Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.499
Revised: January 17, 2024
Accepted: April 2, 2024
Published online: May 26, 2024
Processing time: 176 Days and 5.5 Hours
Bone healing is a complex process involving early inflammatory immune regu
To investigate the underlying mechanism by which hydrogel-loaded exosomes derived from bone marrow mesenchymal stem cells (BMSCs) facilitate the process of fracture healing.
Hydrogels and loaded BMSC-derived exosome (BMSC-exo) gels were characterized to validate their properties. In vitro evaluations were conducted to assess the impact of hydrogels on various stages of the healing process. Hydrogels could recruit macrophages and inhibit inflammatory responses, enhance of human umbilical vein endothelial cell angiogenesis, and promote the osteogenic differentiation of primary cranial osteoblasts. Furthermore, the effect of hydrogel on fracture healing was confirmed using a mouse fracture model.
The hydrogel effectively attenuated the inflammatory response during the initial repair stage and subsequently facilitated vascular migration, promoted the formation of large vessels, and enabled functional vascularization during bone repair. These effects were further validated in fracture models.
We successfully fabricated a hydrogel loaded with BMSC-exo that modulates macrophage polarization and angiogenesis to influence bone regeneration.
Core Tip: We adopted a new method to enhance tissue repair and promote bone regeneration through hydrogels loaded with mesenchymal stem cell (MSC) exosomes. This experiment demonstrated that bone marrow-derived MSC (BMSC)-derived exosome (BMSC-exo) hydrogel significantly promoted the proliferation, migration and osteogenesis of mouse osteoblast progenitor cells, and at the same time enhanced the M2 polarization of macrophages in bone marrow, thus translating into accelerated fracture healing and angiogenesis in vivo. In addition, BMSC-exo hydrogels successfully enhance the strength and toughness of regenerated bone, with higher maximum load, stiffness and damage absorption energy.
- Citation: Zhang S, Lu C, Zheng S, Hong G. Hydrogel loaded with bone marrow stromal cell-derived exosomes promotes bone regeneration by inhibiting inflammatory responses and angiogenesis. World J Stem Cells 2024; 16(5): 499-511
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/499.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.499
Repair of large bone defects remains challenging for orthopedic surgeons[1]. Autogenous bone grafts currently represent the gold standard treatment for bone defects; however, their use is limited by resource scarcity and complications associated with the harvesting process[2]. Recently, the use of exosomes derived from mesenchymal stem cells (MSCs) has garnered significant attention in bone regenerative medicine. MSCs have inherent self-healing capabilities and multidirectional differentiation potential, and are regarded as promising therapeutic strategies for numerous diseases. Extracellular vesicles, such as exosomes derived from these multifunctional cells, are considered capable of promoting tissue regeneration[3]. However, because of their rapid degradation, short half-life, and unpredictable side effects, these exogenous factors are difficult to widely use in clinical practice[4]. To facilitate the potential of extracellular vesicles, hydrogels loaded with MSC-exosomes have emerged as a novel approach, demonstrating augmented proregenerative effects on repaired tissue[3].
Bone marrow-derived MSC (BMSC), a type of MSC, play a crucial role in osteogenesis and tissue repair[5]. BMSCs can migrate from their niche into the peripheral circulation and cross vessel walls to reach target tissues[6,7]. The homing and repair ability of BMSCs in injured tissues is a complex process. Previous studies have demonstrated that angiogenesis factors interact with BMSCs, thereby regulating downstream osteogenesis. Specifically, vascular endothelial growth factor A (VEGFA) stimulates platelet-derived growth factor receptors, thus modulating the migration and proliferation of BMSCs[8].
Furthermore, the polarization of M1/M2 macrophages can contribute to the process whereby BMSC-derived exosomes (BMSC-exos) promote bone healing[9]. MSCs can polarize macrophages to an anti-inflammatory M2 phenotype via paracrine mechanisms to facilitate the regenerative healing of tissues[10].
Given the established evidence of BMSC-exos promoting tendon-bone healing in previous studies, this study investigated the underlying mechanism whereby BMSC-exos influence fracture repair. Exosomes were encapsulated within hydrogels to establish a potentially stable therapeutic system, whose efficacy was subsequently validated in murine fracture models[9,11]. Given the crucial roles of vascularization and macrophage polarization in bone regeneration, this study provides insights into the intricate interplay between BMSC-exos, angiogenesis, and macrophage regulation[12].
All animal studies were performed according to the principles and procedures approved by the Ethics Committee of Zhejiang University of Chinese Medicine (Zhejiang, China). C57BL/6 mice within 24 h of birth were used to acquire osteoblasts, and 8-wk-old male BALB/c mice were used to establish a fracture model. After acclimatization, BALB/c mice were operated on under anesthesia. An 8-mm long skin incision was made on the femur, and blunt dissection of the muscle was used to expose the midshaft of the femur. Mid-diaphyseal femur fractures were created using a rotary Dremel with a diamond blade attachment. A 25-gauge needle was inserted into the medullary canal of the femur from the distal end. BMSC-exo hydrogels (20 μL) were wrapped around the fractured femur, and the incision was closed with nylon sutures. Buprenorphine (0.05 mg/kg IP) was administered every 6-12 h over 3 d postfracture. After 30-d maintenance, callus formation and fracture healing in mice were detected by radiographs and micro-computed tomography.
BMSCs (ATCC PCS-500-012) were plated at a density of 60%-80% confluence in RPMI-1640 supplemented with 15% foetal bovine serum (FBS). Human embryonic kidney cells (HEK293, ATCC CRL-1573) were grown in Dulbecco’s modified Eagle medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were cultured at 37 °C in a 5% CO2 atmosphere for at least 24 h before further processing.
Mouse osteoblast progenitor cells (mOPCSs) were isolated from the calvaria of newborn mice. Briefly, mice were decapitated within 24 h after birth. Non-osseous tissue, dura mater, and the periosteum were then thoroughly removed to harvest the calvaria. After washing with phosphate buffered saline (PBS) and digesting with 0.1% collagenase A plus 0.2% dispase, bone fragments were spread on 100-mm culture plates and cultured in alpha minimal essential medium supplemented with 10% FBS to release osteoblasts. The medium was placed at 37 °C in a 5% CO2 atmosphere and refreshed every 2 d. Second-generation cells were then used in this study. Mouse RAW264.7 macrophages (ATCC TIB-71) were used to determine the influence of BMSC-exo hydrogels on macrophage polarization.
Exosomes were isolated from the cell culture medium as previously described. Exosomes were isolated and purified from the supernatants of BMSC and HEK293 cells via ultracentrifugation. The morphology of the exosomes was observed via transmission electron microscopy (TEM, Hitachi). The size distribution and concentration of exosomes were analyzed using nanoparticle tracking analysis technology, and the specific markers of exosomes were identified via western blotting.
Hydrogels were prepared as described in previous studies[13]. A 3% (w/w) solution of glycol chitosan (GCS) was prepared by dissolving a specific amount of chitosan in distilled and deionized water. The freeze-dried silk fibroin (SF) powder was then dissolved in this chitosan solution to produce a 2% SF and 3% GCS mixed solution. Subsequently, chondroitin-6-sulfate (CHA) was added to the mixed solution, forming a viscous solution. A 20% (w/w) solution of dialysis-purified maleic anhydride-modified polyethylene glycol (DF-PEG) (2.0 g of DF-PEG dissolved in 8.0 g of distilled and deionized waste) was prepared, and 1 mL of DF-PEG solution was then added to the GCS (2.8 g)/SF (1.86 g)/CHA (5 g) solution. The resulting mixture was continuously stirred, and within 60 s, the hydrogel formed at room temperature.
Hydrogel characterization was conducted using scanning electron microscopy (SEM), X-ray diffraction (XRD), energy-dispersive spectroscopy (EDS), and Fourier-transform infrared (FTIR) analyses. Briefly, the prepared CHA/SF/GCS/DF-PEG hydrogel was first prefrozen at -20 °C for 24 h and was then moved to -80 °C for another 24 h, followed by lyophilization at -55 °C until the water was completely sublimated. An FE-SEM system equipped with an EDS system was used to examine the surface structure and morphology of the freeze-dried hydrogels using EDS. The freeze-dried CHA/SF/GCS/DF-PEG hydrogel was crushed and ground to a particle size for sifting through a 300 mesh, and the powder was then analyzed via XRD and FTIR to determine the phase composition of the material.
Protein samples were mixed with 5× loading buffer and heated at 100 °C for 10 min in a water bath. Marker and protein samples were loaded onto a polyacrylamide gel for electrophoresis. The protein was then transferred to a polyvinylidene fluoride membrane and blocked with 5% skim milk powder at room temperature for 1 h. The primary antibody was added for overnight incubation at 4 °C. After incubation, the horseradish-peroxidase-conjugated secondary antibody was incubated at room temperature for 2 h. Immunoreactions were detected using enhanced chemiluminescence detection.
BMSC-exo and HEK293-derived exosomes (HEK293-exo) were fluorescently labeled with PKH256 red dye (Sigma-Aldrich) for tracking of cellular internalization via fluorescence microscopy. Labeled exosomes were supplemented with mOPCSs, incubated for 6 h, and stained with 4’,6’-diaminido-2-phenylindole (DAPI, Thermo Fisher). A fluorescence microscope (Olympus) was used to observe the uptake of labeled exosomes by recipient mOPCSs.
The alkaline phosphatase (ALP) activity kit was employed to assess the osteogenesis of mOPCSs. ECM mineralization was determined by Alizarin red (AR) staining. On days 7 and 14, mOPCSs were stained with 40 mmol/L AR in Tris-HCl solution (pH = 4.2) for 10 min at room temperature, followed by three washes with DI water. For quantitative analysis, 10% cetylpyridinium chloride was added to dissolve the AR staining, and the absorbance was measured at 542 nm. All stained images were captured using a stereomicroscope.
Cell proliferation was analyzed by using an EdU assay. Briefly, mOPCSs with different treatments were incubated in an EdU assay (Ribobio) for 2 h at 37 °C and then incubated with DAPI at room temperature for 5 min. Finally, cells were mounted and imaged under a fluorescence microscope.
Cell migration was determined by Transwell and scratch wound assays. For Transwell assays, after transfection and treatment with high glucose, mOPCSs with different treatments were seeded into the upper chamber of 12-well Transwell plates (2.5 × 103 cells/well) while the lower chamber contained culture medium. After a 24-h incubation period, cells on the upper surface of the filter were removed, and migrated cells in the lower chamber were fixed, stained with crystal violet, and observed under a microscope.
Scratch wound assays were performed in 6-well plates. mOPCSs were seeded in 6-well plates at 1 × 105 cells/well and cultured in serum-free medium to reach 90% confluence. Cells were then starved for 24 h. A 10-μL pipette tip was used to create a scratch in the plate, simulating a wound. mOPCSs were then cultured for an additional 24 or 48 h with PBS or hydrogels with or without BMSC-exo. The area of the blank space at 0, 24, and 48 h was measured using ImageJ software to calculate the migration rate.
Angiogenesis was assessed by cell migration and tube formation. For the tube formation assays, mOPCSs were suspended in pregel solutions (8 mg/mL) at 2 × 106 mL. The suspension was then transferred to a 24-well culture plate and incubated for 30 min at 37 °C to form a cell-loaded hydrogel. After 14 d of culture, immunofluorescence staining was performed. The primary antibody was used to locate endothelial cells. The 96-well plates were precoated with Matrigel at 37 °C for 2 h, and 100 μL of human umbilical vein endothelial cell (HUVEC) suspension was added per well with hydrogel solutions. After 6-h incubation, an inverted microscope was used for cell observation and photographing. Three different fields per well of tube formation were randomly selected and photographed for calculation, and the branch points of capillaries surrounded by cells were quantified using Image J software.
