Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.525
Revised: February 5, 2024
Accepted: April 7, 2024
Published online: May 26, 2024
Processing time: 156 Days and 15.4 Hours
Acute kidney injury (AKI) is a common clinical syndrome with high morbidity and mortality rates. The use of pluripotent stem cells holds great promise for the treatment of AKI. Urine-derived stem cells (USCs) are a novel and versatile cell source in cell-based therapy and regenerative medicine that provide advantages of a noninvasive, simple, and low-cost approach and are induced with high multidifferentiation potential. Whether these cells could serve as a potential stem cell source for the treatment of AKI has not been determined.
To investigate whether USCs can serve as a potential stem cell source to improve renal function and histological structure after experimental AKI.
Stem cell markers with multidifferentiation potential were isolated from human amniotic fluid. AKI severe combined immune deficiency (SCID) mice models were induced by means of an intramuscular injection with glycerol. USCs isolated from human-voided urine were administered via tail veins. The functional changes in the kidney were assessed by the levels of blood urea nitrogen and serum creatinine. The histologic changes were evaluated by hematoxylin and eosin staining and transferase dUTP nick-end labeling staining. Meanwhile, we compared the regenerative potential of USCs with bone marrow-derived mesenchymal stem cells (MSCs).
Treatment with USCs significantly alleviated histological destruction and functional decline. The renal function was rapidly restored after intravenous injection of 5 × 105 human USCs into SCID mice with glycerol-induced AKI compared with injection of saline. Results from secretion assays conducted in vitro demonstrated that both stem cell varieties released a wide array of cytokines and growth factors. This suggests that a mixture of various mediators closely interacts with their biochemical functions. Two types of stem cells showed enhanced tubular cell proliferation and decreased tubular cell apoptosis, although USC treatment was not more effective than MSC treatment. We found that USC therapy significantly improved renal function and histological damage, inhibited inflammation and apoptosis processes in the kidney, and promoted tubular epithelial proliferation.
Our study demonstrated the potential of USCs for the treatment of AKI, representing a new clinical therapeutic strategy.
Core tip: This study reveals that urine-derived stem cells (USCs) significantly enhance renal function and histological recovery in severe combined immune deficiency mice with glycerol-induced acute kidney injury (AKI). By comparing USCs with bone marrow-derived mesenchymal stem cells, the research highlights USCs’ potential as a novel, non-invasive cell source for AKI treatment. The findings suggest that USCs, through their multidifferentiation potential and secretion of various cytokines and growth factors, offer a promising therapeutic strategy for AKI, potentially altering clinical approaches to renal repair.
- Citation: Li F, Zhao B, Zhang L, Chen GQ, Zhu L, Feng XL, Gong MJ, Hu CC, Zhang YY, Li M, Liu YQ. Therapeutic potential of urine-derived stem cells in renal regeneration following acute kidney injury: A comparative analysis with mesenchymal stem cells. World J Stem Cells 2024; 16(5): 525-537
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/525.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.525
Acute kidney injury (AKI) is a complex syndrome marked by the rapid decline in renal function. Despite improvements in intensive care and dialysis methods, the incidence of heart disease and sepsis has risen, resulting in consistently high levels of mortality and morbidity linked to AKI. This worrisome trend could be attributed to the aging population, which poses additional challenges[1]. The worldwide occurrence of AKI stands at 21 million per 100000 person-years, notably affecting hospitalized individuals at a higher frequency. Notably, AKI cases necessitating renal replacement therapy are associated with mortality rates ranging from 50% to 80%, resulting in an estimated annual global death toll of 2 million. Moreover, patients diagnosed with AKI face a ninefold augmented risk of developing chronic kidney disease and a twofold increased risk of mortality in comparison to those without AKI[2]. Even among AKI patients who receive renal replacement therapy, the mortality rate remains alarmingly high, ranging between 50% and 60%[3]. Consequently, the development of more efficacious therapeutic strategies for treating AKI is imperative.
Renal impairment tubular is commonly seen as the primary pathological change linked to AKI. The prognosis of AKI patients is greatly influenced by the effective regeneration of necrotic tubular epithelial cells. In recent times, novel methods have been explored to aid in the restoration of renal tubular epithelial cells. One potential approach involves the utilization of stem cells or progenitor cells, such as embryonic stem cells (ESCs), bone marrow-derived mesenchymal stem cells (BMSCs), stem cells sourced from human amniotic fluid (hAFSCs), and induced pluripotent stem cells (iPSCs), with the goal of reinstating kidney functionality[4-7]. ESCs present an unlimited reservoir for cell replacement therapy; however, their usage is mired in controversy due to ethical concerns surrounding embryo use. Furthermore, the retrieval procedure of BMSCs and hAFSCs can cause discomfort and ethical conflicts. Despite the simple production of iPSCs from human cells, which helps in preventing immune rejection, apprehensions persist about their safety due to the possible development of teratomas. Therefore, investigators continue to explore novel, less invasive cell sources for application.
Urine-derived stem cells (USCs), which are isolated from urine, have a high proliferative capacity. USCs are drawing much research attention due to the advantages of noninvasive harvesting and strong differentiation potential. The applicability of USCs in the treatment of bladder dysfunction and in cell-based urological tissue regeneration has been demonstrated[8-10]. USCs have the potential to differentiate and may be a promising treatment option for kidney disorders[11,12]. In this work, we investigated whether USCs could serve as a potential stem cell source to improve renal function and histological structure in mice with AKI induced by glycerol injection in nonimmune-competent severe combined immune deficiency (SCID) mice. Furthermore, our goal was to assess the regenerative capacity of USCs in comparison to human BMSCs.
This protocol was approved by the Institutional Review Board of Chongqing Medical University and performed in accordance with the ethical standards prescribed by the Helsinki Declaration of the World Medical Association. USCs were isolated as reported[13]. Nonpolluted samples were collected from 30 healthy donors (male; 24-28 years old). All urine samples were fresh morning urine samples, and the urine was stored at 4 °C for less than half an hour. After the collected voided urine samples were centrifuged, the cell pellets were washed with phosphate buffered saline (PBS) and plated in 24-well tissue culture plates. Medium for USCs composed of keratinocyte serum-free medium and progenitor cell medium at a 1:1 ratio was used to culture USCs. Keratinocyte serum-free medium contained 50 ng/mL bovine pituitary extract (Euroclone, Wetherby, United Kingdom) + 5 ng/mL epidermal growth factor (Gibco, Carlsbad, CA) + 30 ng/mL choleratoxin (Gibco, Grand Island, United States) + 100 U/mL penicillin (Gibco, Grand Island, United States) + 1 mg/mL streptomycin (Gibco, Grand Island, United States). Progenitor cell medium contained 1/4 Dulbecco’s modified Eagle’s medium (DMEM), 1/4 Hamm’s F12 (Gibco, Grand Island, United States) + 10% fetal bovine serum (FBS) (Gibco, Grand Island, United States) + 0.4 μg/mL hydrocortisone (Gibco, Grand Island, United States) + 5 ng/mL insulin (Corning, Bedford, United States) + 1.8 × 10-4 M adenine (Corning, Bedford, United States) + 5 μg/mL transferrin (Corning, Bedford, United States) plus 2 × 10-9 M 3,39,5-triiodo-L-thyronine (Corning, Bedford, United States) + 10 ng/mL epidermal growth factor (Gibco, Carlsbad, CA) + 1% penicillin-streptomycin (Gibco, Grand Island, United States).
Bone marrow cells were collected from healthy donors with informed consent. This protocol was approved by the Institutional Review Board of Chongqing Medical University and performed in accordance with the ethical standards prescribed by the Helsinki Declaration of the World Medical Association. BMSCs were isolated, cultured, and characterized as described previously[14]. In our study, BMSCs were plated in 24-well tissue culture plates at approximately 500 cells per well with advanced DMEM (Gibco, Grand Island, United States) with 10% FBS (Gibco, Grand Island, United States). After 5 d of culture, the medium was changed. After expansion of the BMSCs for three successive passages, cells were seeded at a density of 2 × 104 cells/cm. The cells were analyzed for immunophenotype by cytofluorimetric analysis and immunofluorescence. The osteogenic, adipogenic and chondrogenic differentiation abilities of BMSCs were determined.
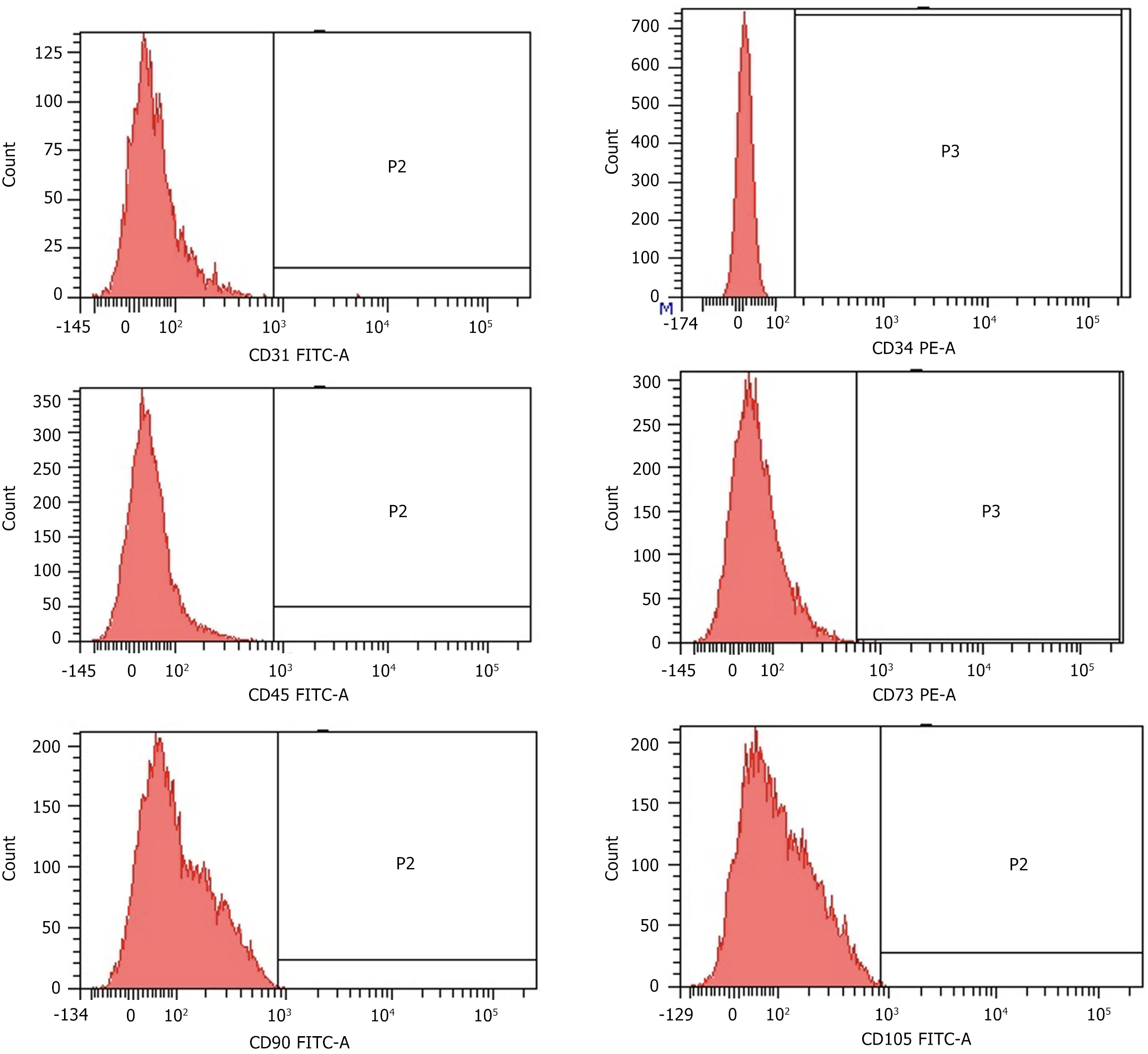
Cytofluorimetric analysis of USCs was performed as described previously[15]. At passage 3, 20000 cells were collected and suspended in 500 μL of staining buffer (SB; PBS with 1% FBS) before being incubated in the dark at 4 °C for 30 min with 20 μg/mL. Subsequently, the cells were labeled with specific anti-human antibodies including: CD31-FITC, CD34-FITC, CD45-FITC, CD73-PE, CD90-FITC, and CD105-FITC (BD Pharmingen). Isotype control antibodies IgG-FITC and IgG-PE conjugates were utilized to assess baseline fluorescence levels. Data analysis was performed using a FACSCalibur™ analytical fluorescence-activated cell sorter.
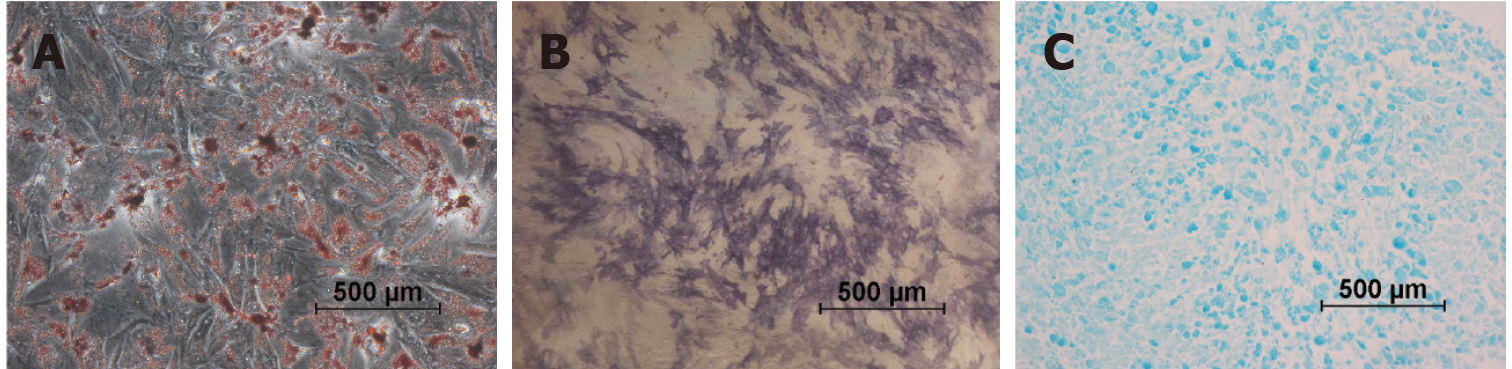
To assess the differentiation potential, USCs were stimulated to differentiate into bone, fat, and cartilage cells using the differentiation induction and maintenance medium provided by OriCell®. The process of osteogenic differentiation was evidenced through the use of alkaline phosphatase (ALP) from Sigma Aldrich, following a 7-d incubation period in osteogenic differentiation media. Adipogenic differentiation was achieved by incubating the cells in OriCell® adipogenic induction and maintenance medium, alternating every 3 to 4 d, for a total of 21 d. Upon completion of adipogenesis, the cultured cells were fixed in 4% PFA and stained with Oil Red O solution from Cyagen. For chondrogenic differentiation, chondrogenesis was induced using the pellet culture method with differentiation medium (Invitrogen). The pelleted aggregates were grown as freely floating cells kept in suspension culture for 2 to 3 wk, with the medium being changed every three days. To prepare for microscopy, the aggregates were embedded in paraffin, and then cut into 4 μm sections before being stained with Alcian blue from Sigma Aldrich.
To measure the cytokines produced by USCs and BMSCs separately, we performed a multiplex cytokine array measured by using a Proteome Profiler™ Human XL Cytokine Array Kit (R&D Systems). Specifically, when cultured to a confluence of 90%, 5 × 106 cells at passage 3 were washed with PBS three times and incubated with 10 mL serum-free DMEM in a 10 cm dish for 24 h (n = 3). Then, the medium without cells was harvested and lyophilized for detection. Special reagents (Proteome Profiler™ Human XL Cytokine Array Kit) were utilized in our study, which consisted of 55 antibodies targeting various cytokines. These antibodies were arranged in duplicate on nitrocellulose membranes, where they were designed to bind specifically to their corresponding cytokine proteins. Following the capture of the cytokine proteins, biotinylated detection antibodies were employed to identify them, and visualization was achieved through the use of chemiluminescent detection reagents. Subsequently, the membranes were exposed to X-ray film for a duration of 10 min. Quantity one software was used to record the film on the diaphragm site display and classify the cytokine. BMSCs served as a control.
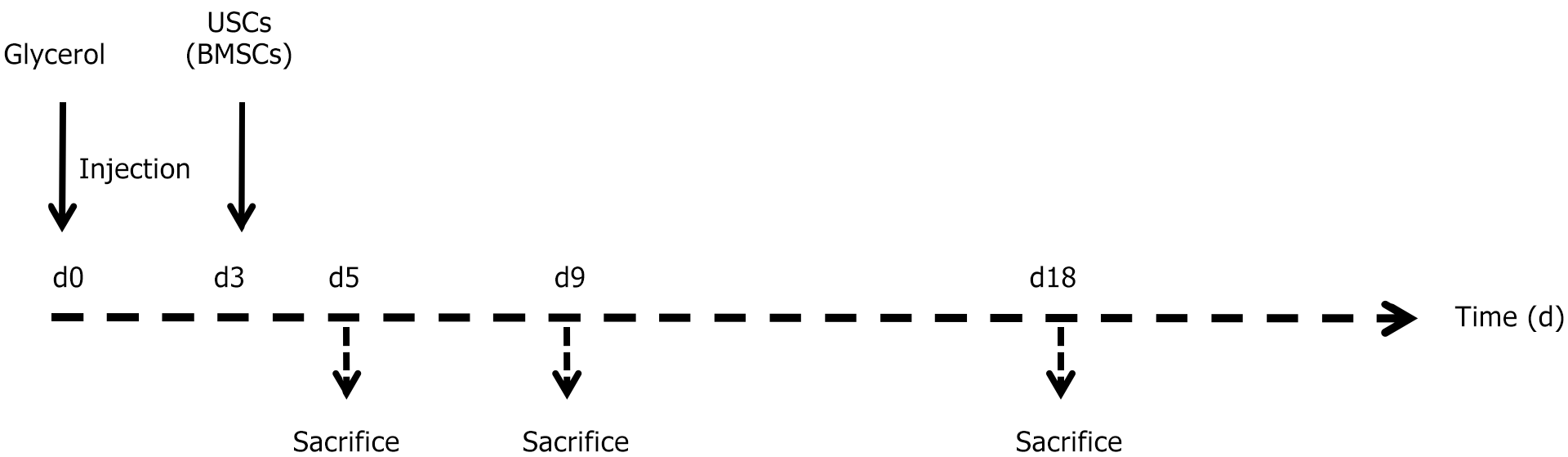
Animal experiments were executed under the policies of the Laboratory Animal Ethical Commission of Chongqing Medical University. This protocol was approved by the Institutional Review Board of Chongqing Medical University and performed in accordance with the ethical standards prescribed by the Helsinki Declaration of the World Medical Association. The rhabdomyolysis model was induced in a total of 80 male SCID (severe combined immune deficiency) BALB/c mice (Charles River, China). In short, after 24 h of free access to water, the mice (body weight 22 ± 3 g) were anesthetized and injected with a dose of 8 μL of 50% glycerol/g body weight into the left and right hind femoral muscles. Three days after induction of experimental injury, the mice were injected with 5 × 105 USCs or BMSCs (Figure 1) or saline as a control. Mice were sacrificed as follows: Day 3 (n = 5), day 5 (CTRL n = 5, USC n = 10, and BMSC n = 10), day 9 (CTRL n = 5, USC n = 10, and BMSC n = 10), and day 18 (CTRL n = 5, USC n = 10, and BMSC n = 10). The kidney tissue was fixed in 10% formaldehyde or frozen in OCT (Thermo, United States). Finally, the tissues were sectioned into 4 mm samples using a cryostat microtome. The sections were stained with hematoxylin-eosin (HE). To record and compare the renal histological structure changes, in more than 20 non-overlapping high-power fields (× 40), the quantity of luminal hyaline casts and necrosis was evaluated. The counting of casts and tubular profiles containing necrotic cells was performed in a manner where the tester was unaware of the data.
To quantify tubular apoptosis, the section was stained by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) using the TUNEL Apo-Green Detection Kit (Biotool, United States) according the manufacturer’s protocol, and the percentage of apoptotic cells was calculated.
To assess renal function, serum from sacrificed mice in metabolic cages was collected and measured for blood nitrogen and creatinine. These data were determined in the clinical laboratory of Chongqing General Hospital by using an automatic biochemical analyzer (Olympus, Japan). Blood samples were collected at various time points (days 0, 3, 5, 9, and 18), and serum was isolated from the supernatant through centrifugation at 3000 rpm for 5 min.
In order to investigate the intrarenal distribution and quantify the localized human USCs or BMSCs, cells were labeled using a Vedeng Cell Tracer Kit (Vedeng, China) and then injected into SCID mice models (n = 3 per group). The mice were euthanized at 1, 3, and 5 d post transplantation, followed by kidney tissue collection and embedding in OCT medium. Tissue sections (5 μm) were fixed in acetone (10 min). To confirm the human origin of USCs or BMSCs, kidney sections of mice injected with human leucocyte antigen (HLA) class I polyclonal antibody (Becton, Dickinson) were analyzed. HLA-positive cell scoring involved tallying the quantity of positive cells per field across 10 randomly selected segments of kidney cortex from three mice in each group, utilizing 40 × magnification. The results are presented as the count of HLA-positive cells per high-powered field (hpf).
All values are presented as the means ± SD. Statistical analyses were performed with SPSS software (version 18.0). One-way analysis of variance was applied to the differences among different mouse groups. A P value of less than 0.05 was considered statistically significant.
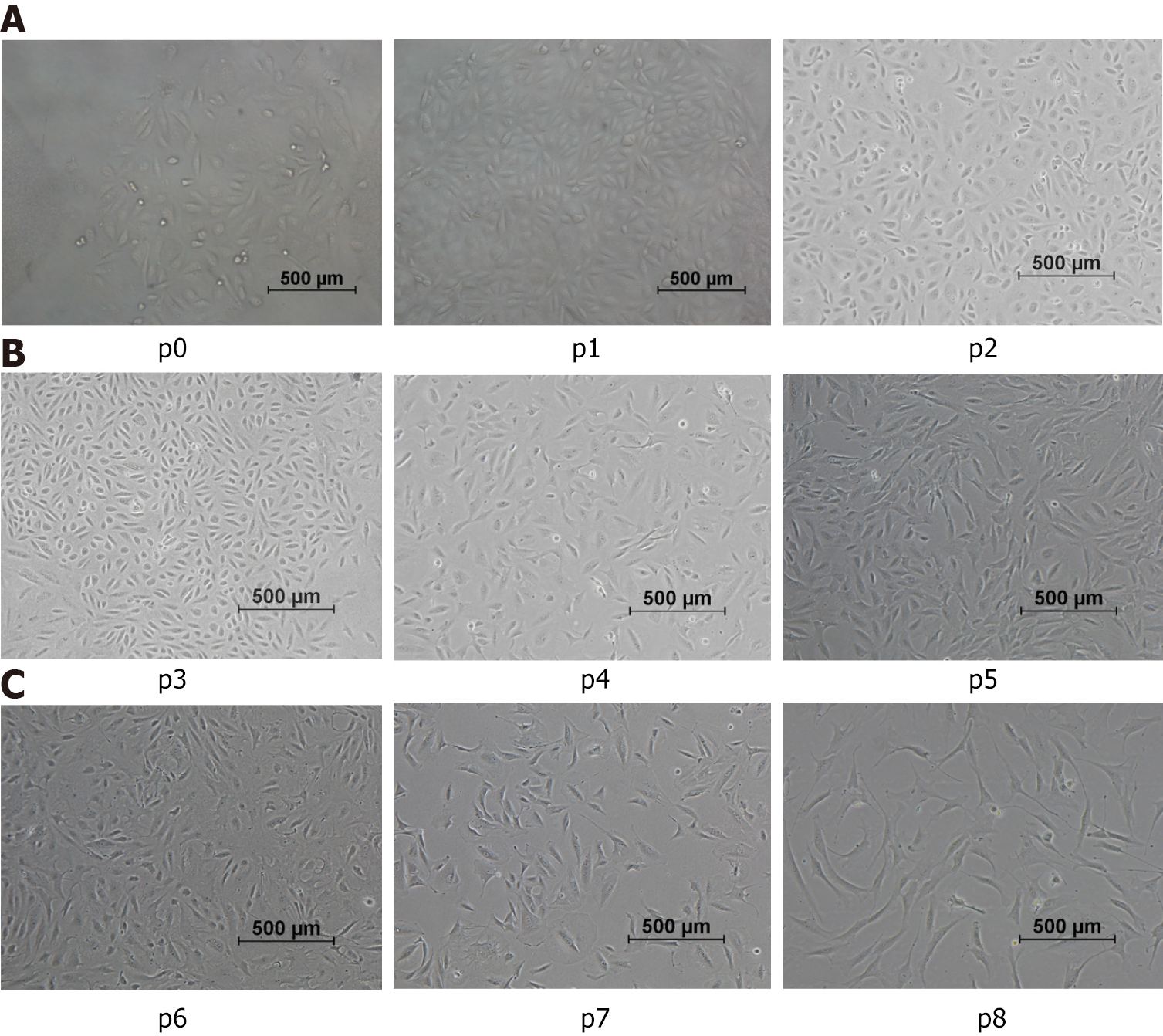
After being induced in medium for 7 d, mostly individual cells adhered to the culture plates. The cell morphologies were changed into rice-like cell clones (Figure 2, p0). Passaging culture can be performed for approximately 8-9 d, with subsequent passages conducted every 3-5 d. With an increasing passage number, the morphology of normoxic group cells gradually changed from the original “rice-like” shape to a “spindle-like” shape (Figure 2A), accompanied by an increase in cell volume. As shown in Figure 2B, the morphology of cells displayed changes that suggest a potential for diverse differentiation, as indicated by variations in shape resembling,but not confirmed to be, endothelial and smooth muscle cell morphologies. Cell populations from passages 7 through 8 maintained a fibrocyte-like morphology, exhibiting a long fusiform shape while proliferating uniformly (Figure 2C).
The USCs were acquired as detailed in a previous study and assessed based on their phenotype and differentiation potential. Analysis via flow cytometry revealed that the cells exhibited uniform weak positivity for the surface antigens CD73, CD90, and CD105, commonly observed in MSCs. USCs were negative for CD31 (a marker of endothelial cells), CD34 (a marker of hematopoietic stem cells) and CD45 (a marker of hematopoietic lineage cells and fibroblasts). A representative fluorescence-activated cell sorter analysis of USCs is shown in Figure 3.
By employing specific differentiation media, successful differentiation of USCs into osteocytes, adipocytes and chondrocytes was achieved. The differentiated cells exhibited distinct phenotypic characteristics, as demonstrated in Figure 4. Osteogenic differentiation was demonstrated by ALP staining (Figure 4A), while adipogenic differentiation was confirmed through intracellular lipid vesicles visualized by Oil Red O staining (Figure 4B). Chondrogenic differentiation in the pelletized USCs was observed through Alcian blue staining, demonstrating the presence of hyaluronic acid and sialomucin expression (Figure 4C).
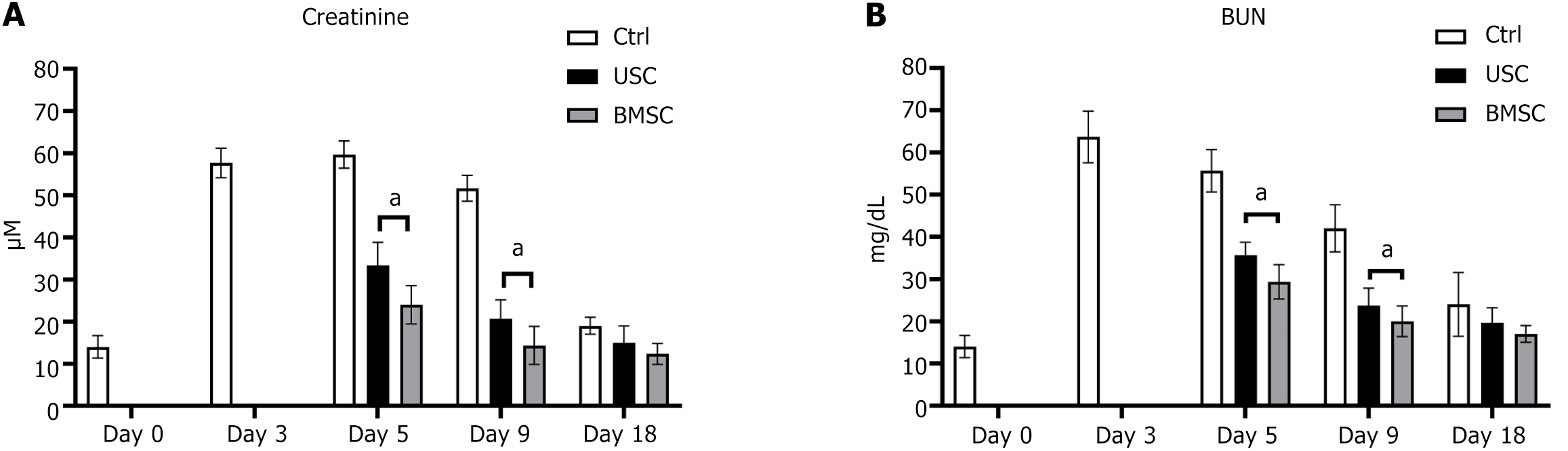
The potential beneficial effect of USCs on improving renal function and histological structure following AKI was investigated through the induction of experimental AKI via glycerol injection, as previously described[16]. Intramuscular injection of glycerol triggers rhabdomyolysis in muscular tissue, resulting in substantial release of enzymes, hemoglobin, and myoglobin to the kidney. The kidney’s exposure to myoglobin and iron subsequently leads to tubular injury. Following glycerol injection, there was an increase in serum creatinine (SCr) and blood urea nitrogen (BUN) levels, reaching a peak at 72 h and maintaining an elevated state for 10 d before normalizing on day 18. Three days post-AKI induction, mice were intravenously administered 5 × 105 USCs or BMSCs in 200 μL of PBS through the tail vein, while control animals received only PBS. The experimental timeline is depicted in Figure 1. Animals were euthanized on days 3, 5, 9, and 18 post-AKI induction.
Following the AKI procedure, within a 72-h period, the serum concentrations of creatinine and BUN (both indicative of renal dysfunction) progressively increased in the three groups. Mice that received USC injections demonstrated a significant reduction in creatinine starting from day 5 (48 h post-treatment) and continuing throughout the duration of the experiment (Figure 5A) compared to the animals in the control group. Moreover, USC treatment led to the normalization of BUN levels as early as day 5 (Figure 5B). The functional recovery observed with USCs was comparable to that achieved through the infusion of BMSCs. The results of this study suggest that USCs provide an additional benefit of protecting the kidneys from the deleterious effects of AKI.
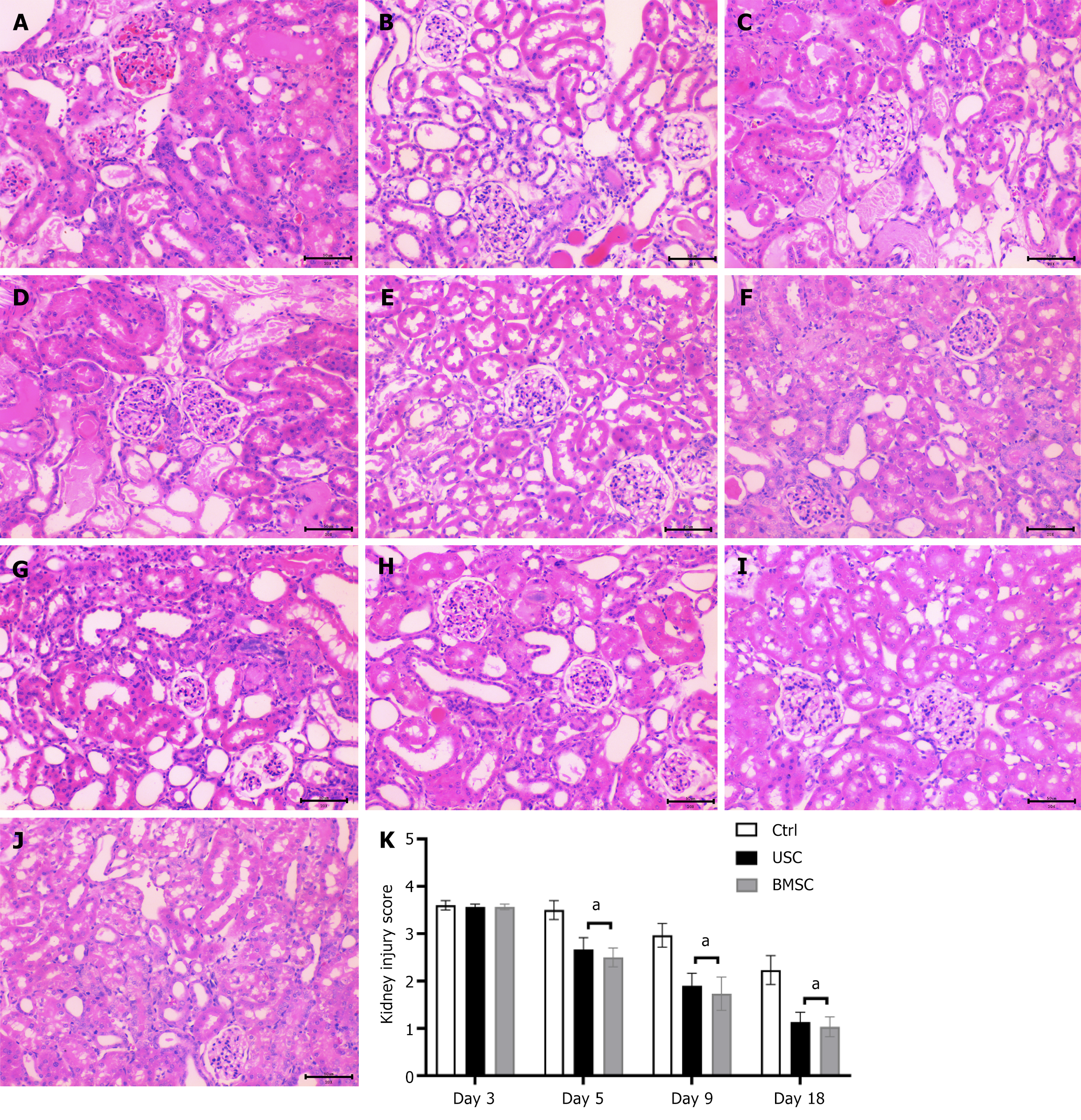
On day 3 post-injury, histological examination of the kidneys from glycerol-injected SCID mice revealed epithelial damage characterized by tubular necrosis and tubular epithelial cell vacuolization. Additionally, proximal tubular cells exhibited a loss of brush border and intratubular protein casts (Figure 6A). Mice administered USCs or BMSCs displayed a significant reduction in tissue damage compared to untreated controls. On day 5 post-glycerol injection (48 h following stem cell injection), mice treated with either USCs (Figure 6B) or BMSCs (Figure 6C) demonstrated diminished tissue damage compared to untreated control mice (Figure 6D). By the 9th d, mice that received USCs or BMSCs showed nearly normal renal structure (Figure 6E and F) in contrast to untreated animals (Figure 6G). By the 18th d, normal histology was reinstated in all the experimental groups (Figure 6H-J).
To assess the regenerative response of kidney tissue in USC-injected animals, we quantified the findings above by kidney injury score. This score indicated a significant reduction in damage and recovery of the renal structure in USC- and BMSC-injected mice compared with untreated control mice (Figure 6K). However, there was no significant difference between the USC and BMSC groups.
Table 1 presents a morphometric evaluation on day 5 after AKI induction comparing injury in AKI mice injected with or without USCs or BMSCs. Mice injected with stem cells had a significantly lower count of cast-containing tubules and fewer necrotic tubules than untreated AKI mice. There was no significant difference between mice administered USCs and those treated with BMSCs.
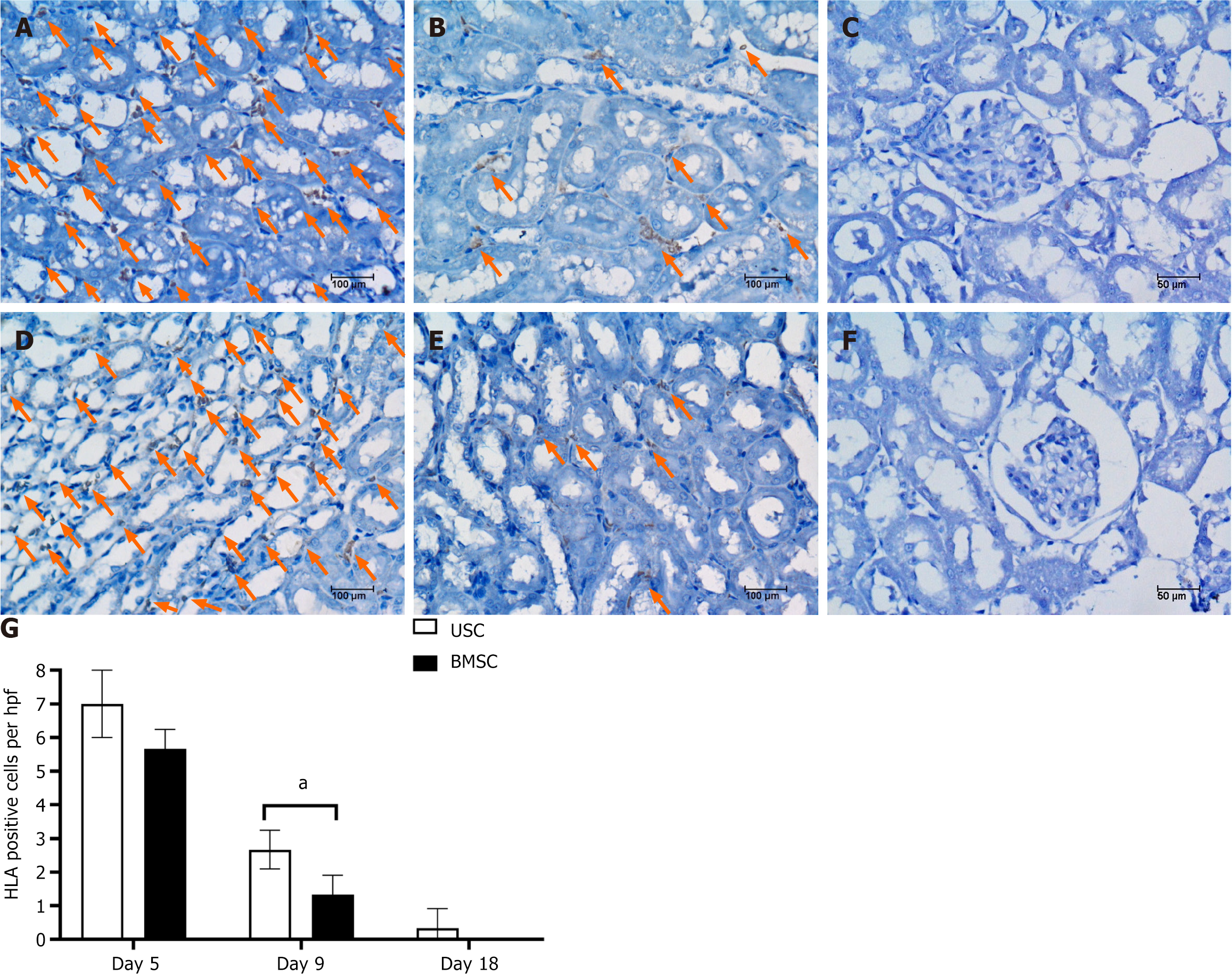
To assess the engraftment of USCs and BMSCs in the kidneys of AKI mice, the stem cells were prelabeled with the HLA Cell Tracer Kit prior to in vivo infusion. HLA-positive cells, 8 ± 0.82 USCs/hpf and 5.67 ± 0.47 BMSCs/hpf, were detected in the renal tissues of glycerol-treated mice on day 5 (2 d after cell injection) (Figure 7A and D). These cells were predominantly located in peritubular capillaries (Figure 7B and E). On AKI day 9 (6 d after cell injection), HLA-positive USCs and HLA-positive BMSCs were found at 3.14 ± 0.47 hpf and 1.33 ± 0.47 hpf, respectively. As HLA-positive USCs continued to remain significantly reduced, few USCs could also be detected on day 18, whereas BMSCs were entirely absent (Figure 7C and F). No signals were detected in HLA-positive cells within the kidneys of control mice. Quantification of HLA-positive cells is depicted in Figure 7G.
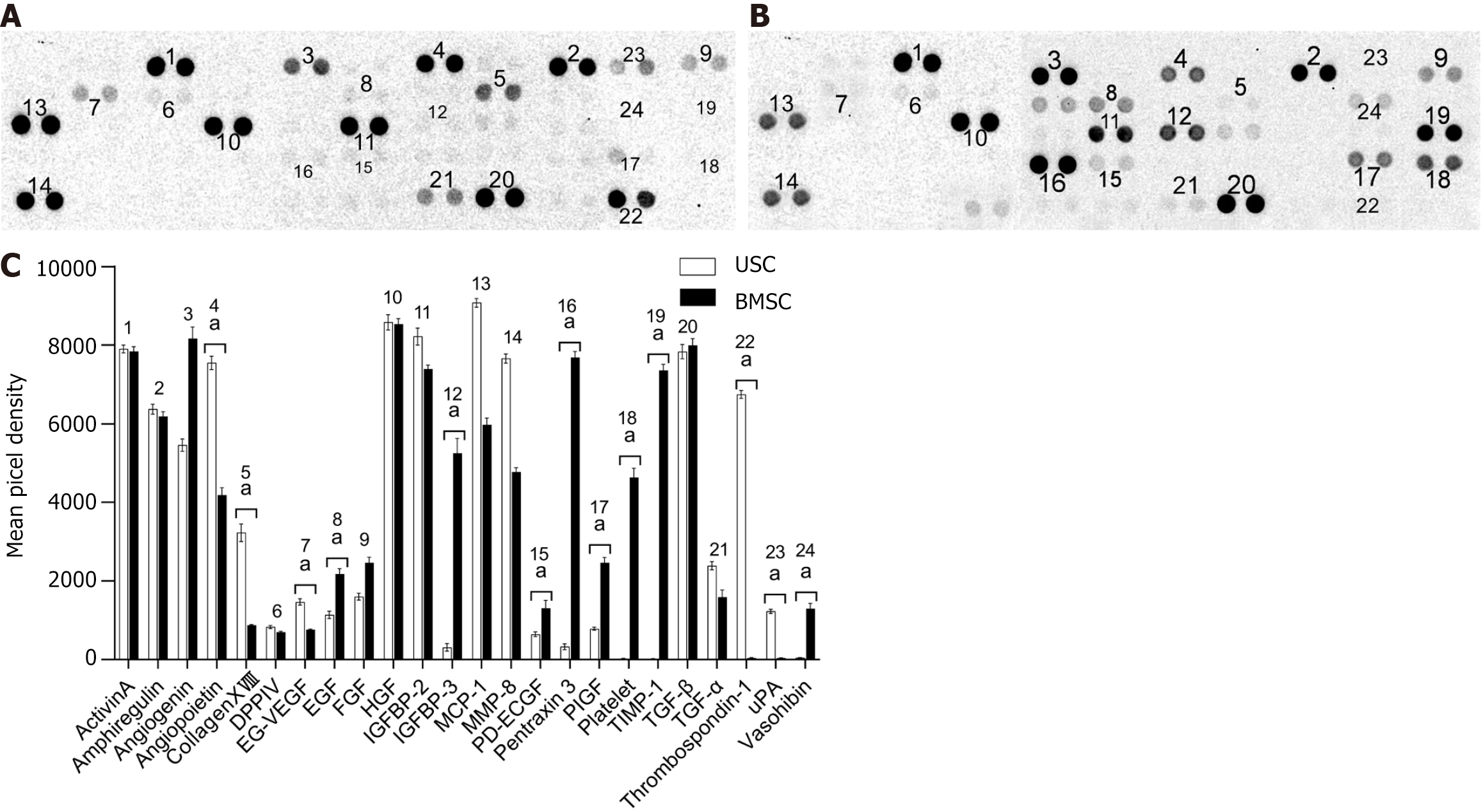
More than 20 types of angiogenesis-related proteins, such as amphiregulin, hepatocyte growth factor, insulin-like growth factor binding protein-1, transforming growth factor-β and others, were detected in the supernatant of the culture medium of USCs and BMSCs (Figure 8A and B). The results showed that the expression of certain angiogenesis-related proteins, including angiopoietin, collagen XVIII, EG-VEGF, thrombospondin-1 and uPA, in USCs was significantly higher than that in BMSCs (Figure 8C, Student’s t test: aP < 0. 05, n = 3).
Extensive studies have been conducted to explore the role and potential of stem cells in AKI. Most of the research has concentrated on BMSCs and BMSCs expanded in vitro. These cells have been utilized in different AKI mouse models[17,18]. In clinical practice, ischemia/reperfusion (I/R) injury is a major contributor to AKI, leading to acute tubular necrosis, apoptosis, and inflammation as the main pathological changes. At present, renal replacement therapy is the established method for treating renal I/R injury[19,20]. The successful healing of renal I/R injury greatly depends on replacing necrotic tubular epithelial cells with operational alternatives. However, the regenerative capacity of the kidney itself to increase the number of tubular cells after AKI is limited[21,22]. Although there have been many drugs and biological factors identified as effective in treating AKI in animal models, there is still a lack of a significantly successful new treatment that has been implemented in clinical settings[23].
MSCs were first discovered in the bone marrow and subsequently found in various tissues of the body, such as bone marrow, fat, and muscle, during development[24]. However, MSCs have some disadvantages, including ethical issues, invasive harvesting and limited donors[25]. In recent years, Zhang et al[26], the consultant of our study, first isolated a type of adult stem cell with good self-proliferation and multidirectional differentiation capabilities from voided urine; the cells were called USCs[26]. USCs express a diverse array of surface markers characteristic of MSCs and renal system markers, and they exhibit the capacity to differentiate into various cell types under specific conditions. Recognized as stem cells of immense potential, USCs have been utilized in numerous disease models[27]. For example, studies on repair of the neurogenic bladder rat model revealed that in the USC transplantation group, the bladder function is better recovered and that the repaired neurogenic bladder has the ability to spontaneously urinate, as detected through urodynamic experiments and isolated bladder detrusor muscle strip experiments[28]. Additionally, in a rabbit model with urethral defects, USCs were transplanted onto a three-dimensional tissue scaffold (SIS), and subsequent surgical evaluations conducted at the 2nd, 3rd, 4th, and 12th wk revealed significant enhancements in the urethral caliber length, urothelial regeneration rate, smooth muscle mass, and vascular density among the rats in the USC group[29]. In a rat model of diabetic nephropathy, USCs exhibited short-term survival in the kidneys, resulting in decreased levels of SCr and BUN, as well as reduced collagen deposition and inflammatory cell infiltration. Consequently, USC transplantation successfully improved renal function[30]. However, the therapeutic effect of USCs on AKI has rarely been reported thus far. In this study, we found that injection of USCs can improve renal function and histological structure in SCID mouse models of AKI. In addition, we compared this effect with that of BMSCs. Compared with BMSCs, USCs have different levels of promotion effect on histological and functional improvement of kidney injury. In addition, USCs exert an obvious antifibrotic effect on kidney cells, whereas BMSCs were more effective.
This study demonstrated that the administration of USCs was able to improve renal function and histological structure in AKI recovery in an SCID mouse model. In comparison to the control group, the group treated with USC showed notable reductions in levels of SCr and BUN, a lower Paller’s tubular injury score, a higher count of actively dividing cells, and a decreased count of cells undergoing apoptosis. In our further research, the expression levels of some anti-inflammatory factors were significantly upregulated, and the expression levels of the fibration factors were significantly reduced compared with those in the control group.
Many researchers have attempted to discover the mechanism of using MSC transplantation to treat AKI, and the prospective mechanisms primarily focus on two aspects. First, exogenous MSCs home to the site of kidney injury and proliferate and transdifferentiate into renal tubules and other cells. In some studies, researchers noticed that many transplanted cells were implanted into damaged tissues. In animal experiments, Ezquer et al[31] found that after renal I/R injury, bone marrow MSCs move to the area of damaged tubules and differentiate into tubular epithelial cells. In a rat model using cisplatin to induce acute renal failure in mice, Morigi et al[32] found that treatment by injection of MSCs can improve renal function and that MSCs can directly participate in renal reconstruction by transdifferentiating into tubular cells. Duffield et al[33] observed that injected exogenous BMSCs existed only in the middle area of endothelial cells near the capillaries of renal tubules and expressed the endothelial cell surface markers CD31 and VWf. Second, the cytokines secreted by exogenous BMSCs play a crucial role in reducing inflammation and promoting the repair of kidney injury. Among the diverse cytokines, the production of inflammatory cytokines is considered a critical factor in the occurrence and development of AKI. The effect of MSCs is mainly mediated by complex paracrine effects, including the secretion of beneficial factors and the activation of signaling proteins[34]. The paracrine/endocrine effects of stem cells are supported by the following evidence: A previous study elucidated the potential of adipose-derived stem cell therapy in improving functional parameters and attenuating the advancement of renal fibrosis in both the initial and later stages following injury. In addition, the therapy was found to reduce the levels of interleukin (IL)-6 and tumor necrosis factor (TNF), while simultaneously boosting the levels of IL-4, IL-10, and heme oxygenase-1[35]. Kim et al[36] reported that culture medium from MSCs alone has a beneficial effect on the regeneration of kidney injury; Bruno et al[14] showed that the release of microvesicles may account for the positive effect of MSCs in the recovery of AKI.
BMSCs also affect T-cell-mediated inflammation. Research shows that cocultivation of BMSCs with Th1, Th2 or natural killer cells can reduce the secretion of inflammatory factors such as TNF-α and interferon-γ and increase the secretion of tolerogenic and inhibitory factors such as IL-10[37]. These effects are mediated by BMSC production of prostaglandin E2.
Therefore, the therapeutic effect of BMSCs on AKI is related to their role in regulating T-cell function. During the treatment of animal kidney I/R injury with BMSCs, Semedo et al[38] found that the concentration of anti-inflammatory cytokines in kidney extracts increased, which supports the previous view. Furthermore, by analyzing the cytokines and human angiogenesis-related factors released by USCs and BMSCs in conditioned medium, it was shown that they had differences in the series of factors secreted. Specifically, USCs produce lower angiogenesis-related growth factors and cytokines than BMSCs but more matrix metalloproteinases. Matrix metalloproteinases have been proven to be a crucial factor for the repair of kidney tissue after AKI. Different growth factor combinations may be responsible for the difference in tubular epithelial cell proliferation and apoptosis after stem cell therapy.
In the present study, we tried to explain the difference in the protective mechanism of USCs and BMSCs by observing the dynamics of kidney localization in vivo and the production of different soluble factors in vitro. We found that neither USCs nor BMSCs were integrated into the kidney tissue but remained in the renal interstitium for a short time. Previous studies have also presented similar findings regarding MSCs[39,40]. Essentially, the positioning of transplanted stem cells near the tubules indicates that repair mechanisms for different cell types largely depend on the localized discharge of soluble factors, rather than exclusively depending on tissue integration.
To summarize, this study successfully demonstrated the ability of USCs to differentiate into renal cells and alleviate renal I/R injury in rats. The therapeutic effects of USCs were primarily attributed to their antiapoptotic and mitogenic properties, as well as their influence on cytokine production. The positive outcomes achieved in treating I/R injury with USCs strongly suggest their potential as a highly suitable stem cell option for the treatment of kidney ailments.
In conclusion, this investigation revealed that USCs improved the renal function and histological structure in AKI recovery. The anti-apoptotic and mitogenic actions of USCs mediated their effects, along with their impact on cytokine production. The successful treatment of AKI with USCs strongly indicates that USCs may be a well-suited type of stem cell for the treatment of kidney disease. Further studies are needed to investigate the mechanism and clinical applications.
The authors express their gratitude to the individuals who participated in this study.
| 1. | Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14:607-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 963] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 2. | Farrar A. Acute Kidney Injury. Nurs Clin North Am. 2018;53:499-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med. 2008;36:S146-S151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Mata-Miranda MM, Bernal-Barquero CE, Martinez-Cuazitl A, Guerrero-Robles CI, Sanchez-Monroy V, Rojas-Lopez M, Vazquez-Zapien GJ. Nephroprotective Effect of Embryonic Stem Cells Reducing Lipid Peroxidation in Kidney Injury Induced by Cisplatin. Oxid Med Cell Longev. 2019;2019:5420624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Chen X, Huang J, Wu J, Hao J, Fu B, Wang Y, Zhou B, Na T, Wei J, Zhang Y, Li Q, Hu S, Zhou J, Yu J, Wu Z, Zhu H, Cao J, Wang L, Peng Y, Liang L, Ma A, Zhao T, Xiang AP. Human mesenchymal stem cells. Cell Prolif. 2022;55:e13141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Ashour RH, Saad MA, Sobh MA, Al-Husseiny F, Abouelkheir M, Awad A, Elghannam D, Abdel-Ghaffar H, Sobh M. Comparative study of allogenic and xenogeneic mesenchymal stem cells on cisplatin-induced acute kidney injury in Sprague-Dawley rats. Stem Cell Res Ther. 2016;7:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Osafune K. iPSC technology-based regenerative medicine for kidney diseases. Clin Exp Nephrol. 2021;25:574-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Wan Q, Xiong G, Liu G, Shupe TD, Wei G, Zhang D, Liang D, Lu X, Atala A, Zhang Y. Urothelium with barrier function differentiated from human urine-derived stem cells for potential use in urinary tract reconstruction. Stem Cell Res Ther. 2018;9:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 9. | Tu M, Wang R, Zhu P, Wang Q, Sun B, Lu K, Zhang J, Xie W, Guo H, Li S, Wu Y, Wang X. Human Urine-Derived Stem Cells Improve Partial Bladder Outlet Obstruction in Rats: Preliminary Data and microRNA-mRNA Expression Profile. Stem Cell Rev Rep. 2022;18:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Zhao Z, Liu D, Chen Y, Kong Q, Li D, Zhang Q, Liu C, Tian Y, Fan C, Meng L, Zhu H, Yu H. Ureter tissue engineering with vessel extracellular matrix and differentiated urine-derived stem cells. Acta Biomater. 2019;88:266-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Kang HS, Choi SH, Kim BS, Choi JY, Park GB, Kwon TG, Chun SY. Advanced Properties of Urine Derived Stem Cells Compared to Adipose Tissue Derived Stem Cells in Terms of Cell Proliferation, Immune Modulation and Multi Differentiation. J Korean Med Sci. 2015;30:1764-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Xiong G, Tang W, Zhang D, He D, Wei G, Atala A, Liang XJ, Bleyer AJ, Bleyer ME, Yu J, Aloi JA, Ma JX, Furdui CM, Zhang Y. Impaired Regeneration Potential in Urinary Stem Cells Diagnosed from the Patients with Diabetic Nephropathy. Theranostics. 2019;9:4221-4232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 13. | Ouyang B, Sun X, Han D, Chen S, Yao B, Gao Y, Bian J, Huang Y, Zhang Y, Wan Z, Yang B, Xiao H, Songyang Z, Liu G, Deng C. Human urine-derived stem cells alone or genetically-modified with FGF2 Improve type 2 diabetic erectile dysfunction in a rat model. PLoS One. 2014;9:e92825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 1048] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 15. | Culenova M, Nicodemou A, Novakova ZV, Debreova M, Smolinská V, Bernatova S, Ivanisova D, Novotna O, Vasicek J, Varga I, Ziaran S, Danisovic L. Isolation, Culture and Comprehensive Characterization of Biological Properties of Human Urine-Derived Stem Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035-1041. [PubMed] |
| 17. | Zhou S, Qiao YM, Liu YG, Liu D, Hu JM, Liao J, Li M, Guo Y, Fan LP, Li LY, Zhao M. Bone marrow derived mesenchymal stem cells pretreated with erythropoietin accelerate the repair of acute kidney injury. Cell Biosci. 2020;10:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Yang Y, Gao J, Wang S, Wang W, Zhu FL, Wang X, Liang S, Feng Z, Lin S, Zhang L, Chen X, Cai G. Efficacy of umbilical cord mesenchymal stem cell transfusion for the treatment of severe AKI: a protocol for a randomised controlled trial. BMJ Open. 2022;12:e047622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Corona A, Cattaneo D, Latronico N. Antibiotic Therapy in the Critically Ill with Acute Renal Failure and Renal Replacement Therapy: A Narrative Review. Antibiotics (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 20. | Wang Z, Zhang C. From AKI to CKD: Maladaptive Repair and the Underlying Mechanisms. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 21. | Yao M, Qin S, Xiong J, Xin W, Guan X, Gong S, Chen J, Liu Y, Zhang B, Zhao J, Huang Y. Oroxylin A ameliorates AKI-to-CKD transition through maintaining PPARα-BNIP3 signaling-mediated mitochondrial homeostasis. Front Pharmacol. 2022;13:935937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 22. | Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16:269-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 656] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 23. | Li N, Wang Y, Wang X, Sun N, Gong YH. Pathway network of pyroptosis and its potential inhibitors in acute kidney injury. Pharmacol Res. 2022;175:106033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 24. | Al-Azab M, Idiiatullina E, Safi M, Hezam K. Enhancers of mesenchymal stem cell stemness and therapeutic potency. Biomed Pharmacother. 2023;162:114356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 25. | Turner L, Snyder J. Ethical issues concerning a pay-to-participate stem cell study. Stem Cells Transl Med. 2021;10:815-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (3)] |
| 27. | Zhang D, Wei G, Li P, Zhou X, Zhang Y. Urine-derived stem cells: A novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014;1:8-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 28. | Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, Fan Y, Lu X, Zhou X, Liu H, Atala A, Rohozinski J, Zhang Y. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells. 2013;31:1840-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 29. | Liu Y, Ma W, Liu B, Wang Y, Chu J, Xiong G, Shen L, Long C, Lin T, He D, Butnaru D, Alexey L, Zhang Y, Zhang D, Wei G. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res Ther. 2017;8:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 30. | Dong X, Zhang T, Liu Q, Zhu J, Zhao J, Li J, Sun B, Ding G, Hu X, Yang Z, Zhang Y, Li L. Beneficial effects of urine-derived stem cells on fibrosis and apoptosis of myocardial, glomerular and bladder cells. Mol Cell Endocrinol. 2016;427:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Ezquer F, Giraud-Billoud M, Carpio D, Cabezas F, Conget P, Ezquer M. Proregenerative Microenvironment Triggered by Donor Mesenchymal Stem Cells Preserves Renal Function and Structure in Mice with Severe Diabetes Mellitus. Biomed Res Int. 2015;2015:164703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 536] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 33. | Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (5)] |
| 34. | Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31-F42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 884] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 35. | Köken T, Serteser M, Kahraman A, Akbulut G, Dilek ON. Which is more effective in the prevention of renal ischemia-reperfusion-induced oxidative injury in the early period in mice: interleukin (IL)-10 or anti-IL-12? Clin Biochem. 2004;37:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Kim YS, Aum J, Kim BH, Jang MJ, Suh J, Suh N, You D. Therapeutic Effect of Three-Dimensional Cultured Adipose-Derived Stem Cell-Conditioned Medium in Renal Ischemia-Reperfusion Injury. Int J Stem Cells. 2023;16:168-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 38. | Semedo P, Wang PM, Andreucci TH, Cenedeze MA, Teixeira VP, Reis MA, Pacheco-Silva A, Câmara NO. Mesenchymal stem cells ameliorate tissue damages triggered by renal ischemia and reperfusion injury. Transplant Proc. 2007;39:421-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 396] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 40. | Morigi M, De Coppi P. Cell therapy for kidney injury: different options and mechanisms--mesenchymal and amniotic fluid stem cells. Nephron Exp Nephrol. 2014;126:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Li SC, United States; Mohammadzadeh I, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD