1 μg of total RNA using a reverse transcription kit (Takara, Japan) following the manufacturer’s instructions. Gene expression levels were quantified by quantitative real-time polymerase chain reaction (qRT-PCR) using a CFX96 Touch system. Primer sequences are listed in Table 2. Glyceraldehyde-3-phosphate dehydrogenase served as the internal control.
| Genes | Primers types | Sequence (5’-3’) |
| ALP | Forward | TGTGGAAGGCTCTGGAAAGT |
| Reverse | TGTCAACAGGATCCAGGCAT | |
| OPG | Forward | ACCCCAGAGCGAAATACAGT |
| Reverse | TGTCGTGTGTTGCATTTCCT | |
| Osterix | Forward | CAAGGTGTATGGCAAGGCTT |
| Reverse | GCAGGCAGGTGAACTTCTTC | |
| SOX9 | Forward | GAACAAGCCGCACGTCAA |
| Reverse | CGCGGCTGGTACTTGTAATC | |
| COL2A1 | Forward | TCGTGGTGACAAAGGTGAAA |
| Reverse | CCAGCCTTTTCATCAAATCC | |
| ACAN | Forward | ACAAGGTCTCACTGCCCAAC |
| Reverse | AAGTCGAGGGTGTAGCGTGT | |
| PPAR/γ | Forward | GCCAAGAACATCCCCAACTTC |
| Reverse | GCAAAGATGGCCTCATGCA | |
| C/EBPs | Forward | CCGAGAATGGGAAGCTTGTC |
| Reverse | AAGCACCAACGAGAGGAGAA | |
| AP2 | Forward | GGGTGTCGATGCTGACATTA |
| Reverse | TACTCTGGCGACGAAGTATTTG | |
| SOST | Forward | AGAACAACAAGACCATGAAC |
| Reverse | GCGGCACGGCCCATCGGTC | |
| GADPH | Forward | CCATCTTCCAGGAGCGAGAT |
| Reverse | GGTCATGAGTCCTTCCACGA |
Total protein was extracted from tissue samples using radioimmunoprecipitation assay buffer. Protein lysates (30 μg per lane) were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% non-fat milk in Tris-buffered saline with 0.1% Tween-20 (TBST, pH = 7.6) for 1 hour at room temperature, then incubated overnight at 4 °C with primary antibodies against osteoprotegerin (OPG, 1:1000), β-catenin (1:2000), receptor activator of nuclear factor kappa B (RANK) ligand (RANKL, 1:1500), SOST (1:1000), peroxisome proliferator-activated receptor gamma (PPARγ, 1:800), CCAAT/enhancer-binding protein (C/EBP, 1:1000), and glyceraldehyde-3-phosphate dehydrogenase (1:5000, loading control). After three washes with TBST, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G secondary antibody (1:10000) for 2 hours at room temperature. Protein bands were visualized using enhanced chemiluminescence substrate and quantified with a ChemiDoc XRS+ imaging system (Bio-Rad Laboratories, CA, United States).
Statistical analysis was conducted using GraphPad Prism 9.1 software (GraphPad Software, Inc., La Jolla, CA, United States). Data are presented as mean ± SD. Each experiment included at least three replicates and was independently repeated three times unless otherwise specified. One-way analysis of variance (ANOVA) followed by Fisher’s least significant difference post hoc test was used to compare differences among multiple groups. A P-value < 0.05 was considered statistically significant.
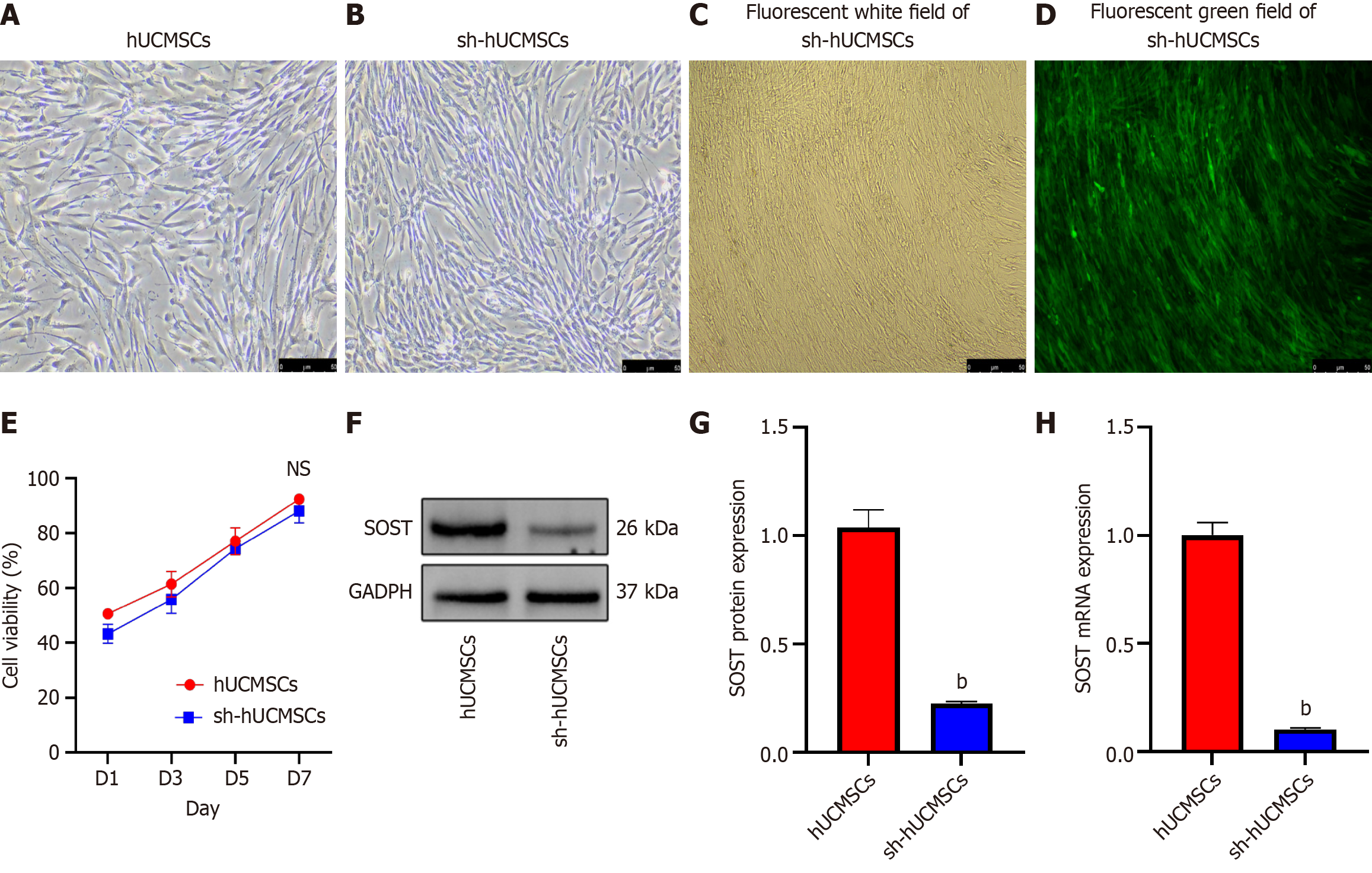
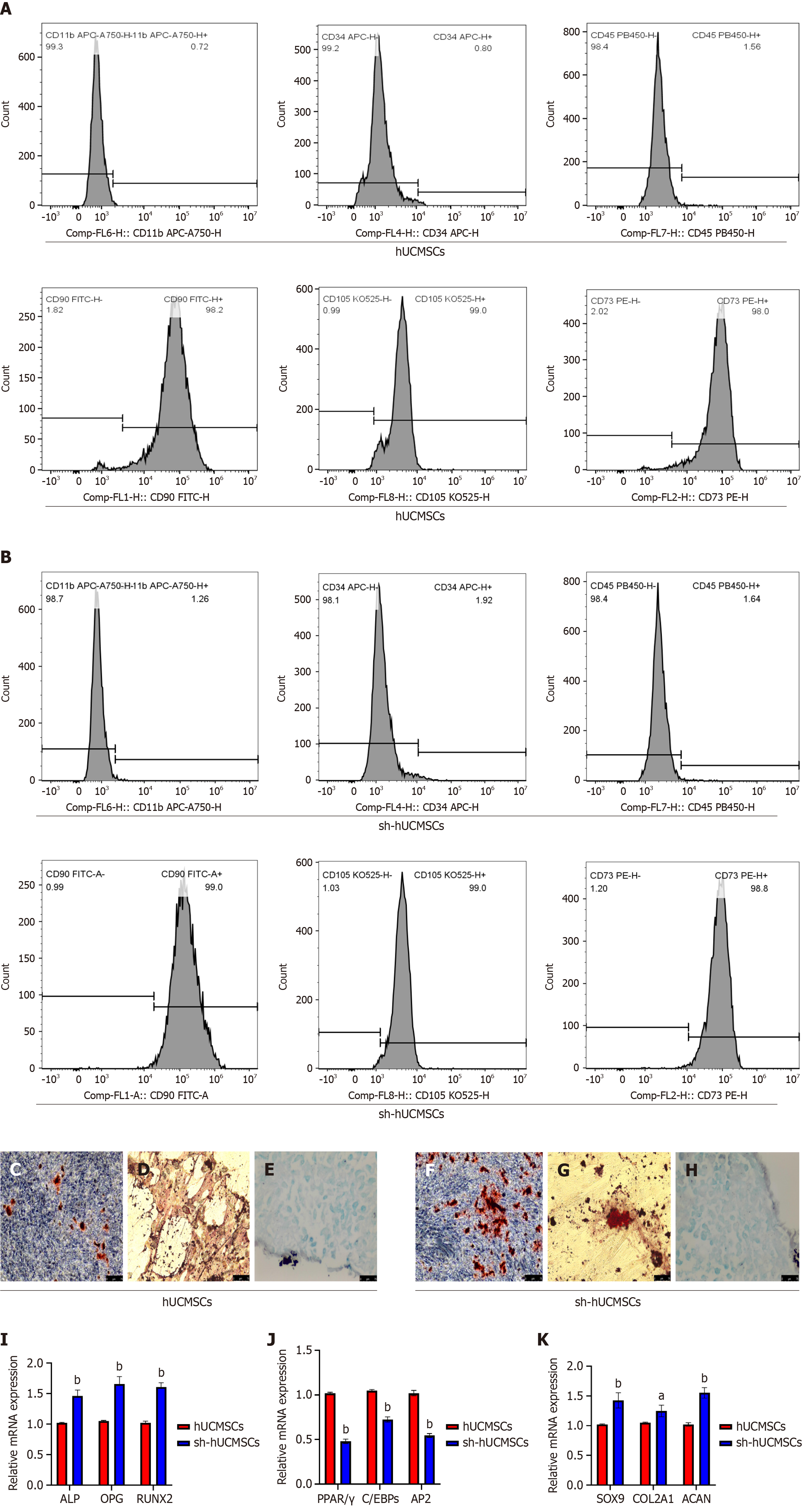