Data were analyzed using SPSS 21.0 software. The normality of the data distribution was assessed using the Z test, and data conforming to a normal distribution are presented as mean ± SD. For comparison between two groups, Student’s t-test was employed, and one-way ANOVA was used for comparisons involving three or more groups. Post hoc pairwise comparisons were conducted using Tukey’s test. Significance was defined as P < 0.05.
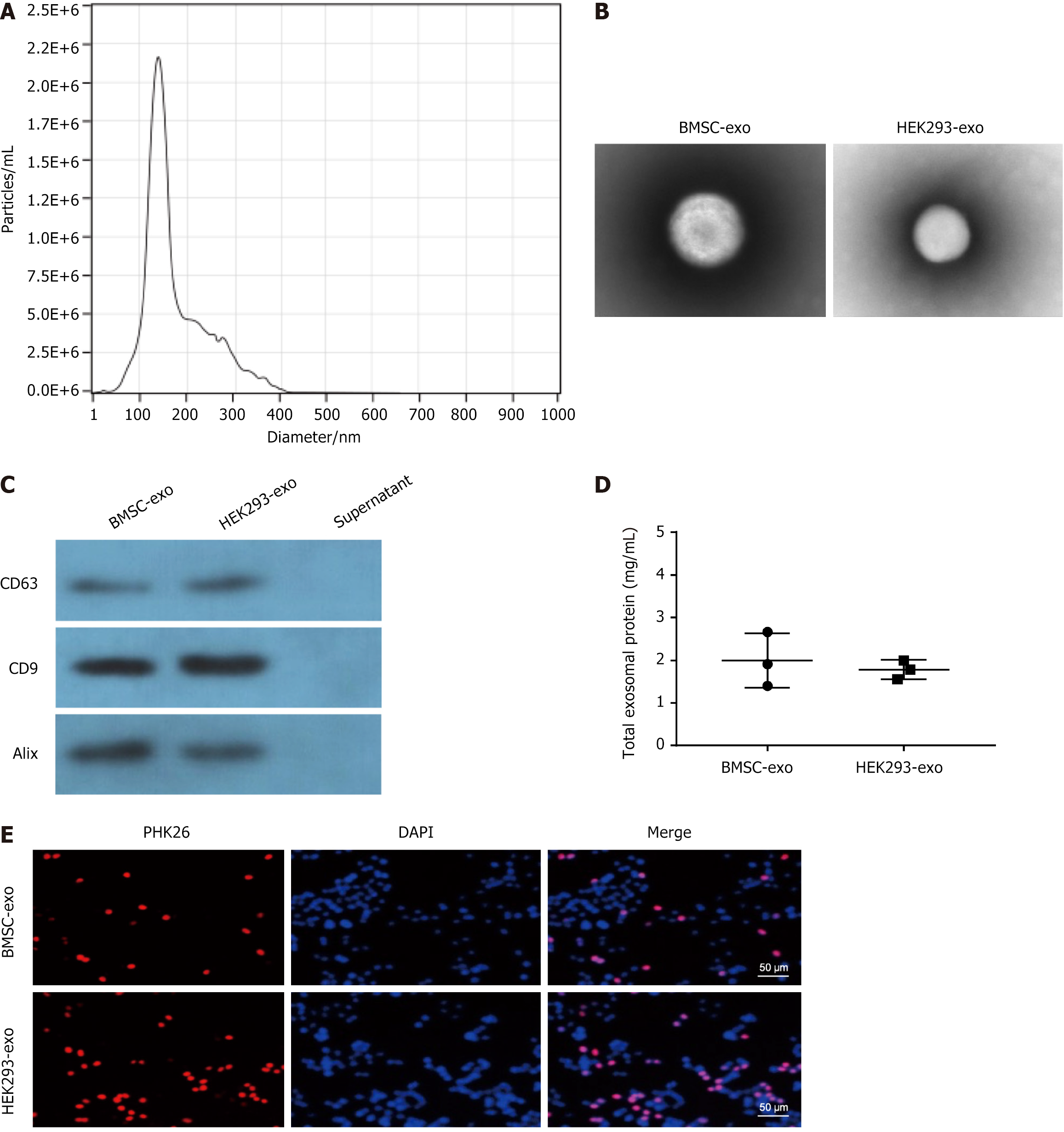
Exosomes were isolated and purified from the supernatants of BMSC or HEK293 cells, followed by identification using TEM. The analysis revealed an average particle diameter of 150 nm (Figure 1A and B). The presence of specific axosomal markers, including CD63, CD9, and ALIX, was confirmed in both BMSC-exo and HEK293-exo but was absent in the residual supernatant (Figure 1C). The total axosomal protein was also confirmed in BMSC-exo and HEK293-exo (Figure 1D). The PKH26-labeled BMSC-exo and HEK293-exo were effectively internalized by mOPCSs as shown via fluorescence microscopy (Figure 1E).
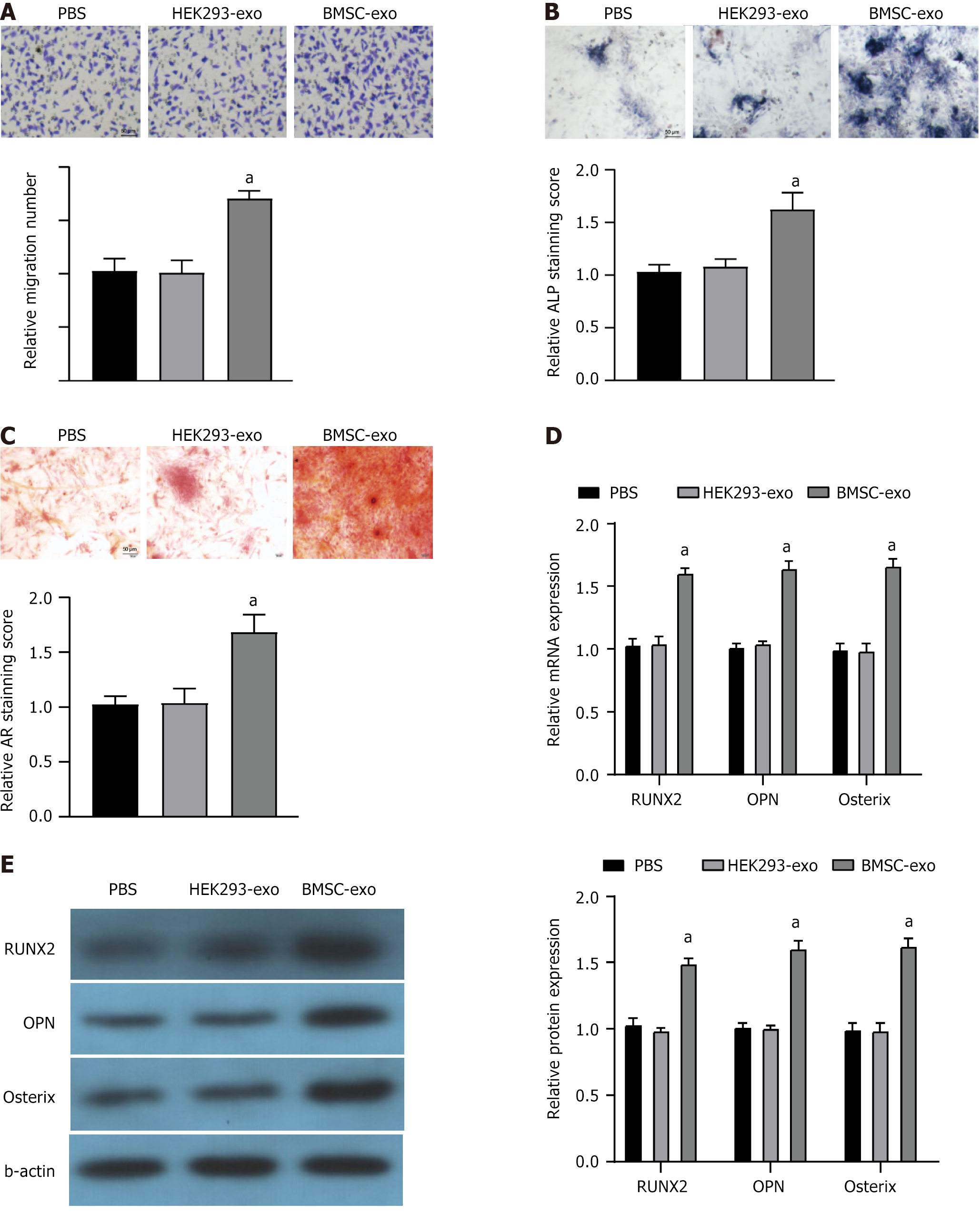
Based on the Transwell cell migration assay, BMSC-exo enhanced migration, whereas HEK293-exo did not affect the migration of mOPCSs (Figure 2A). The ALP and AR staining of mOPCSs treated by BMSC-exo indicated the formation of dense mineral nodules and a high degree of mineralization (Figure 2B and C). BMSC-exo significantly upregulated the expression of genes associated with osteogenesis (Runx2, OPN, and Osterix) in mOPCSs, whereas HEK293-exo-treated mOPCSs expressed similar levels of the above genes as those of PBS control (Figure 2D and E). Therefore, BMSC-exo was selected for hydrogel formation and used in subsequent trials.
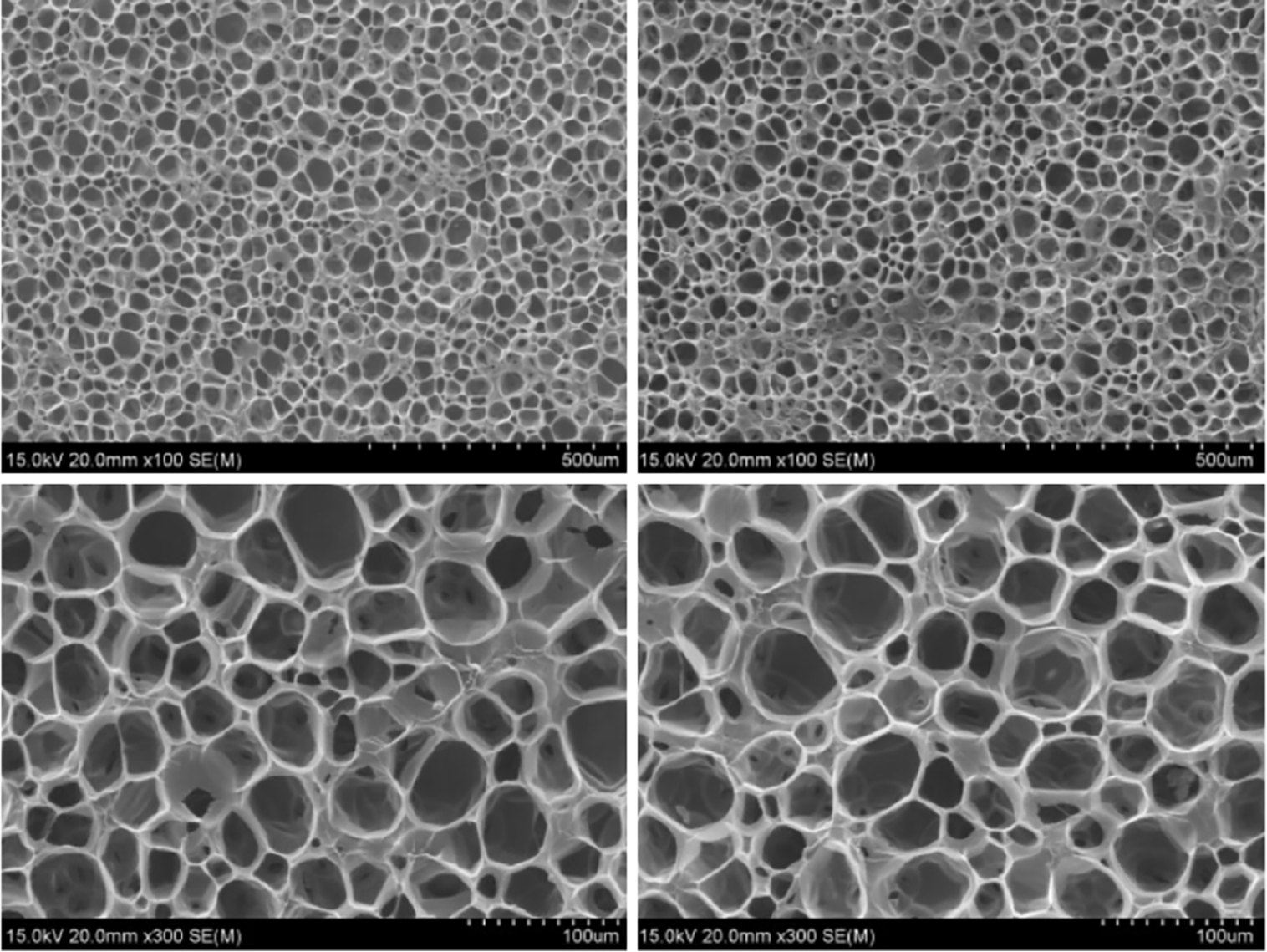
The analysis of SEM images revealed that the pores in the CHA/SF/GCS/DF-PEG hydrogel exhibited predominantly circular or elliptical shapes with uniform sizes and were well interconnected. The pore walls demonstrated consistent thickness while the surface appeared smooth with a porosity of 91.24% ± 12.85%. Furthermore, the coral remained intact without any signs of cracking. Additionally, SEM images showed an abundance of spherical crystals adhering to the surface of CHA following hydrothermal reaction treatment (Figure 3). Collectively, these data confirm the successful preparation of the CHA/SF/GCS/DF-PEG hydrogel. Supplementary Figure 1 indicates that the prepared hydrogel exerted no side effects on biological processes, including osteogenesis, chondrogenesis, or adipogenesis. The vital organs of mice were also not influenced by hydrogel treatment.
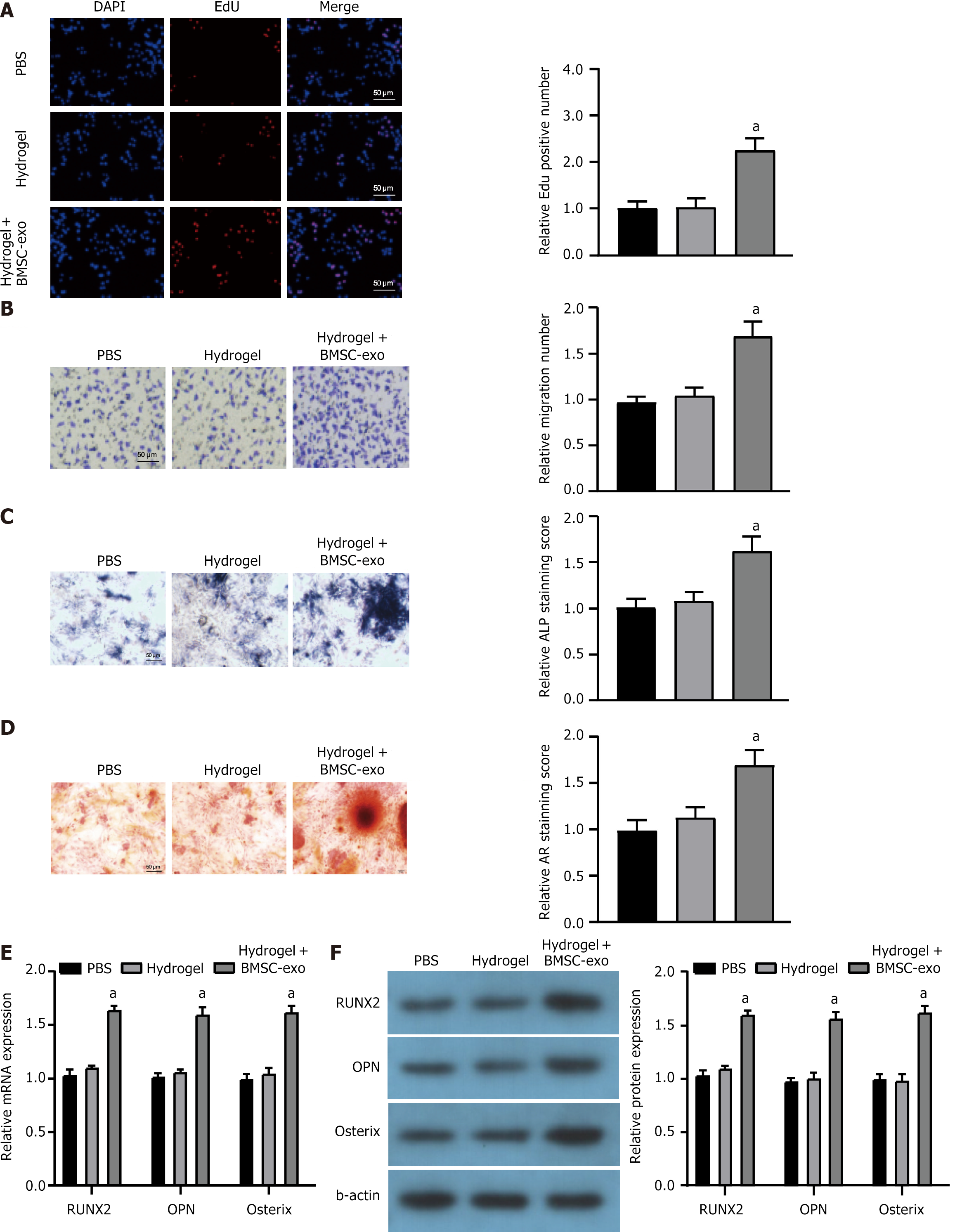
Using the EdU assay, we observed a marked increase in the proliferation of mOPCSs cells in the hydrogel + BMSC-exo group compared with that in the PBS control group or the hydrogel-only group (Figure 4A). The Transwell cell migration assay revealed that hydrogel treatment with BMSC-exo enhanced the migration of mOPCSs (Figure 4B). ALP and AR staining of mOPCSs treated with hydrogel + BMSC-exo displayed significantly denser mineral nodules and a higher degree of mineralization (Figure 4C and D). Notably, hydrogel + BMSC-exo significantly upregulated the expression of genes associated with osteogenesis (Runx2, OPN, and Osterix) in mOPCSs (Figure 4E and F). Therefore, the BMSC-exo hydrogel was successfully generated and showed significant reparative potential in vitro.
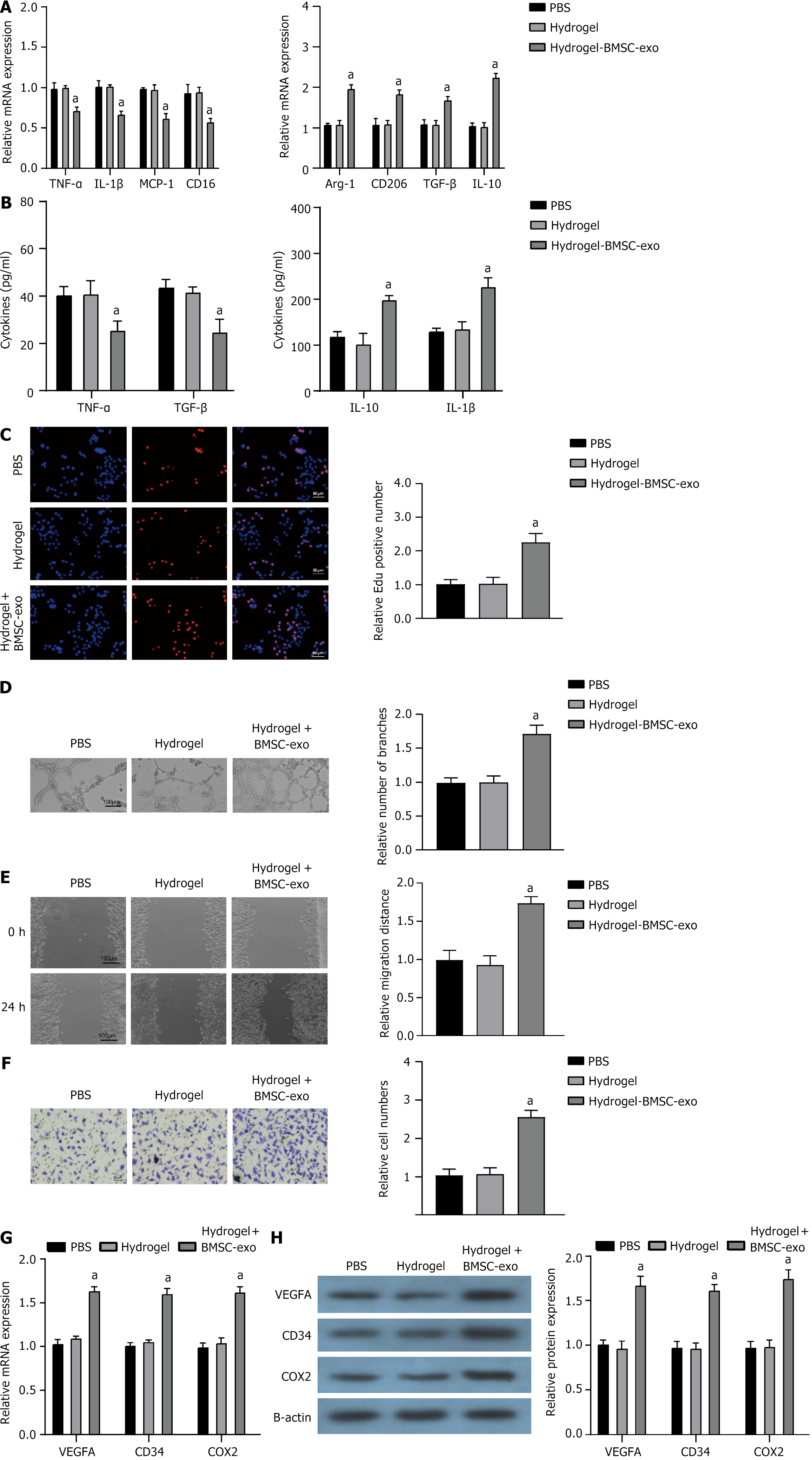
The BMSC-exo hydrogel exhibited significant inhibition of the inflammatory response in RAW264.7 macrophages, as evidenced by the downregulation of tumor necrosis factor-α, interleukin (IL)-1β, monocyte chemoattractant protein-1, and CD16 expression, along with the upregulation of arginase-1, CD206, transforming growth factor-β, and IL-10 expression (Figure 5A and B). When co-cultured with HUVECs, mOPCSs incubated on hydrogel + BMSC-exo exhibited enhanced proliferation and tube formation (Figure 5C and D). Scratch wound and Transwell assays and assays both showed that the migration ability of mOPCSs was significantly enhanced in the hydrogel + BMSC-exo group compared with that in the PBS control and the hydrogel-only groups (Figure 5E and F). Furthermore, the expression of angiogenesis factors [VEGFA, CD3, CD4, and cyclooxygenase-2 (COX2)] was increased in mOPCSs on hydrogel + BMSC-exo (Figure 5G and H). Hence, the BMSC-exo hydrogel could promote angiogenesis in vitro.
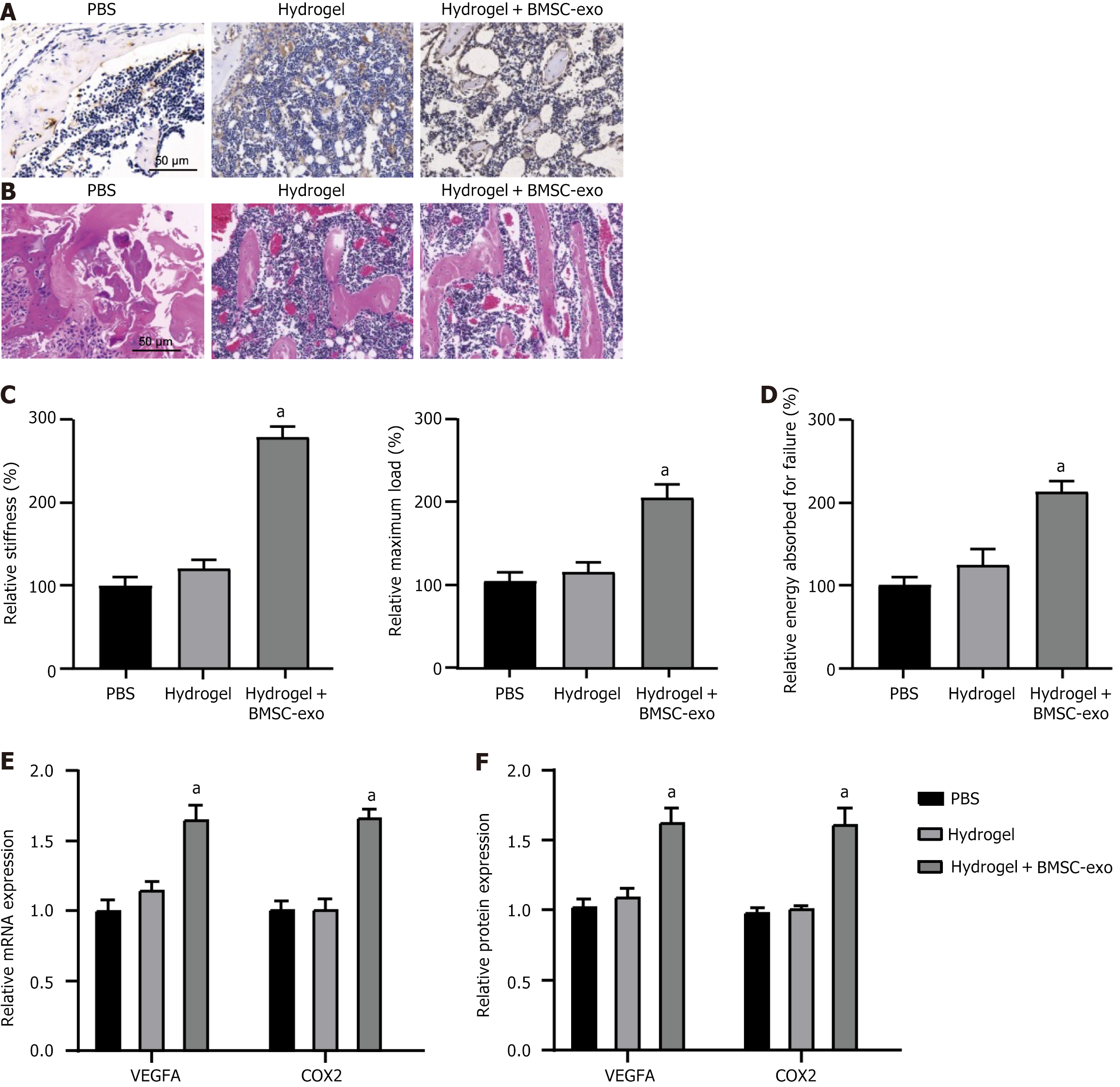
To evaluate hydrogel performance in vivo, mouse diaphysial fractures were treated with hydrogels containing or lacking BMSC-exo. Subsequently, the regenerated bone tissue from the mouse femur was collected for histological analysis, which revealed that treatment with BMSC-exo hydrogel increased bone formation and reduced cartilage at 30 d postinjury compared with both the PBS and hydrogel-only groups (Figure 6A and B). The reparative capacity of bone was assessed from multiple perspectives, demonstrating that BMSC-exo hydrogel enhanced the strength and toughness of regenerated bone by exhibiting higher maximum load, stiffness, and energy absorption upon failure (Figure 6C and D). Consistent with the in vitro findings, angiogenic markers (VEGFA and COX2) exhibited high expression levels in bone tissue treated with BMSC-exo hydrogels (Figure 6E and F). Therefore, BMSC-exo hydrogels could effectively promote fracture healing and angiogenesis in vivo.
Angiogenesis is a crucial step in regenerating and restoring tissues[14]. As a highly vascularized connective tissue, bone relies on blood vessels to deliver essential nutrients, oxygen, immune system cells, MSCs, hormones, neurotransmitters, and growth factors to support the metabolic needs of bone cells. Consequently, angiogenesis is critical in bone regeneration, and successful outcomes of bone repair procedures heavily depend on the formation of functional blood vessels through this process. To address this, various proangiogenic factors have been used to promote angiogenesis in repairing large bone defects. Among these approaches, the use of exosomes derived from MSCs has emerged as a promising cell-free strategy to enhance angiogenesis across multiple tissue regenerations[15,16]. Nevertheless, the development of effective exosome-based strategies for treating large bone defects remains to be accomplished. This study incorporated BMSC-exo into an alloplastic material (CHA/SF/GCS/DF-PEG hydrogels) and evaluated its efficacy in promoting bone regeneration using a rat fracture model. Furthermore, the underlying mechanism of this innovative strategy was investigated.
The BMSC-exo hydrogel significantly enhanced the proliferation, migration, and osteogenesis of mOPCSs while suppressing the inflammatory response of macrophages, thereby accelerating fracture healing and angiogenesis in vivo. The BMSC-exo hydrogel effectively improved the strength and toughness of the regenerated bone by increasing the maximum load, stiffness, and energy absorption upon failure. Although our in vitro and in vivo studies demonstrated the substantial effects of BMSC-exo-loaded hydrogels on fracture healing, further validation using clinical samples is warranted. This will be the primary focus of future investigations along with an in-depth exploration of the molecular mechanisms underlying this therapeutic effect.
Therefore, this novel material loaded with BMSC-exo presents a potential approach for enhancing angiogenesis and bone regeneration in clinical settings, thus holding promise for optimizing therapeutic outcomes for patients with fractures.
Repairing large bone defects is a major challenge for plastic surgeons, and autologous bone transplantation is currently the best treatment option. However, its application is limited due to scarce resources and complications. Exosomes derived from mesenchymal stem cells (MSCs) have shown great potential in bone regenerative medicine, but they face issues such as rapid degradation, short half-life, and unpredictable side effects. Bone marrow stromal cells play a crucial role in bone formation and tissue repair by migrating from their niche to surrounding tissues and promoting regeneration. The interaction between angiogenic factors and bone marrow-derived MSCs (BMSCs) regulates downstream osteogenesis; for example, vascular endothelial growth factor A can stimulate platelet-derived growth factor receptors to modulate the migration and proliferation of BMSCs.
To find a more effective method for bone defect repair to overcome the limitation of autologous bone grafting. Develop and optimize extracellular vesicle technology to improve its usability and safety in clinical practice. To further investigate the role of BMSCS in bone regeneration and explore their wider application in tissue repair. To reveal the mechanism of interaction between angiogenic factors and BMSCS and provide a theoretical basis for future therapeutic strategies.
Bone healing is an intricate physiological process initiated by early inflammatory immune regulation encompassing multiple events, including angiogenesis, osteogenic differentiation, and biommineralization. In this study, we fabricated a BMSC-derived exosomes (BMSC-exos)-loaded hydrogel that dynamically integrates diverse biological functions and thus operates at distinct stages of the fracture healing process.
The characterization of hydrogels and loaded BMSC-exo gels was verified to validate their properties. In vitro evaluations were conducted to assess the impact of hydrogels on various stages of the healing process. Hydrogels demonstrated the ability to recruit macrophages and inhibition of inflammatory responses, enhance human umbilical vein endothelial cell angiogenesis, and promote osteogenic differentiation of primary cranial osteoblasts. Furthermore, the effect of hydrogel on fracture healing was confirmed using a mouse fracture model.
During the initial stage of repair, the hydrogel loaded with BMSC-exos effectively attenuated the inflammatory response. Furthermore, they significantly enhanced vascular migration and angiogenesis. These effects were further corroborated in a fracture model.
The present study proposes a novel approach for loading BMSC-exos, aiming to optimize patient outcomes by enhancing angiogenesis and bone regeneration capacity in a clinical setting. Simultaneously, continuous development and optimization of extracellular vesicle technology in clinical practice are necessary to improve its usability and safety.
Further in-depth study of its possible mechanism of action and gradually spread to clinical application.
| 1. | Epple C, Haumer A, Ismail T, Lunger A, Scherberich A, Schaefer DJ, Martin I. Prefabrication of a large pedicled bone graft by engineering the germ for de novo vascularization and osteoinduction. Biomaterials. 2019;192:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (35)] |
| 2. | Kawecki F, Clafshenkel WP, Fortin M, Auger FA, Fradette J. Biomimetic Tissue-Engineered Bone Substitutes for Maxillofacial and Craniofacial Repair: The Potential of Cell Sheet Technologies. Adv Healthc Mater. 2018;7:e1700919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (35)] |
| 3. | Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18:100522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 255] [Article Influence: 85.0] [Reference Citation Analysis (37)] |
| 4. | Liao HT, Chen CT. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6:288-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (16)] |
| 5. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 730] [Article Influence: 104.3] [Reference Citation Analysis (36)] |
| 6. | Funari A, Alimandi M, Pierelli L, Pino V, Gentileschi S, Sacchetti B. Human Sinusoidal Subendothelial Cells Regulate Homing and Invasion of Circulating Metastatic Prostate Cancer Cells to Bone Marrow. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (36)] |
| 7. | Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1832] [Cited by in RCA: 1650] [Article Influence: 86.8] [Reference Citation Analysis (13)] |
| 8. | Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177:489-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (36)] |
| 9. | Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B, Chen L. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 94] [Reference Citation Analysis (38)] |
| 10. | Chamberlain CS, Clements AEB, Kink JA, Choi U, Baer GS, Halanski MA, Hematti P, Vanderby R. Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem Cells. 2019;37:652-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (16)] |
| 11. | Liu L, Guo S, Shi W, Liu Q, Huo F, Wu Y, Tian W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng Part A. 2021;27:962-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 12. | Filipowska J, Tomaszewski KA, Niedźwiedzki Ł, Walocha JA, Niedźwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20:291-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 393] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, Li L, Xu S, Xia Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl Mater Interfaces. 2021;13:18472-18487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 14. | Liu L, You Z, Yu H, Zhou L, Zhao H, Yan X, Li D, Wang B, Zhu L, Xu Y, Xia T, Shi Y, Huang C, Hou W, Du Y. Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nat Mater. 2017;16:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 15. | Brennan MÁ, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 16. | Fan J, Lee CS, Kim S, Chen C, Aghaloo T, Lee M. Generation of Small RNA-Modulated Exosome Mimetics for Bone Regeneration. ACS Nano. 2020;14:11973-11984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elchaninov AV, Russia; Li SC, United States S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD