Published online Jun 26, 2024. doi: 10.4252/wjsc.v16.i6.728
Revised: March 17, 2024
Accepted: April 19, 2024
Published online: June 26, 2024
Processing time: 150 Days and 23.9 Hours
Necrotizing enterocolitis (NEC) is a severe gastrointestinal disease that affects premature infants. Although mounting evidence supports the therapeutic effect of exosomes on NEC, the underlying mechanisms remain unclear.
To investigate the mechanisms underlying the regulation of inflammatory response and intestinal barrier function by umbilical cord mesenchymal stem cell (UCMSCs) exosomes, as well as their potential in alleviating NEC in neonatal mice.
NEC was induced in 5-d-old C57BL/6 pups through hypoxia and gavage feeding of formula containing lipopolysaccharide (LPS), after which the mice received human UCMSC exosomes (hUCMSC-exos). The control mice were allowed to breastfeed with their dams. Ileal tissues were collected from the mice and analyzed by histopathology and immunoblotting. Colon tissues were collected from NEC neonates and analyzed by immunofluorescence. Molecular biology and cell culture approaches were employed to study the related mechanisms in intestinal epithelial cells.
We found that autophagy is overactivated in intestinal epithelial cells during NEC, resulting in reduced expression of tight junction proteins and an increased inflammatory response. The ability of hUCMSC-exos to ameliorate NEC in a mouse model was dependent on decreased intestinal autophagy. We also showed that hUCMSC-exos alleviate the inflammatory response and increase migration ability in intestinal epithelial cells induced by LPS.
These results contribute to a better understanding of the protective mechanisms of hUCMSC-exos against NEC and provide a new theoretical and experimental foundation for NEC treatment. These findings also enhance our understanding of the role of the autophagy mechanism in NEC, offering potential avenues for identifying new therapeutic targets.
Core Tip: Based on observations of clinical samples, this study revealed a new mechanism by which human umbilical cord mesenchymal stem cells-derived exosomes reduce the inflammatory response and intestinal barrier dysfunction in neonatal mice with necrotizing enterocolitis (NEC) from the perspective of intestinal epithelial cell autophagy. These findings offer new insights for the clinical application of exosomes in NEC treatment and contribute to the establishment of a solid theoretical foundation.
- Citation: Zhu L, He L, Duan W, Yang B, Li N. Umbilical cord mesenchymal stem cell exosomes alleviate necrotizing enterocolitis in neonatal mice by regulating intestinal epithelial cells autophagy. World J Stem Cells 2024; 16(6): 728-738
- URL: https://www.wjgnet.com/1948-0210/full/v16/i6/728.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i6.728
Necrotizing enterocolitis (NEC) is a gastrointestinal disorder that predominantly affects newborns, particularly prema
Extensive research has shown substantiated that NEC perturbs the equilibrium of proinflammatory and anti-inflammatory signals within the organism, precipitating an intensified succession of inflammatory reactions. This heightened inflammatory reaction exacerbates NEC and contributes to multiple organ failure. Moreover, this reaction adversely affects the clinical prognosis and induces exacerbates harm to the intestinal epithelial barrier[3]. Hence, a severe inflammatory response and impaired functionality of the intestinal mucosal epithelial barrier are two pivotal elements in the development of NEC.
Autophagy exerts varied influences on the gastrointestinal mucosal barrier. Investigative studies have shown elucidated that autophagy upholds the equilibrium of the intestinal mucosal milieu via its connections to the intestinal epithelium[4]. Malfunctions in the autophagy signalling pathway may induce harm to Paneth cells within the intestines and diminish the mucosa’s capacity to counteract pathogens[5]. Furthermore, autophagy has both inflammatory and anti-inflammatory effects. Under normal circumstances, autophagy governs the secretion of inflammatory cytokines and maintains cellular homeostasis. Nonetheless, excessive autophagy activation during times of stress can trigger the activation of inflammasomes, excessive cytokine secretion, and increased inflammation[6]. Suboptimal maturation of the intestinal mucosal barrier and weakened resistance of the intestinal epithelium to pathogens in premature infants are critical risk factors for the onset of NEC[7]. The influence of autophagy on the intestinal mucosa and inflammatory factors is dual-faceted, indicating its substantial regulatory role in the initiation of disease.
In recent years, stem cell therapy has shown promising results in treating various diseases, including gastrointestinal diseases. Significant progress has been made in this field of animal research. Stem cells have been found to possess several beneficial effects such as anti-inflammatory, anti-apoptotic, and intestinal barrier-enhancing effects. These effects have potential clinical implications for NEC, thus providing a protective effect. A meta-analysis of nine animal experiments demonstrated that stem cells and stem cell-derived exosomes can reduce the incidence of NEC, particularly stage II NEC, by improving intestinal motility and reducing intestinal permeability. Umbilical cord mesenchymal stem cells (UCMSCs) are widely used due to their easy accessibility and noninvasive isolation method. These cells possess various differentiation capabilities and have shown success in animal models of ischemia reperfusion and NEC[8]. However, the specific mechanism of their action is still unclear. Notably, exosomes, which are bioactive factors secreted by stem cells, have shown comparable, or even superior, therapeutic effects. Therefore, the aim of our study was to investigate the mechanisms underlying the regulation of the inflammatory response and intestinal barrier function by UCMSC-derived exosomes, as well as their potential for alleviating NEC in neonatal mice.
We collected colon tissue samples from neonates with NEC and used the colon tissue removed during the first operation (the colon tissue was separated 3-5 mm from the edge of the obviously damaged or necrotic site) as the experimental group (NEC). When the stoma was selectively closed, the functional end tissue of the colon served as a control group (Ctr). The present study was approved by the ethical standards of Qilu Hospital of Shandong University ethics committee. This study was performed in accordance with the International Ethical Guidelines for Human Biomedical Research (2012). Information regarding the patients with NEC was provided by the guardians of the patients. Written informed consent was obtained from the participants involved in the study.
Human UCMSCs (hUCMSCs) purchased from Cyagen Biosciences (Guangzhou, China) were cultured in MSC medium (OriCell®, Cyagen Biosciences, Guangzhou, China) at 37 °C in a 5% CO2 incubator. hUCMSCs from passages 3 and 6 were used throughout the experiments.
IEC-18 represent rat ileal epithelial cells. The cells were routinely cultured in DMEM with high glucose containing 10% foetal bovine serum (HyClone) and 1% penicillin/streptomycin (Gibco) in a humidified incubator under 5% CO2 at 37 °C. IEC-18 cells were exposed to lipopolysaccharide (LPS, 50 μg/mL, Sigma) for 12 h to induce epithelial injury in vitro. hUCMSCs exosomes (hUCMSC-exos) (50 μL) were added separately to relieve the injury of IEC-18 cells. Rapamycin (30 nM) were added to clarify the role of autophagy in the above phenomenon.
Exosomes were extracted using an exosome extraction kit (ExoQuick-TC, SBI, United States) according to the manu
C57BL/6J mice were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China). Five- to nine-day-old neonatal pups were used to stablish an experimental NEC model according to a previous study[10]. Experimental NEC mice was induced in mice via combined ‘artificial feeding + hypoxia + LPS’ stimulation. Newborn mice underwent the following procedures: (1) Artificial feeding: Formula milk was administered through a gastric tube inserted into the mouth. Artificial feeding was performed every 8 h, with a dose of 40 μL/g of mouse body weight; (2) Hypoxia: After each feeding, the mice were placed in an experimental animal hypoxia chamber for 0.5 h, where they received hypoxia treatment (95% N2 + 5% O2 for 10 min); and (3) LPS: On the 2nd and 3rd d of modelling, LPS (4 μg/g of mouse body weight) was added to the formula milk during the second artificial feeding at 6 d (D6) and 7 d (D7) after birth. The modelling process lasted for a total of 96 h and ended on the 9th day of age (D9). All surviving mice were anesthetized with an intraperitoneal injection of pentobarbital (3%, 1 μL/g of mouse body weight) and underwent cardiac puncture. Blood samples were collected, and intestinal samples were obtained by opening the abdominal cavity. The exclusion criteria were as follows: Mice that died due to asphyxia caused by errors in gastric tube insertion during artificial feeding and mice that died within 24 h of the start of modelling. NEC mice were divided into 2 groups: (1) The NEC group: No treatment group; and (2) The Exo group: During the NEC induction process, D6 and D7 mice were intraperitoneally injected with 100 μL of exo.
All procedures involving animals used in this study were performed and monitored in accordance with the guidelines of the Chinese Council on Animal Care and were approved by the Institutional Animal Care and Use Committee of the Qilu Hospital of Shandong University. Throughout the study, all efforts were made to minimize any suffering of the animals.
Fixation of the distal ileum tissues was carried out for one night using a solution of 4% paraformaldehyde. These tissues were subsequently embedded in paraffin and sliced into 3 μm thick sections. Afterwards, hematoxylin and 3% eosin were used to individually stain the prepared sections. The histopathological state of the terminal ileum tissues in each group of young animals was meticulously examined and documented via the capture of accompanying photographic evidence.
After deparaffinization and rehydration, the tissue sections were placed in a repair box filled with antigen retrieval buffer containing citric acid (pH = 6.0) and heated in a microwave oven to facilitate antigen retrieval. Subsequently, the slides were transferred to a solution for antigen retrieval and incubated with a primary antibody overnight at 4 °C. The primary antibodies used were against ZO-1 (Cat# GB111402-100, Servicebio) and LC3 (Cat#ab192890, Abcam). After overnight incubation, a secondary antibody was applied at room temperature for 1 h. For immunohistochemical staining, 3,3-diaminobenzidine (Sigma, St. Louis, MO) was used. Counterstaining with haematoxylin was performed, and the sections were examined under an optical microscope. Alternatively, for immunofluorescence staining, Alexa Fluor 488-conjugated goat anti-rabbit antibody (1:400; Servicebio, China) or Cy3-conjugated goat anti-rabbit antibody (1:400; Servicebio, China) was utilized. The cell nuclei were labelled using DAPI solution (SouthernBiotech). The slides were visualized using a fluorescence microscope and the results were quantified using Image-Pro Plus 6.0 software. The expression levels of LC3 and ZO-1 were quantified based on the mean density of 5 randomly selected fields from each group.
Ad-mRFP-GFP-LC3 (synthesized by Obio Technology, Shanghai, China)[11] was used to monitor the induction of autophagy and autophagic flux by detecting the degradation of GFP signals under acidic conditions in the lysosome lumen. The experiment commenced by introducing cells into 12-well culture plates at a concentration of 1 × 105 cells/ml in DMEM. Once the cells reached 30% confluence, Ad-mRFP-GFP-LC3 was added to each well at a multiplicity of infection of 50, following the manufacturer’s instructions. All treatment procedures were conducted 48 hours after infection. To observe the cells, they were washed three times with phosphate buffered saline (PBS) and then fixed with 4% paraformaldehyde for 30 min. Finally, confocal microscopy (Olympus, FV3000) was used to capture images of the cells.
For immunoblot analysis, mouse ileal tissues and IEC-18 cells were lysed using a total protein extraction kit according to the manufacturer’s instructions. The IEC-18 cells were divided into the Ctr, LPS (50 μg/mL, 12 h), LPS + Exo (1 mL culture medium + 50 μL hUCMSC-exos, 24 h), Rap (30 nM, cells pretreated for 1 h), Rap + LPS, and Rap + LPS + Exo. Total lysates were resolved by electrophoresis using 4%-15% precast polyacrylamide gels, transferred to polyvinylidene fluoride membranes, and incubated overnight at 4 °C with antibodies against p62 (Cat# ab109012, Abcam), LC3 (Cat# ab192890, Abcam), ZO-1 (ZO-1, Cat# GB111402-100, Servicebio), Caudin1 (Cat# ab211737, Abcam), E-Cadherin (Cat# ab231303, Abcam), Ki67 (Cat# ab16667, Abcam,) and GAPDH (Cat# AB-P-R001, Goodhere Biotech). The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti-mouse IgG-HRP, Cat# SA00001-1, Proteintech; goat anti-rabbit IgG-HRP, Cat# A0208, Beyotime) for 1 h at room temperature and detected using an enhanced chemiluminescence substrate. The analysis of immunoblot images was performed using Bio-Rad’s Image Lab software.
TEM (HITACHI, HT7700, Chiyoda, Tokyo, Japan) was employed to uncover the morphological changes in autophagic structures throughout the dynamic maturation process, spanning from the phagophore to the autolysosome. Cells in each group were collected by cell scrapers and then fixed in 2.5% glutaraldehyde following centrifugation at 800 rpm for 5 min. Subsequently, a postfixation step was performed at room temperature for 2 h using 1% osmium tetroxide (OsO4) in 0.1 M PBS. The sequential procedures of dehydration, infiltration, embedment, and sectioning of ultrathin slices were then carried out. Finally, the ultrathin sections were treated with uranyl acetate and lead citrate for 15 min each, before being observed and imaged using TEM.
According to the manufacturer’s protocol, the levels of interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α in mouse serum and the levels of secreted IL-6, IL-8 and TNF-α in IEC-18 cell culture supernatants were determined using an enzyme-linked immunosorbent assay (ELISA) kit (mouse IL-6 ELISA kit, Cat# CSB-E04639m; rat IL-6 ELISA kit, CSB-E04640r; mouse TNF-α ELISA kit, Cat# CSB-E04741m; rat TNF-α ELISA kit, CSB-E11987r; all purchased from CUSABIO, Wuhan, China. Mouse IL-8 ELISA kit, Cat# SEKM-0046 and rat IL-8 ELISA kit, Cat# SEKR-0014 purchased from Suolaibao (Beijing, China). Three times ELISA analyzes were repeated three times for each sample. A Multiskan FC photometer (Thermo Scientific) was used to detect the absorbance of each sample at 450 nm.
Cell migration was evaluated through the use of a scratch assay. IEC-18 cells were seeded in 6-well plates at a density of 1.2 × 105 cells/mL and cultivated until they reached complete confluence. To establish a baseline value, five parallel scratches were created in each well using a 200 μL pipette tip, and the width of each scratch was determined. Subse
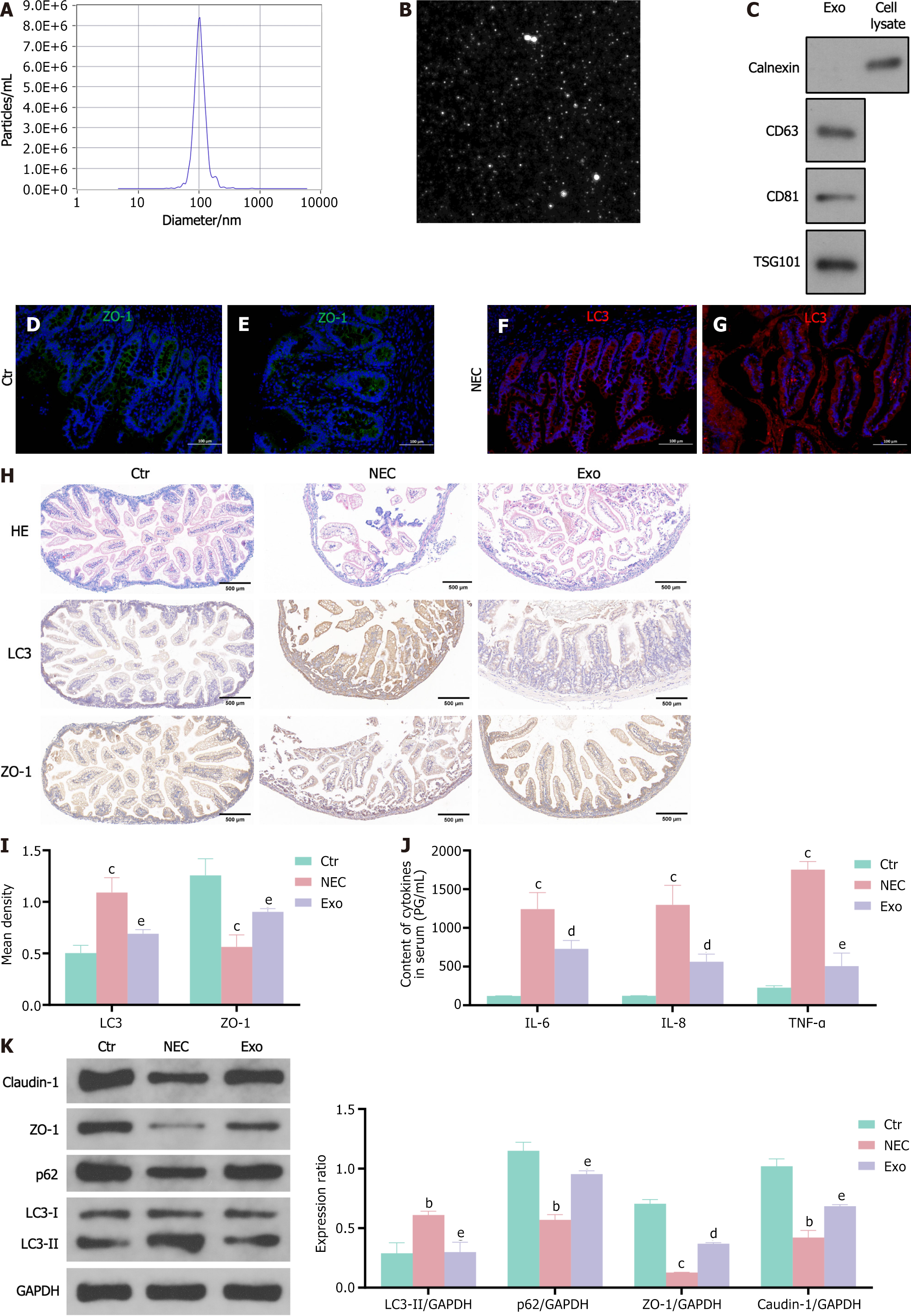
To investigate the potential roles of hUCMSC-exos in neonatal NEC, hUCMSC-exos were first isolated and verified via TEM, western blotting, and nanoparticle tracking analysis (NTA). The NTA data indicated that the diameters of the hUCMSC-exos were mostly approximately 100 nm (Figure 1A). TEM revealed that hUCMSC-exos exhibited a round morphology (Figure 1B), which is consistent with the typical exosomal morphology. Western blot results indicated that the levels of specific exosome surface markers (CD81 and CD63) were significantly increased in the hUCMSC-exos (Figure 1C). Collectively, these results confirmed that the hUCMSC-exos were successfully isolated and identified.
To demonstrate the role of intestinal epithelial cell autophagy in the development of NEC, we conducted a study using colon tissue samples obtained from neonates with NEC. The intestinal villi were seriously damaged in the NEC tissue sections (Figure 1D and E). Immunofluorescence analysis revealed decreased expression of the tight junction protein ZO-1 (Figure 1D and E) and increased expression of the autophagy protein LC3 (Figure 1F and G) in the intestinal epithelial cells of NEC neonates, demonstrating that autophagy may be involved in the development of NEC.
Similar to the finding in NEC neonates, blunting of villous tips and submucosal separation/oedema were observed, and the expression of ZO-1 and LC3 was altered in NEC mice (Figure 1H and I). The concentrations of IL-6, IL-8 and TNF-α were significantly increased in the NEC group (Figure 1J). However, the intraperitoneal administration of hUCMSC-exos during the generation of mouse model of NEC resulted in a significant reduction in intestinal injury severity and cytokines levels and prevented the decreases in ZO-1 and claudin-1 expression compared with those in NEC controls (Figure 1H and I), supporting the protective role of hUCMSC-exos in a mouse of NEC model. We found that the expression of the autophagy markers LC3 and p62 was reversed in the hUCMSC-exos treated group (Figure 1K). Next, we investigated whether the beneficial effects of hUCMSC-exos were associated with enterocyte autophagy.
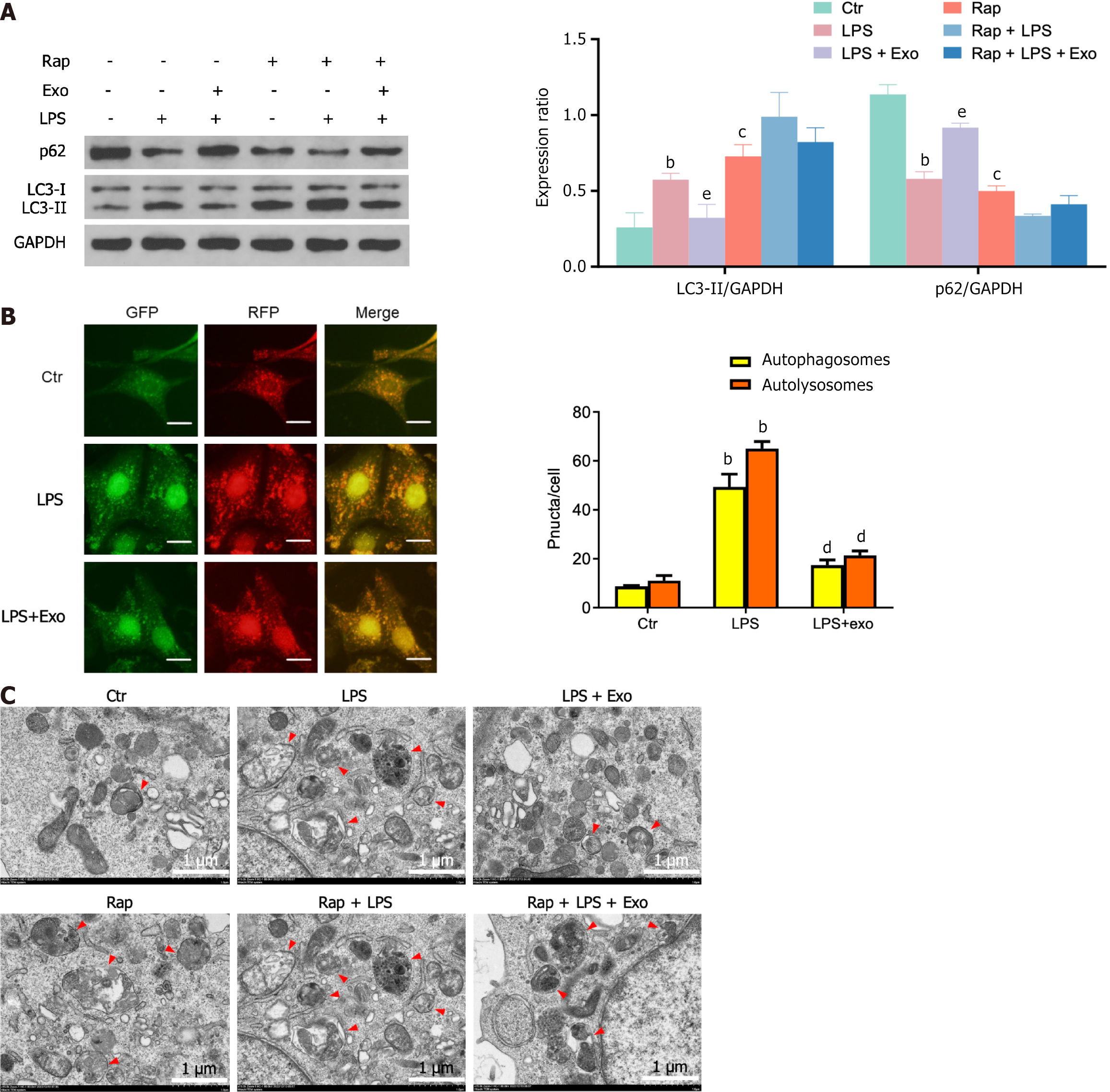
LPS is a commonly used factor in in vitro experiments to simulate intestinal epithelial cell damage. The stimulation of IEC-18 cells by LPS notably enhanced autophagy, as indicated by the elevated expression of the autophagy marker protein LC3-II and decreased expression of p62 (Figure 2A). Further examination via confocal microscopy revealed a significant increase in the presence of autophagosomes and autophagic lysosomes (Figure 2B). TEM also confirmed a significant increase in the number of autophagic vacuoles (Figure 2C). Additionally, an inverse expression pattern was observed in the hUCMSC-exos- treated group, demonstrating that hUCMSC-exos inhibit the autophagy of enterocyte induced by LPS. Furthermore, after the mechanistic target of rapamycin (mTOR) inhibitor rapamycin activated autophagy, the effects of hUCMSC-exos were abolished, indicating that hUCMSC-exos may regulate autophagy in intestinal epithelial cells through the mTOR signalling pathway. However, whether the regulation of enterocyte autophagy by hUCMSC-exos is involved in its protective effect on NEC is unclear.
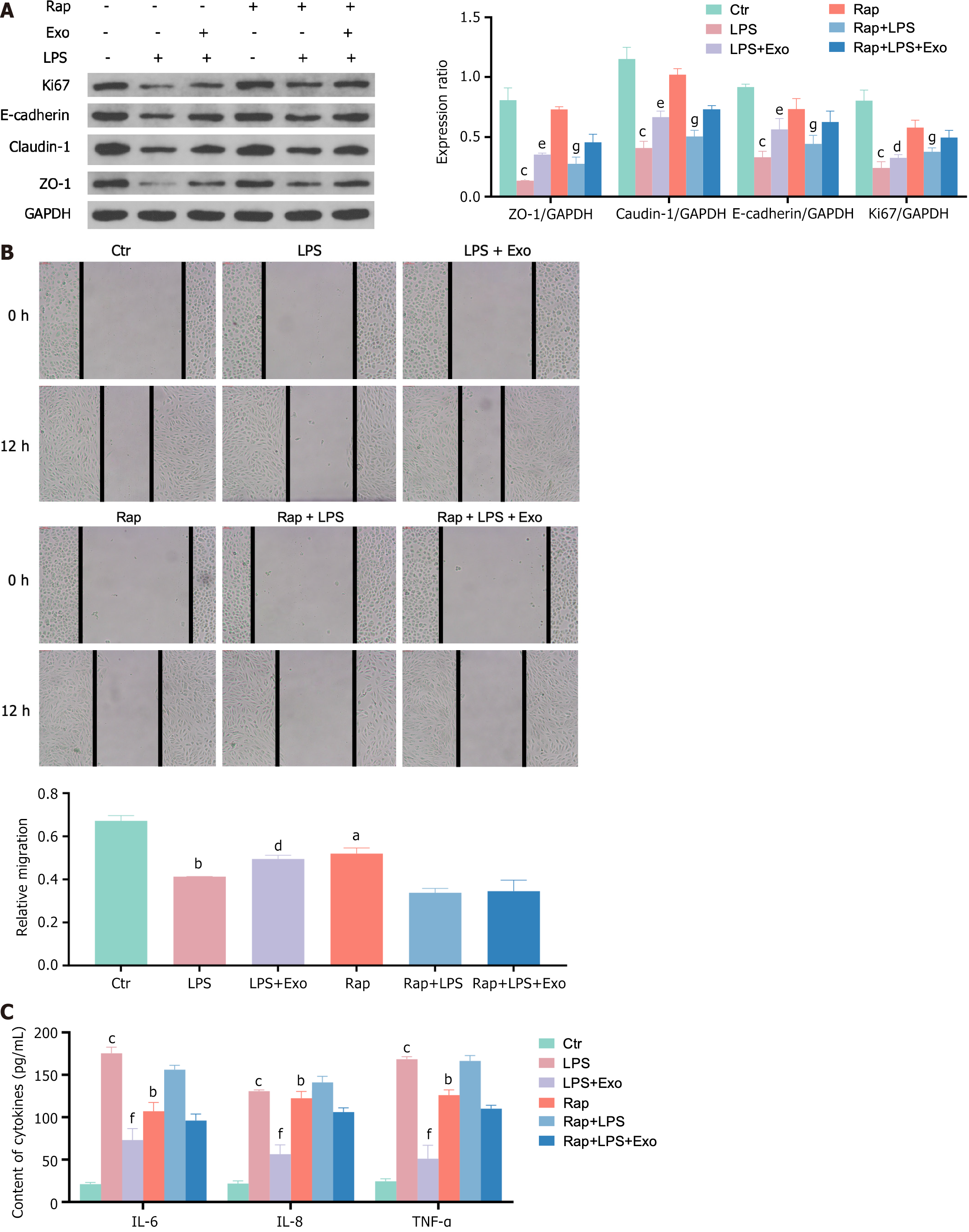
As shown in Figure 3, hUCMSC-exos enhanced the expression of tight junction proteins (E-cadherin, claudin-1, and ZO-1) and the cell proliferation protein Ki67, which was inhibited by LPS, in IEC-18 cells. They also improved the migration ability of IEC-18 cells and reduced the inflammatory reaction caused by LPS. However, when autophagy is activated by Rap, the effects of the hUCMSC-exos mentioned above are nullified. This indicates that autophagy plays a significant role in the protective effect of hUCMSC-exos in intestinal epithelial cells.
In the present study, we observed overactivated autophagy in neonates and mice with NEC, which is in line with earlier studies[12,13]. Additionally, we have provided evidence showing the ability of hUCMSC-exos to mitigate inflammatory responses and intestinal damage in NEC mice and to enhance the migratory capacity of intestinal epithelial cells in vitro. Specifically, we found that hUCMSC-exos ameliorate NEC in a manner dependent on enterocyte autophagy. These findings illuminate the underlying mechanism through which hUCMSC-exos confer protection against NEC, which involves the inhibition of excessive intestinal epithelial cell autophagy.
NEC still represents a devastating disease for preterm neonates, and limited preventive and therapeutic strategies are currently available. In recent years, stem cell therapy has emerged as a promising approach for treating various diseases, including gastrointestinal diseases. Animal research has made significant progress in understanding the potential benefits of stem cells in treating NEC[14]. UCMSCs are extensively employed in investigations owing to their convenient accessibility and noninvasive isolation technique. These cells exhibit diverse abilities to differentiate and have been effectively employed in animal models of ischemia-reperfusion and NEC[8]. However, the specific mechanism of action is still unclear.
Exosomes, which are vesicles formed by the fusion of multivesicular bodies and plasma membranes, are secreted by various cells including stem cells. They serve as an efficient communication medium for transmitting genetic information between cells. Exosomes contain proteins and RNAs, which effectively regulate gene expression in target cells and impact their physiological state. In animal models, stem cells have been shown to primarily reduce the incidence of NEC through paracrine mechanisms. Stem cell-derived exosomes, which are bioactive factors secreted by stem cells, have the potential to achieve similar or even superior therapeutic effects[15]. Our study revealed that hUCMSC-exos significantly reduced plasma inflammatory factor levels, alleviated intestinal damage, and increased the expression of tight junction proteins in intestinal epithelial cells in NEC mice.
Autophagy plays a significant role in maintaining the balance of the gastrointestinal mucosal barrier. The regulation of intestinal epithelial connections helps maintain the homeostasis of the intestinal mucosal environment[4]. In NEC rats, typical autophagy phenomena have been observed in intestinal epithelial cells. Treatment with epidermal growth factor has been found to reduce autophagy and protect the intestines of NEC rats by decreasing the expression of Beclin-1 in ileal epithelial cells[16]. Similarly, vitamin D-3 has been shown to inhibit overactivated autophagy in intestinal epithelial cells of NEC rats, thereby promoting intestinal mucosal integrity[12]. Clinical studies have indicated that the autophagy protein Beclin-1 is highly expressed in neonatal NEC patients with severe intestinal necrosis, while the autophagy protein p62 is expressed at low levels. Furthermore, genetic mutations in the autophagy-related gene ATG16 have been found to be associated with NEC in premature infants, suggesting that reduced autophagy caused by these mutations may have a protective effect on NEC[13]. Similarly, we found that the expression of the autophagy marker protein LC3 was signi
Specifically, excessive autophagy in intestinal epithelial cells can hinder cell migration, damage the intestinal barrier, and increase intestinal permeability by degrading tight junction proteins such as claudin-210. The autophagy-inducing factor rapamycin has been shown to significantly inhibit the migration of various cells, including intestinal epithelial cells, both in vitro and in vivo[17]. Therefore, we verified the effects of hUCMSC-exos on autophagy, migration and functional proteins in intestinal epithelial cells in vitro.
We found that hUCMSC-exos can reduce the expression of autophagy and inflammatory factors induced by LPS in IEC-18 cells. Additionally, hUCMSC-exos increased the expression of tight junction proteins and cell proliferation proteins and enhanced cell migration. However, when autophagy is activated by Rap, the aforementioned effects of hUCMSC-exos are weakened. These results highlight the role of autophagy in mediating the protective effect of hUCMSC-exos on intestinal epithelial cells. Considering the significance of intestinal epithelial cells in the development and progression of NEC, these findings strongly suggest that autophagy may play a crucial role in alleviating NEC caused by hUCMSC-exos.
In conclusion, we provide concrete evidence that downregulated autophagy in intestinal epithelial cells reduces intestinal inflammation and damage in NEC mice, and that targeting autophagy, such as via hUCMSC-exos, could serve as a potential therapeutic strategy for modulating NEC pathogenesis. The specific mechanism by which exosomes regulate autophagy is still unclear, and the components of exosomes are complex. Our main research focus in the future will be to clarify which components play a major role in the aforementioned effects of exosomes.
We thank the patients and their families for their cooperation and contribution.
| 1. | Alsaied A, Islam N, Thalib L. Global incidence of Necrotizing Enterocolitis: a systematic review and Meta-analysis. BMC Pediatr. 2020;20:344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 2. | Frost BL, Modi BP, Jaksic T, Caplan MS. New Medical and Surgical Insights Into Neonatal Necrotizing Enterocolitis: A Review. JAMA Pediatr. 2017;171:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Wei J, Meng Z, Li Z, Dang D, Wu H. New insights into intestinal macrophages in necrotizing enterocolitis: the multi-functional role and promising therapeutic application. Front Immunol. 2023;14:1261010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 4. | Yamoto M, Lee C, Chusilp S, Yazaki Y, Alganabi M, Li B, Pierro A. The role of autophagy in intestinal epithelial injury. Pediatr Surg Int. 2019;35:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Bel S, Hooper LV. Secretory autophagy of lysozyme in Paneth cells. Autophagy. 2018;14:719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Guan Y, Zhang L, Li X, Zhang X, Liu S, Gao N, Li L, Gao G, Wei G, Chen Z, Zheng Y, Ma X, Siwko S, Chen JL, Liu M, Li D. Repression of Mammalian Target of Rapamycin Complex 1 Inhibits Intestinal Regeneration in Acute Inflammatory Bowel Disease Models. J Immunol. 2015;195:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Markel TA, Martin CA, Chaaban H, Canvasser J, Tanner H, Denchik H, Good M. New directions in necrotizing enterocolitis with early-stage investigators. Pediatr Res. 2020;88:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Drucker NA, Te Winkel JP, Shelley WC, Olson KR, Markel TA. Inhibiting hydrogen sulfide production in umbilical stem cells reduces their protective effects during experimental necrotizing enterocolitis. J Pediatr Surg. 2019;54:1168-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int J Nanomedicine. 2020;15:5911-5926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 361] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 10. | Ma F, Hao H, Gao X, Cai Y, Zhou J, Liang P, Lv J, He Q, Shi C, Hu D, Chen B, Zhu L, Xiao X, Li S. Melatonin ameliorates necrotizing enterocolitis by preventing Th17/Treg imbalance through activation of the AMPK/SIRT1 pathway. Theranostics. 2020;10:7730-7746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Zhu L, Duan W, Wu G, Zhang D, Wang L, Chen D, Chen Z, Yang B. Protective effect of hydrogen sulfide on endothelial cells through Sirt1-FoxO1-mediated autophagy. Ann Transl Med. 2020;8:1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Chen L, Lv Z, Gao Z, Ge G, Wang X, Zhou J, Sheng Q. Human β-defensin-3 reduces excessive autophagy in intestinal epithelial cells and in experimental necrotizing enterocolitis. Sci Rep. 2019;9:19890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Sampath V, Bhandari V, Berger J, Merchant D, Zhang L, Ladd M, Menden H, Garland J, Ambalavanan N, Mulrooney N, Quasney M, Dagle J, Lavoie PM, Simpson P, Dahmer M. A functional ATG16L1 (T300A) variant is associated with necrotizing enterocolitis in premature infants. Pediatr Res. 2017;81:582-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Di SJ, Wu SY, Liu TJ, Shi YY. Stem cell therapy as a promising strategy in necrotizing enterocolitis. Mol Med. 2022;28:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 15. | McCulloh CJ, Olson JK, Wang Y, Zhou Y, Tengberg NH, Deshpande S, Besner GE. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. 2018;53:1215-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Maynard AA, Dvorak K, Khailova L, Dobrenen H, Arganbright KM, Halpern MD, Kurundkar AR, Maheshwari A, Dvorak B. Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G614-G622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Neal MD, Sodhi CP, Dyer M, Craig BT, Good M, Jia H, Yazji I, Afrazi A, Richardson WM, Beer-Stolz D, Ma C, Prindle T, Grant Z, Branca MF, Ozolek J, Hackam DJ. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol. 2013;190:3541-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/