After the tissue blocks adhered to the wall, fibroblast-like cells were observed separating from the surrounding tissues one week later. These cells appeared elongated and spindle-shaped, exhibiting a spiral or radial growth pattern when cell confluence was high (Figure 2A). No significant morphological changes were noted in the fibroblast-like cells following the electroporation of the SOST-RNAi plasmid (Figure 2B). A small amount of fluorescence was detectable within 24-48 hours, while a substantial increase in fluorescence expression was evident in the culture flask after 5-6 days of continuous puromycin screening (Figure 2C and D). This finding indicates that the fibroblast-like cells in the culture flask were successfully electroporated with the SOST-RNAi plasmid. We assessed cell proliferation using the Cell Counting Kit-8 assay (Figure 2E) and observed no significant difference between the two groups at days 1, 3, 5, and 7 (P > 0.05). The efficiency of SOST-silenced was confirmed by western blot and qRT-PCR analyses (Figure 2F-H). SOST expression in sh-hUCMSCs was significantly reduced compared to hUCMSCs, with a highly significant difference (P < 0.001). Surface antigen analysis of the two fibroblast groups showed positive rates above 98% for CD90, CD73, and CD105, and below 2% for CD45, CD34, and CD11b (Figure 3A and B), consistent with established MSC surface markers.
Both hUCMSCs and sh-hUCMSCs were induced to differentiate into osteogenic, adipogenic, and chondrogenic lineages in vitro. Figure 3C-E show the results for hUCMSCs, and Figure 3F-H for sh-hUCMSCs. Following osteogenic induction, Alizarin Red S staining revealed mineralized nodules indicative of calcium deposition (Figure 3C and F). Oil Red O staining demonstrated lipid droplet formation under adipogenic conditions (Figure 3D and G). Alcian Blue staining confirmed proteoglycan synthesis under chondrogenic induction (Figure 3E and H). These findings verify the multipotent differentiation capacity of both cell types and confirm their identity as MSCs. QRT-PCR analysis revealed that osteogenic marker genes - including Runt-related transcription factor 2, osteopontin, and alkaline phosphatase (ALP) - were upregulated in both groups following osteogenic induction, with significantly higher expression observed in sh-hUCMSCs compared to hUCMSCs (P < 0.0001) (Figure 3I). Similarly, adipogenic markers such as PPARγ, C/EBP, and adipocyte protein 2 were also induced (Figure 3J); however, their expression levels were significantly lower in sh-hUCMSCs than in hUCMSCs (P < 0.0001). In addition, chondrogenic markers, including SRY-box transcription factor 9, aggrecan, and type II collagen alpha 1 chain, were upregulated in both groups, with higher expression in sh-hUCMSCs (P < 0.01) (Figure 3K).
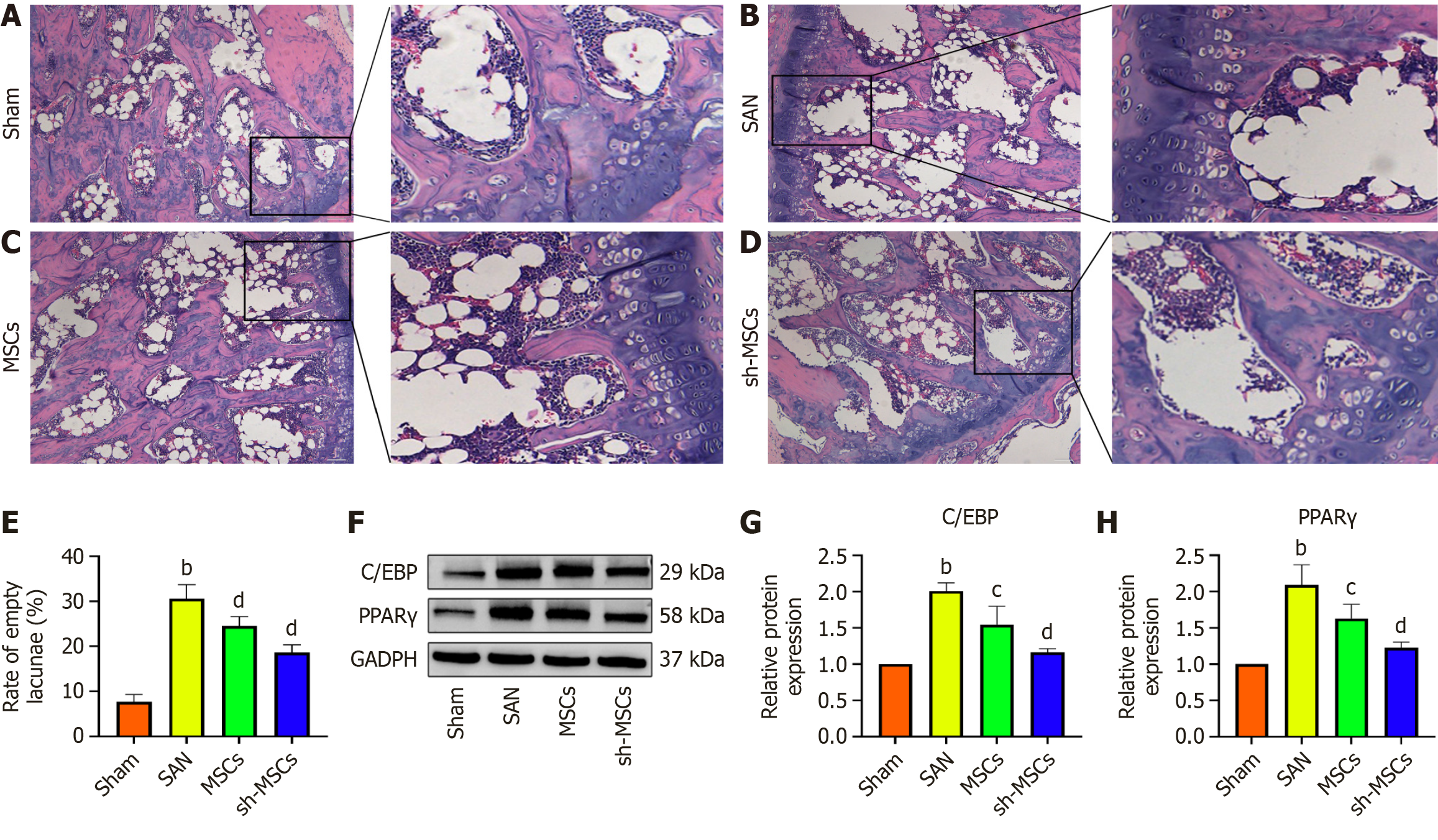
HE staining revealed that the femoral head cartilage in the sham group exhibited a relatively thick structure, with regularly arranged subchondral trabeculae and no significant presence of adipocytes in the medullary cavity. In contrast, the SAN group showed extensive bone marrow cell necrosis and marked adipocyte infiltration. Some adipocytes displayed hypertrophy and coalesced into cyst-like structures, indicating dexamethasone-induced necrotic changes. In the MSCs and sh-MSCs groups, lipid infiltration in the femoral bone marrow cavity was notably reduced, with the sh-MSCs group exhibiting the lowest level of adipocyte accumulation (Figure 4A-D).
In the SAN group, a marked increase in empty bone lacunae was observed in the femoral head. In contrast, the number of empty lacunae was significantly reduced in both the MSCs and sh-MSCs groups, with the sh-MSCs group showing the lowest count (Figure 4E). Western blot analysis of lipid metabolism-related factors revealed that C/EBP and PPARγ expression levels were lowest in the sham group and highest in the SAN group (Figure 4F). The MSCs group showed higher expression than the sh-MSCs group, with statistically significant differences among groups (P < 0.05) (Figure 4G and H). These findings suggest that both hUCMSCs and sh-hUCMSCs effectively inhibit adipogenic differentiation and reduce adipose tissue accumulation in SANFH mice, with sh-hUCMSCs exhibiting a more pronounced anti-adipogenic effect.
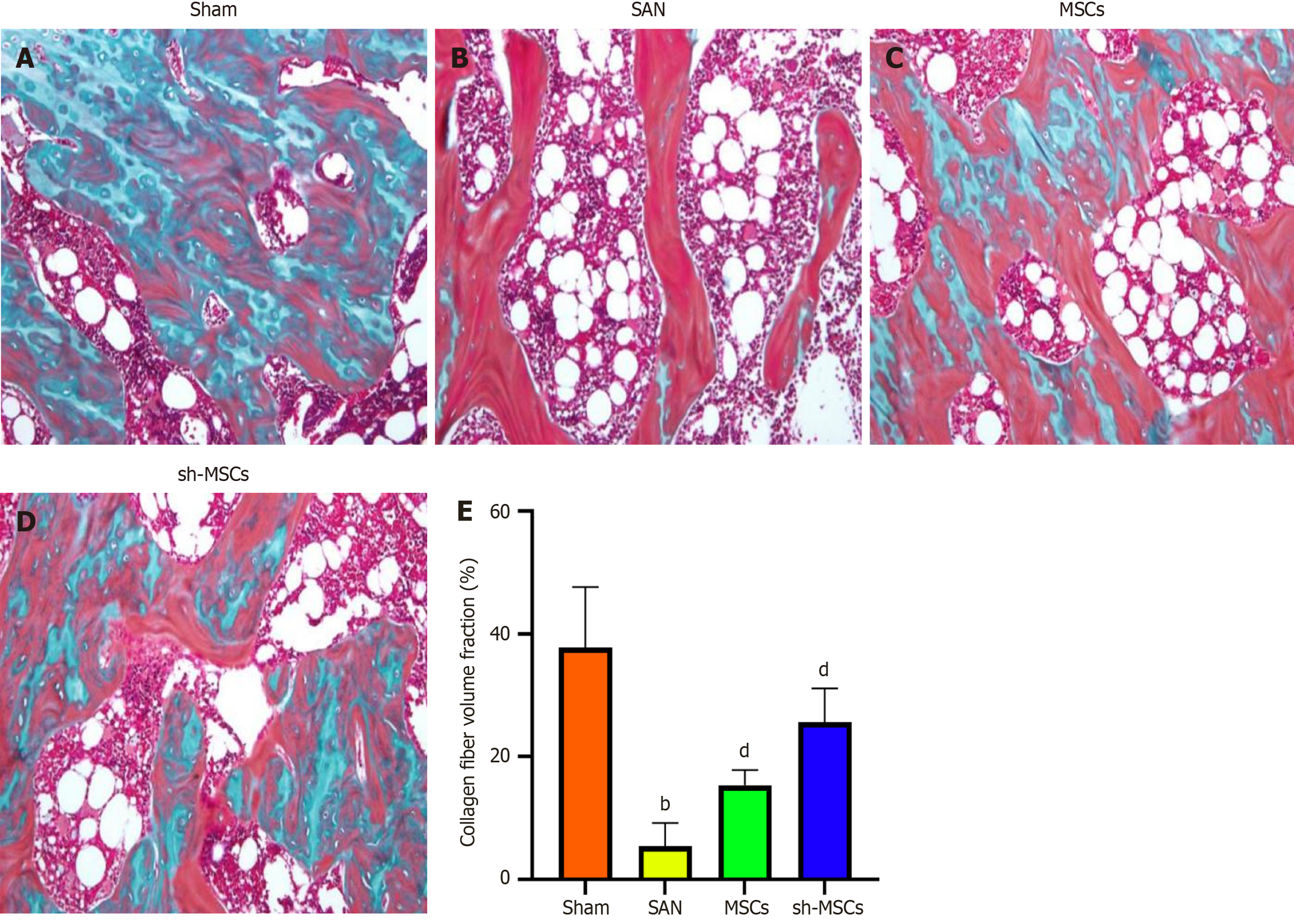
Masson staining revealed the highest collagen fiber content and the most orderly trabecular arrangement in the sham group. In contrast, the SAN group exhibited predominantly mature bone with minimal new bone formation, indicating severe osteonecrosis of the femoral head. Both the MSCs and sh-MSCs groups showed evidence of bone regeneration, with the sh-MSCs group displaying more extensive new bone formation (Figure 5). These findings suggest that both hUCMSCs and sh-hUCMSCs promote new bone formation via osteogenic differentiation, with sh-hUCMSCs demonstrating a superior osteogenic potential.
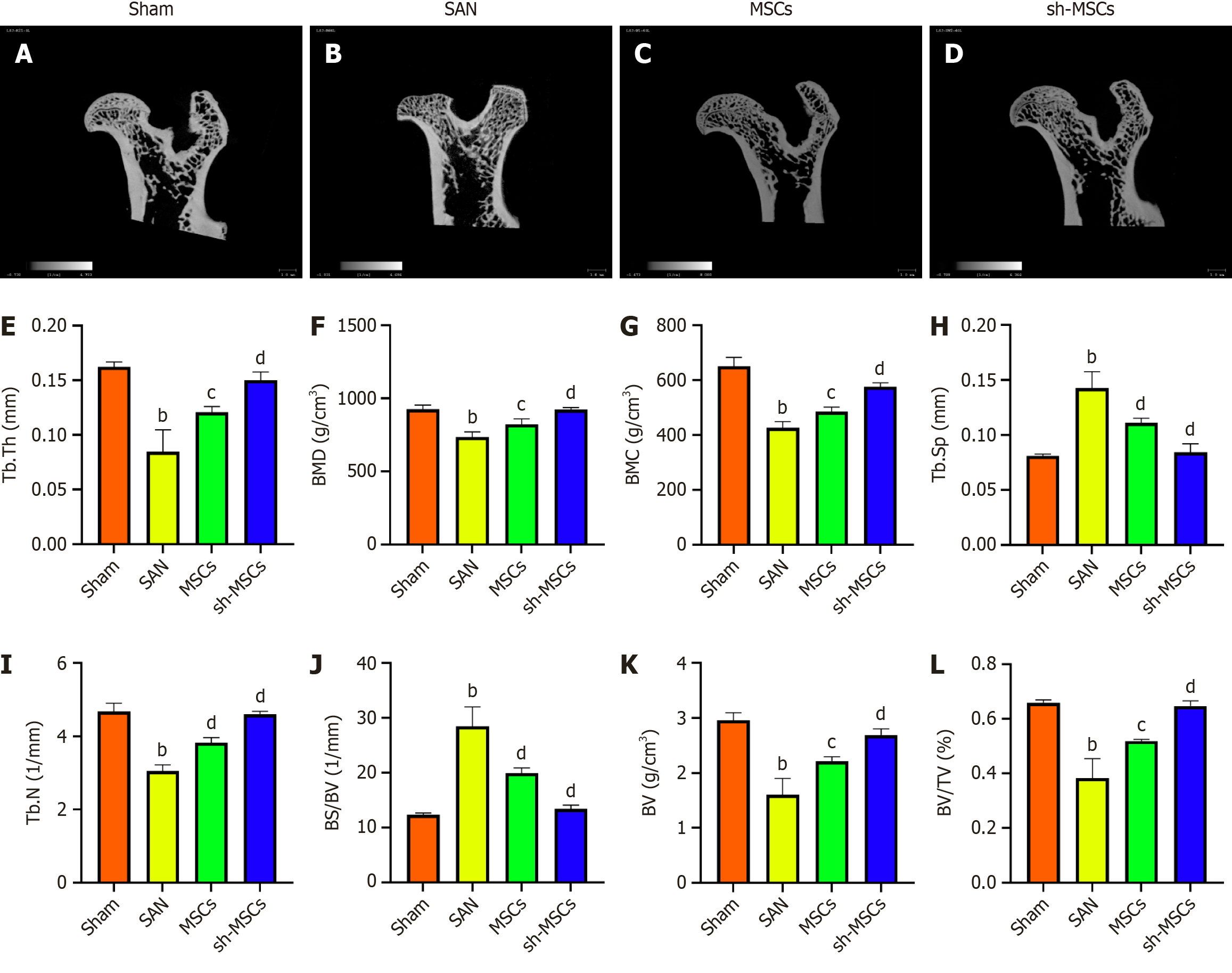
Micro-CT scanning results are shown in Figure 6A-D. In the sham group, the femoral head surface appeared smooth, with an intact structure and well-organized trabecular architecture. In contrast, the SAN group exhibited femoral head collapse, sparse and fragile trabeculae, and cystic changes in the cartilage. Both the MSCs and sh-MSCs groups showed improved femoral head morphology and reduced areas of trabecular cystic degeneration, with more substantial repair observed in the sh-MSCs group. Quantitative parameters such as bone mineral density, BV, BV/TV, and BS/BV were used to assess bone mass changes, while trabecular microarchitecture was evaluated using Tb.N, Tb.Th, and Tb.Sp. Compared with the SAN group, both MSC-treated groups exhibited significant increases in bone mineral density, BV, BV/TV, Tb.Th, Tb.N, and trabecular spacing, with the sh-MSCs group demonstrating greater improvements in cancellous and trabecular bone repair. Additionally, Tb.Sp reflects trabecular spacing, and BS/BV represents the bone surface area per unit volume - higher values indicating a more deteriorated trabecular structure. Both hUCMSCs and sh-hUCMSCs significantly reduced Tb.Sp and BS/BV compared to the SAN group, with more pronounced reductions observed in the sh-MSCs group (Figure 6E-L).
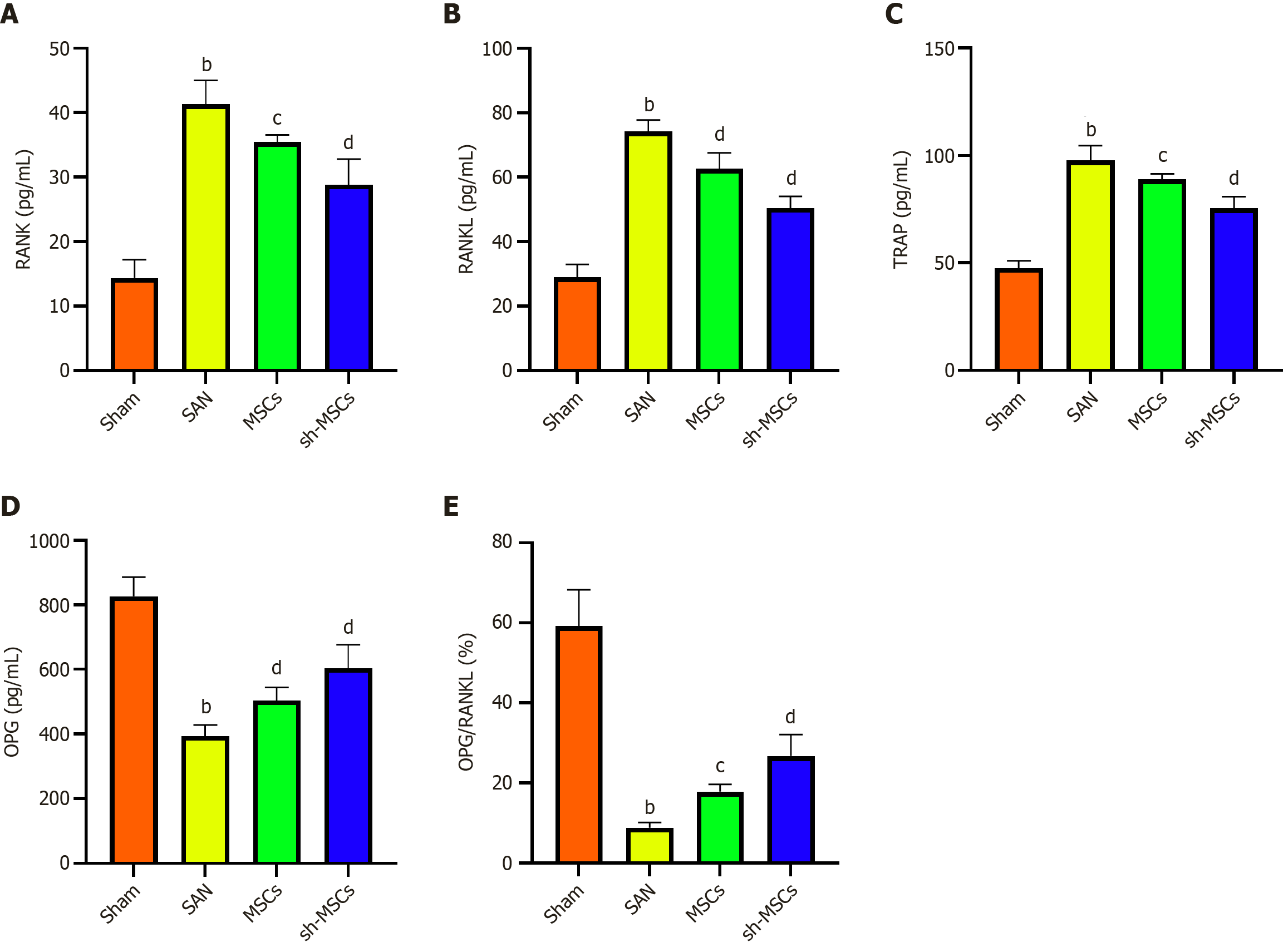
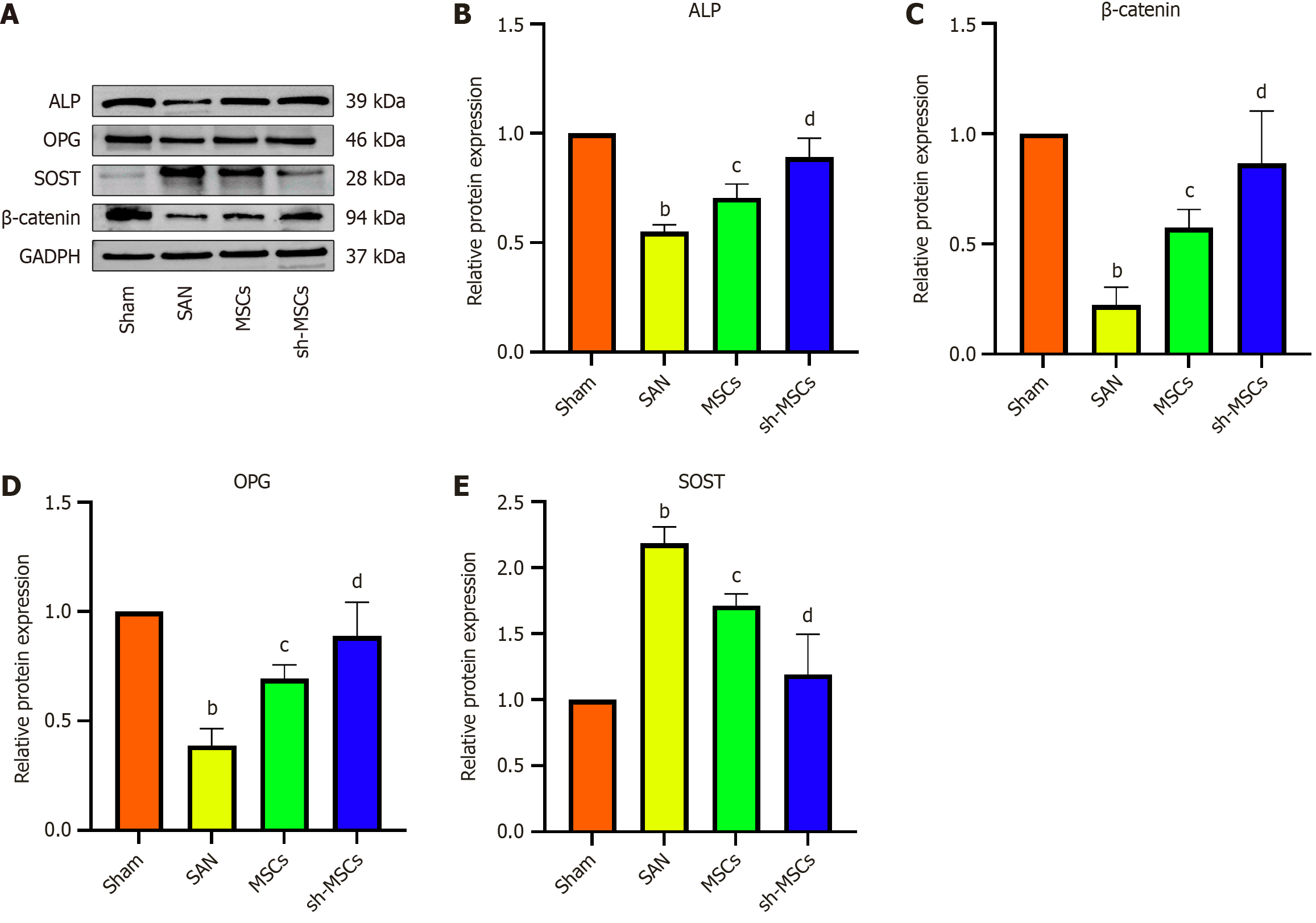
ELISA results (Figure 7) revealed that OPG levels were significantly lowest in the SAN group and highest in the sham group, with the sh-MSCs group exhibiting higher OPG expression than the MSCs group (P < 0.05). Conversely, RANK, RANKL, and tartrate-resistant acid phosphatase (TRAP) levels were lowest in the sham group, highest in the SAN group, and elevated in the MSCs group relative to the sh-MSCs group. These differences were statistically significant among all groups (P < 0.05). Western blot analysis demonstrated that the expression levels of ALP, OPG, and β-catenin were highest in the Sham group, lowest in the SAN group, and markedly higher in the sh-MSCs group compared to the MSCs group (P < 0.05) (Figure 8). In contrast, SOST expression was lowest in the sham group, highest in the SAN group, and significantly reduced in the sh-MSCs group relative to the MSCs group (P < 0.05). These results indicate that SOST-silenced hUCMSCs more effectively restore bone metabolic balance in SANFH mice by upregulating osteogenic markers (OPG, ALP, β-catenin) and downregulating osteoclast-related factors (RANK, RANKL, TRAP, SOST), thereby enhancing osteogenesis and suppressing bone resorption.
This study demonstrated that SOST-silenced hUCMSCs alleviate SANFH by activating the Wnt/β-catenin signaling pathway, thereby promoting osteogenic differentiation and inhibiting adipogenesis to restore bone metabolic balance. We successfully established a cellular model of hUCMSCs with SOST gene silencing, confirming that the modified cells retained typical MSC morphology, surface marker expression, and multilineage differentiation potential. Moreover, SOST-silenced led to significantly increased mRNA expression of osteogenic markers (ALP, OPG, and Runt-related transcription factor 2) and decreased expression of adipogenic markers (PPARγ and C/EBPs), indicating an enhanced osteogenic and suppressed adipogenic phenotype at the transcriptional level.
Micro-CT analysis demonstrated that multi-target SOST gene silencing in modified hUCMSCs significantly enhanced osteoblast activity and proliferation, resulting in increased trabecular bone density and thickness. These changes indicate a more favorable therapeutic effect on early-stage bone repair in the SANFH model. Previous studies have established that glucocorticoids promote the adipogenic differentiation of MSCs while suppressing osteogenic differentiation. In healthy bone tissue, osteocyte-rich lacunae are common, whereas the presence of empty lacunae typically reflects bone loss and lipid accumulation. In this study, HE staining revealed an increased number of osteoblasts on the damaged surface of the femoral head in the sh-MSCs group, accompanied by a marked reduction in vacuolated adipocytes and empty lacunae. These findings suggest that the bone repair observed may result from enhanced osteogenic differentiation and suppressed adipogenesis in SOST-silenced MSCs. Additionally, Masson staining further supported this hypothesis, showing increased new bone formation and orderly collagen fiber arrangement in the femoral heads of the sh-MSCs group. Our findings are supported by previous studies. Koide et al[25] reported that SOST-knockout mice exhibited significantly greater trabecular bone mass compared to wild-type controls, despite no observable change in osteoclast number. Similarly, Chen et al[26] demonstrated that SOST gene methylation enhances the osteogenic differentiation of MSCs while concurrently suppressing their adipogenic differentiation.
Bone is a dynamic connective tissue in which the balance between osteoclastic resorption and osteoblastic reconstruction is essential for maintaining homeostasis[27]. Among the key regulators of osteoclastogenesis, RANK and RANKL play central roles by promoting osteoclast differentiation upon binding[28]. OPG, secreted by osteoblasts, mesenchymal stromal cells, and fibroblasts, acts as a decoy receptor for RANKL, thereby preventing RANK-RANKL interaction and inhibiting osteoclast formation[29]. The OPG/RANKL ratio is thus widely used as an indicator of the dynamic balance between bone formation and resorption over time[30]. TRAP is a classical enzymatic marker of osteoclast activity and bone resorption. In contrast, ALP is a key marker of osteogenic differentiation in stem cells and reflects their osteogenic capacity. Additionally, β-catenin plays a pivotal role in bone metabolism by activating the Wnt/β-catenin pathway, which promotes osteoblast proliferation and differentiation, suppresses osteoclastogenesis, and enhances overall skeletal remodeling[31].
Previous studies have demonstrated that inhibition of SOST significantly upregulates β-catenin and OPG expression, while concurrently reducing TRAP and RANKL levels[32]. Conversely, in vitro overexpression of SOST suppresses β-catenin and increases the RANKL/OPG ratio, consistent with our findings. ELISA results from our study revealed decreased serum OPG and elevated RANKL levels in the SAN group, resulting in a reduced OPG/RANKL ratio compared to the sham group. This suggests that enhanced bone resorption is a hallmark of SANFH pathogenesis. The mechanism underlying these observations involves two primary processes[33]: (1) SOST inhibits osteoblast maturation by suppressing the Wnt/β-catenin signaling pathway, thereby reducing OPG production; and (2) SOST simultaneously enhances RANKL expression while downregulating OPG, leading to an imbalanced OPG/RANKL ratio. Following treatment with MSCs and sh-hUCMSCs, TRAP levels were reduced relative to the SAN group, with a more significant decline observed in the sh-MSCs group. This effect is attributed to the activation of the Wnt/β-catenin signaling pathway via SOST gene silencing, thereby suppressing osteoclast proliferation and TRAP production[32]. Western blot analysis further confirmed that silencing SOST enhanced the expression of ALP, OPG, and β-catenin, while markedly decreasing SOST expression in bone tissue. Recent studies have also highlighted the clinical potential of SOST-targeting antibodies, such as romosozumab and blosozumab[34,35], which have been shown to rapidly increase bone formation markers, reduce bone resorption markers, and significantly improve bone mineral density.
Future studies should further clarify the molecular mechanisms by which SOST regulates cartilage dynamics, with particular emphasis on its physiopathological roles in cell differentiation. Comprehensive validation of SOST as both a biomarker and therapeutic target for osteoarthritis is also warranted. Moreover, investigating the potential of gene editing technologies to modulate SOST for screening and optimizing tissue engineering seed cells represents a promising avenue for influencing cell behavior and enhancing regenerative therapies.
However, this study still has some limitations. First, the sample size of the animal experiments was relatively small, which may affect the generalizability of the results. Second, although we demonstrated the therapeutic effect of SOST-silenced hUCMSCs in a steroid-induced SANFH model, the long-term safety and efficacy of this approach remain to be validated in large-scale animal studies or clinical trials. Third, the molecular mechanisms underlying the interaction between SOST silencing and Wnt/β-catenin signaling require further in-depth investigation. Future studies are warranted to address these issues.
The therapeutic effect of sh-hUCMSCs on SANFH likely involves activating the Wnt/β-catenin signaling pathway by inhibiting SOST gene expression. This activation enhances the osteogenic differentiation, proliferative capacity, and bone forming function of hUCMSCs, while effectively suppressing their adipogenic differentiation potential. Future studies should further clarify the molecular mechanisms by which SOST regulates cartilage dynamics, with particular emphasis on its physiopathological roles in cell differentiation. Comprehensive validation of SOST as both a biomarker and therapeutic target for osteoarthritis is also warranted.
| 1. | Fei Y, Zhao L, Wu L, Zuo X, Li R, Cheng J, Luo H, Wu X, Sun L, Xu J, Zhu Y, Wang Y, Chen Z, Li X, Wang X, Zhang X; PRESS study team. Evaluation and prediction of relapse risk in stable systemic lupus erythematosus patients after glucocorticoid withdrawal (PRESS): an open-label, multicentre, non-inferiority, randomised controlled study in China. Ann Rheum Dis. 2025;84:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Snyder ME, Moghbeli K, Bondonese A, Craig A, Popescu I, Fan L, Tabib T, Lafyatis R, Chen K, Trejo Bittar HE, Lendermon E, Pilewski J, Johnson B, Kilaru S, Zhang Y, Sanchez PG, Alder JK, Sims PA, McDyer JF. Modulation of tissue resident memory T cells by glucocorticoids after acute cellular rejection in lung transplantation. J Exp Med. 2022;219:e20212059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Mengelkoch S, Gassen J, Slavich GM, Hill SE. Hormonal contraceptive use is associated with differences in women's inflammatory and psychological reactivity to an acute social stressor. Brain Behav Immun. 2024;115:747-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? A Ten-Year Update. J Bone Joint Surg Am. 2015;97:1604-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 355] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Zhao DW, Yu M, Hu K, Wang W, Yang L, Wang BJ, Gao XH, Guo YM, Xu YQ, Wei YS, Tian SM, Yang F, Wang N, Huang SB, Xie H, Wei XW, Jiang HS, Zang YQ, Ai J, Chen YL, Lei GH, Li YJ, Tian G, Li ZS, Cao Y, Ma L. Prevalence of Nontraumatic Osteonecrosis of the Femoral Head and its Associated Risk Factors in the Chinese Population: Results from a Nationally Representative Survey. Chin Med J (Engl). 2015;128:2843-2850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 6. | Huang ZQ, Fu FY, Li WL, Tan B, He HJ, Liu WG, Chen WH. Current Treatment Modalities for Osteonecrosis of Femoral Head in Mainland China: A Cross-Sectional Study. Orthop Surg. 2020;12:1776-1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Lu C, Qi H, Xu H, Hao Y, Yang Z, Yu W, Xu P. Global research trends of steroid-induced osteonecrosis of the femoral head: A 30-year bibliometric analysis. Front Endocrinol (Lausanne). 2022;13:1027603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Xu Y, Jiang Y, Wang Y, Jia B, Gao S, Yu H, Zhang H, Lv C, Li H, Li T. LINC00473-modified bone marrow mesenchymal stem cells incorporated thermosensitive PLGA hydrogel transplantation for steroid-induced osteonecrosis of femoral head: A detailed mechanistic study and validity evaluation. Bioeng Transl Med. 2022;7:e10275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Tang Z, Xu X, Shi W, Ren X, Luo H, Xu Y, Li C. Huc-MSC-derived exosomes alleviates alcohol-induced osteonecrosis of the femoral head through targeting the miR-25-3p/GREM1 axis. Genomics. 2025;117:110996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Fan S, Li J, Zheng G, Ma Z, Peng X, Xie Z, Liu W, Yu W, Lin J, Su Z, Xu P, Wang P, Wu Y, Shen H, Ye G. WAC Facilitates Mitophagy-mediated MSC Osteogenesis and New Bone Formation via Protecting PINK1 from Ubiquitination-Dependent Degradation. Adv Sci (Weinh). 2025;12:e2404107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Abatay Sel F, Erol A, Suleymanoglu M, Kuruca DS, Savran Oguz F. Easy and Rapid Methods for Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Human Umbilical Wharton's Jelly-Derived Mesenchymal Stem Cells. Methods Mol Biol. 2024;2736:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Fu YS, Yeh CC, Chu PM, Chang WH, Lin MA, Lin YY. Xenograft of Human Umbilical Mesenchymal Stem Cells Promotes Recovery from Chronic Ischemic Stroke in Rats. Int J Mol Sci. 2022;23:3149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Liu G, García Cenador MB, Si S, Wang H, Yang Q. Influences of umbilical cord mesenchymal stem cells and their exosomes on tumor cell phenotypes. Am J Cancer Res. 2023;13:6270-6279. [PubMed] |
| 14. | Li J, Wu Z, Zhao L, Liu Y, Su Y, Gong X, Liu F, Zhang L. The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Res Ther. 2023;14:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 15. | Xu HH, Li SM, Fang L, Xia CJ, Zhang P, Xu R, Shi ZY, Zou Z, Ge QW, Wang P, Tong PJ, Jin HT. Platelet-rich plasma promotes bone formation, restrains adipogenesis and accelerates vascularization to relieve steroids-induced osteonecrosis of the femoral head. Platelets. 2021;32:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Yu H, Zhu D, Liu P, Yang Q, Gao J, Huang Y, Chen Y, Gao Y, Zhang C. Osthole stimulates bone formation, drives vascularization and retards adipogenesis to alleviate alcohol-induced osteonecrosis of the femoral head. J Cell Mol Med. 2020;24:4439-4451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Zhang S, Wang H, Meng Q, Lee WY, Li Z, Sun S. Recent advances in osteonecrosis of the femoral head: a focus on mesenchymal stem cells and adipocytes. J Transl Med. 2025;23:592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Adapala NS, Yamaguchi R, Phipps M, Aruwajoye O, Kim HKW. Necrotic Bone Stimulates Proinflammatory Responses in Macrophages through the Activation of Toll-Like Receptor 4. Am J Pathol. 2016;186:2987-2999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Nan K, Zhang Y, Zhang X, Li D, Zhao Y, Jing Z, Liu K, Shang D, Geng Z, Fan L. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Res Ther. 2021;12:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Omran A, Atanasova D, Landgren F, Magnusson P. Sclerostin: From Molecule to Clinical Biomarker. Int J Mol Sci. 2022;23:4751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Yamaguchi Y, Kumagai K, Imai S, Miyatake K, Saito T. Sclerostin is upregulated in the early stage of chondrogenic differentiation, but not required in endochondral ossification in vitro. PLoS One. 2018;13:e0201839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Huang J, Ma T, Wang C, Wang Z, Wang X, Hua B, Jiang C, Yan Z. SOST/Sclerostin impairs the osteogenesis and angiogesis in glucocorticoid-associated osteonecrosis of femoral head. Mol Med. 2024;30:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Diegel CR, Kramer I, Moes C, Foxa GE, McDonald MJ, Madaj ZB, Guth S, Liu J, Harris JL, Kneissel M, Williams BO. Inhibiting WNT secretion reduces high bone mass caused by Sost loss-of-function or gain-of-function mutations in Lrp5. Bone Res. 2023;11:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Peng P, Nie Z, Sun F, Peng H. Glucocorticoids induce femoral head necrosis in rats through the ROS/JNK/c-Jun pathway. FEBS Open Bio. 2021;11:312-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Koide M, Yamashita T, Nakamura K, Yasuda H, Udagawa N, Kobayashi Y. Evidence for the major contribution of remodeling-based bone formation in sclerostin-deficient mice. Bone. 2022;160:116401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Chen X, Liu X, Wan J, Hu Y, Wei F. Icariin Facilitates Osteogenic Differentiation and Suppresses Adipogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Enhancing SOST Methylation in Postmenopausal Osteoporosis. J Gene Med. 2025;27:e70010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Che L, Chen X, He Z, Song D, Yuan Y, Liu C. Repurpose dasatinib and quercetin: Targeting senescent cells ameliorates postmenopausal osteoporosis and rejuvenates bone regeneration. Bioact Mater. 2023;25:13-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Yang N, Li M, Li X, Wu L, Wang W, Xu Y, Wang Z, Zhu C, Geng D. MAGL blockade alleviates steroid-induced femoral head osteonecrosis by reprogramming BMSC fate in rat. Cell Mol Life Sci. 2024;81:418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Sun M, Cao Y, Yang X, An F, Wu H, Wang J. DNA methylation in the OPG/RANK/RANKL pathway is associated with steroid-induced osteonecrosis of the femoral head. BMC Musculoskelet Disord. 2021;22:599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Ni X, Wu B, Li S, Zhu W, Xu Z, Zhang G, Cui H, Bai Q, Wang J. Equol exerts a protective effect on postmenopausal osteoporosis by upregulating OPG/RANKL pathway. Phytomedicine. 2023;108:154509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Lu S, Sun C, Miao C, Zhao Z. ERβ compensates for the absence of ERα function to promote osteoblast viability by inhibition of SOST signaling. Exp Ther Med. 2017;14:3387-3392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Jiao Z, Chai H, Wang S, Sun C, Huang Q, Xu W. SOST gene suppression stimulates osteocyte Wnt/β-catenin signaling to prevent bone resorption and attenuates particle-induced osteolysis. J Mol Med (Berl). 2023;101:607-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 33. | Robling AG, Bonewald LF. The Osteocyte: New Insights. Annu Rev Physiol. 2020;82:485-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 34. | Lane NE, Betah D, Deignan C, Oates M, Wang Z, Timoshanko J, Khan AA, Binkley N. Effect of Romosozumab Treatment in Postmenopausal Women With Osteoporosis and Knee Osteoarthritis: Results From a Substudy of a Phase 3 Clinical Trial. ACR Open Rheumatol. 2024;6:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 35. | Anastasilakis AD, Tsourdi E. Τhe story of sclerostin inhibition: the past, the present, and the future. Hormones (Athens). 2025;24:41-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |